Thermal Conductivity Variations in Frozen Hydrate-Bearing Sand upon Heating and Dissociation of Pore Gas Hydrate
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Thermal Conductivity of Frozen Sand: Effect of Gas Hydrate Component
3.2. Thermal Conductivity of Frozen Sand: Effect of Pressure Drop to Below Equilibrium
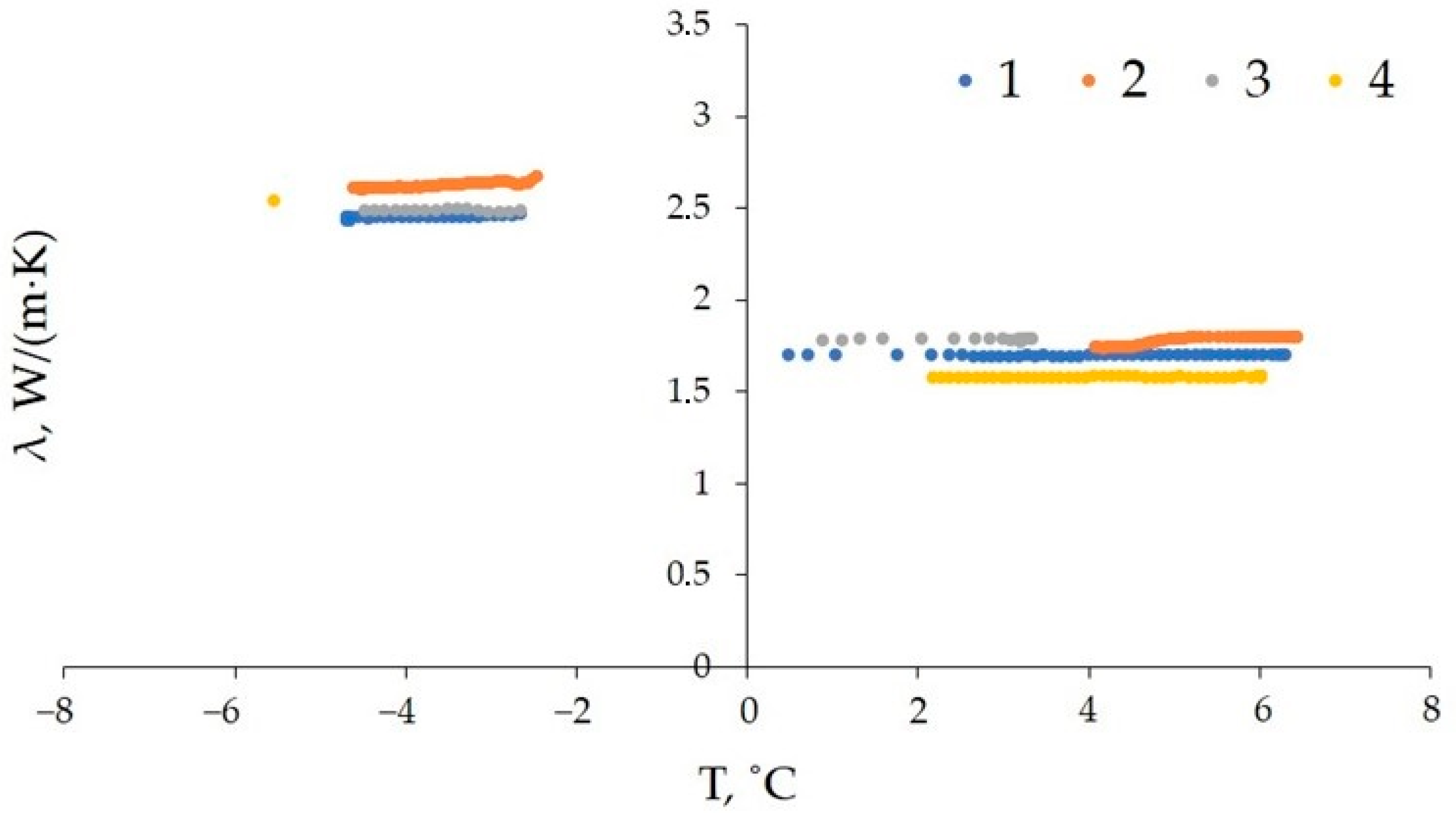
3.3. Thermal Conductivity of Frozen Sand: Effect of Temperature Increase
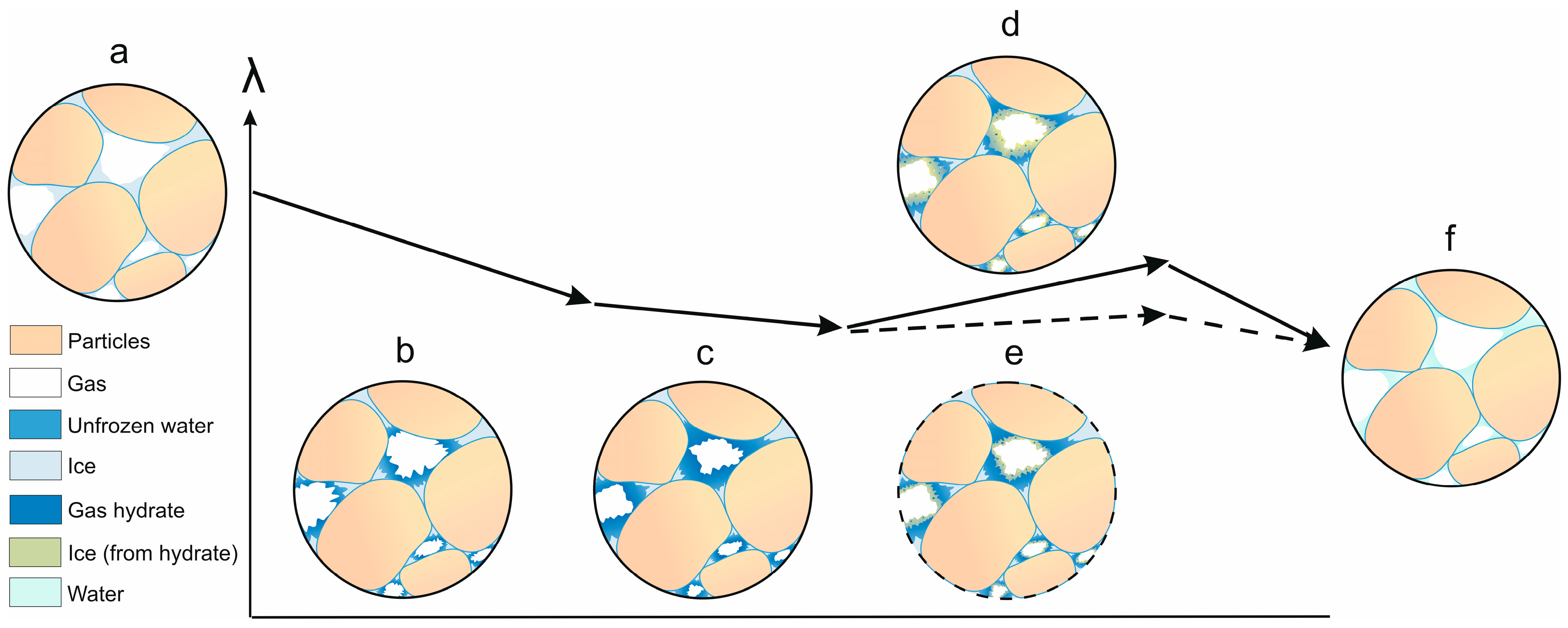
4. Discussion
5. Conclusions
- (1)
- Thermal conductivity increased (up to 20%) during dissociation of pore gas hydrate in frozen hydrate-bearing sand samples as gas pressure dropped below the equilibrium, as the relative percentages of low- and high-conductive components (methane hydrate and ice, respectively) shifted toward the latter. However, thermal conductivity first became a few percent to 10% lower, due to cracking, before increasing as the dissociated gas hydrate converted into ice (via liquid water).
- (2)
- Heating of hydrate-bearing sand containing 20–40% of residual pore hydrate to positive temperatures led to a 30% decrease in its thermal conductivity. The magnitude of thermal conductivity decreases in saline sand free from residual pore hydrate correlated with salinity (it was 40% at a salinity of ~0.14%).
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Collett, T.; Bahk, J.-J.; Baker, R.; Boswell, R.; Divins, D.; Frye, M.; Goldberg, D.; Husebø, J.; Koh, C.; Malone, M.; et al. Methane hydrates in nature—Current knowledge and challenges. J. Chem. Eng. Data 2015, 60, 319–329. [Google Scholar] [CrossRef]
- Boswell, R.; Hancock, S.; Yamomoto, K.; Collett, T.; Pratap, M.; Lee, S.-R. Natural Gas Hydrates: Status of Potential as an Energy Resource; Central Energy Resources Science Center: Denver, CO, USA, 2020; pp. 111–131. [CrossRef]
- Moridis, G.J.; Collett, T.S.; Boswell, R.; Kurihara, M.; Reagan, M.T.; Sloan, E.D.; Koh, C. Toward production from gas hydrates: Assessment of resources and technology and the role of numerical simulation. SPE Reserv. Eval. Eng. 2009, 12, 745–771. [Google Scholar] [CrossRef]
- Konno, Y.; Fujii, T.; Sato, A.; Akamine, K.; Naiki, M.; Masuda, Y.; Yamamoto, K. Key findings of the world’s first offshore methane hydrate production test off the coast of Japan: Toward future commercial production. Energy Fuel 2017, 31, 2607–2616. [Google Scholar] [CrossRef]
- Li, J.; Ye, J.; Qin, X.; Qiu, H.; Wu, N.; Lu, H.; Xie, W.; Lu, J.; Peng, F.; Xua, Z.; et al. The first offshore natural gas hydrate production test in South China Sea. China Geol. 2018, 1, 5–16. [Google Scholar] [CrossRef]
- Boswell, R.; Yoneda, J.; Waite, W.F. India national gas hydrate program expedition 02 summary of scientific results: Evaluation of natural gas-hydrate-bearing pressure cores. Mar. Pet. Geol. 2018, 108, 143–153. [Google Scholar] [CrossRef]
- Merey, S. Evaluation of drilling parameters in gas hydrate exploration wells. J. Pet. Sci. Eng. 2019, 172, 855–877. [Google Scholar] [CrossRef]
- Yamamoto, K.; Wang, X.-X.; Tamaki, M.; Suzuki, K. The second offshore production of methane hydrate in the Nankai Trough and gas production behavior from a heterogeneous methane hydrate reservoir. RSC Adv. 2019, 9, 25987–26013. [Google Scholar] [CrossRef]
- Yamamoto, K.; Boswell, R.; Collett, T.-S.; Dallimore, S.R.; Lu, H. Review of past gas production attempts from subsurface gas hydrate deposits and necessity of long-term production testing. Energy Fuels 2022, 36, 5047–5062. [Google Scholar] [CrossRef]
- Ye, J.-L.; Qin, X.-W.; Xie, W.-W.; Lu, H.-L.; Ma, B.-J.; Qiu, H.-J.; Liang, J.-Q.; Lu, J.-A.; Kuang, Z.-G.; Lu, C.; et al. The Second natural gas hydrate production test in the South China Sea. China Geol. 2020, 3, 197–209. [Google Scholar] [CrossRef]
- Max, M.D.; Johnson, A.H.; Dillon, W.P. Natural Gas Hydrate—Arctic Ocean Deepwater Resource Potential; Springer: Dordrecht, The Netherlands, 2013; p. 113. ISBN 978-3-319-02508-7. [Google Scholar]
- Ruppel, C. Permafrost-associated gas hydrate: Is it really approximately 1% of the global system? J. Chem. Eng. Data 2015, 60, 429–436. [Google Scholar] [CrossRef]
- Cook, J.G.; Leaist, D.G. An exploratory study of the thermal conductivity of methane hydrates. Geophys. Res. Lett. 1983, 10, 397–399. [Google Scholar] [CrossRef]
- Huang, D.; Fan, S. Thermal conductivity of methane hydrate formed from sodium dodecyl sulfate solution. J. Chem. Eng. Data 2004, 49, 1479–1482. [Google Scholar] [CrossRef]
- Rosenbaum, E.J.; English, N.J.; Johnson, J.K.; Shaw, D.W.; Warzinski, R.P. Thermal conductivity of methane hydrate from experiment and molecular simulation. J. Phys. Chem. B 2007, 111, 13193–13205. [Google Scholar] [CrossRef] [PubMed]
- Waite, W.F.; Stern, L.A.; Kirby, S.H.; Winters, W.J.; Mason, D.H. Simultaneous determination of thermal conductivity, thermal diffusivity and specific heat in sI methane hydrate. Geophys. J. Int. 2007, 169, 767–774. [Google Scholar] [CrossRef]
- Petrenko, V.F.; Whitworth, R.W. Physics of Ice, 3rd ed.; OUP: Oxford, UK, 2002; p. 373. [Google Scholar]
- Moridis, G.J.; Reagan, M.T.; Boyle, K.L.; Zhang, K. Evaluation of the gas production potential of challenging hydrate deposits. Transp. Porous Media 2011, 90, 269–299. [Google Scholar] [CrossRef]
- Groysman, A.G. Thermal Properties of Gas Hydrates; Nauka: Novosibirsk, Russia, 1985; p. 94. (In Russian) [Google Scholar]
- Asher, G.B. Development of a Computerized Thermal Conductivity Measurement System Utilizing the Transient Needle Probe Technique: An Application to Hydrates in Porous Media. Ph.D. Thesis, Dissertation T-3335. Colorado School of Mines, Golden, CO, USA, 1987; p. 179. [Google Scholar]
- Wright, J.F.; Nixon, F.M.; Dallimore, S.R.; Henninges, J.; Cote, M.M. Thermal conductivity of sediments within the gas-hydrate-bearing interval at the JAPEX/JNOC/GSC et al. Mallik 5L-38 gas hydrate production research well. In Mallik 5L-38 Gas Hydrate Production Research Well. Geological Survey of Canada, Bulletin 585; Dallimore, S.R., Collett, T.S., Eds.; US Government Printing Office: Washington, DC, USA, 2005; p. 10. [Google Scholar]
- Waite, W.F.; Santamarina, J.C.; Cortes, D.D.; Dugan, B.; Espinoza, D.N.; Germaine, J.; Jang, J.; Jung, J.W.; Kneafsey, T.J.; Shin, H.; et al. Physical properties of hydrate-bearing sediments. Rev. Geophys. 2009, 47, 668. [Google Scholar] [CrossRef]
- Sun, S.; Gu, L.; Yang, Z.; Li, Y.; Zhang, C. A new effective thermal conductivity model of methane hydrate-bearing sediments considering hydrate distribution patterns. Int. J. Heat Mass Transf. 2022, 183, 122071. [Google Scholar] [CrossRef]
- Stoll, R.D.; Bryan, G.M. Physical properties of sediments containing gas hydrates. J. Geophys. Res. 1979, 84, 1629–1634. [Google Scholar] [CrossRef]
- Duchkov, A.D.; Manakov, A.Y.; Kazantsev, S.A.; Permyakov, M.E.; Ogienko, A.G. Thermal conductivity measurement of the synthetic samples of bottom sediments containing methane hydrates. Izv. Phys. Solid Earth 2009, 45, 661–669. [Google Scholar] [CrossRef]
- Li, D.; Liang, D. Experimental study on the effective thermal conductivity of methane hydrate-bearing sand. Int. J. Heat Mass Transf. 2016, 92, 8–14. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, J.; Wang, B.; Liu, W.; Yang, M.; Song, Y. Effective thermal conductivity of methane hydrate-bearing sediments: Experiments and correlations. Fuel 2016, 179, 87–96. [Google Scholar] [CrossRef]
- Fadeeva, I.I.; Duchkov, A.A.; Permyakov, M.E. Thermophysical method for quantitative estimation of hydrate content in samples imitating bottom sediments. Russ. Geol. Geophys. 2016, 57, 984–992. [Google Scholar] [CrossRef]
- Wang, B.; Fan, Z.; Lv, P.; Zhao, J.; Song, Y. Measurement of effective thermal conductivity of hydrate-bearing sediments and evaluation of existing prediction models. Int. J. Heat Mass Transf. 2017, 110, 142–150. [Google Scholar] [CrossRef]
- Li, D.L.; Du, J.W.; He, S.; Liang, D.Q.; Zhao, X.Y.; Yang, X.Y. Measurement and modeling of the effective thermal conductivity for porous methane hydrate samples. Sci. China Chem. 2012, 55, 373–379. [Google Scholar] [CrossRef]
- Sun, S.; Zhao, J.; Zhao, J.; Hao, Y.; Yang, J. The effective thermal conductivity of methane hydrate-bearing sea sand. J. Chem. Thermodynam. 2019, 132, 423–431. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Chen, Z.; Li, Q.; Xia, Z.; Zhang, Y.; Wang, Y.; You, C. Effective thermal conductivity changes of the hydrate-bearing quartz sands in depressurization and soaking. J. Nat. Gas Sci. Eng. 2021, 89, 103878. [Google Scholar] [CrossRef]
- Wei, R.; Xia, X.; Qu, X.; Lv, X.; Fan, Q.; Zhang, L.; Zhang, Y.; Zhao, J.; Yang, L. Dependence of thermal conductivity on the phase transition of gas hydrate in clay sediments. Fuel 2022, 317, 123565. [Google Scholar] [CrossRef]
- Li, X.; Wei, X.; Li, Q.; Pang, W.; Fan, Q.; Chen, Q.; Sun, C. Experimental investigation on the effective thermal conductivities of different hydrate-bearing sediments. Petrol. Sci. 2023, 20, 2479–2487. [Google Scholar] [CrossRef]
- Henninges, J.; Huenges, E.; Burkhardt, H. In situ thermal conductivity of gas-hydrate-bearing sediments of the Mallik 5L–38 well. J. Geophys. Res. 2005, 110, B11206. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yun, T.S. Thermal conductivity of methane hydrate-bearing Ulleung Basin marine sediments: Laboratory testing and numerical evaluation. Mar. Pet. Geol. 2013, 47, 77–84. [Google Scholar] [CrossRef]
- Muraoka, M.; Ohtake, M.; Susuki, N.; Yamamoto, Y.; Suzuki, K.; Tsuji, T. Thermal properties of methane hydrate-bearing sediments and surrounding mud recovered from Nankaitrough wells. J. Geophys. Res. 2014, 119, 8021–8033. [Google Scholar] [CrossRef]
- Muraoka, M.; Susuki, N.; Yamaguchi, H.; Tsuji, T.; Yamamoto, Y. Thermal properties of a supercooled synthetic sand–water–gas–methane hydrate sample. Energy Fuels 2015, 29, 1345–1351. [Google Scholar] [CrossRef]
- Muraoka, M.; Ohtake, M.; Susuki, N.; Morita, H.; Oshima, M.; Yamamoto, Y. Thermal properties of highly saturated methane hydrate-bearing sediments recovered from the Krishna–Godavari Basin. Mar. Pet. Geol. 2019, 108, 321–331. [Google Scholar] [CrossRef]
- Fan, S.; Li, D.; Huang, D.; Liang, D. Thermal conductivity characteristic of methane hydrate below ice point and at atmospheric pressure. In Proceedings of the 6th International Conference on Gas Hydrates, Vancouver, BC, Canada, 6–20 July 2008; pp. 518–531. [Google Scholar]
- Chuvilin, E.; Bukhanov, B. Thermal conductivity variations of gas-hydrated sediments during hydrate formation at different conditions. Part 2. Results. Kriosf. Zemli 2014, 18, 49–55. [Google Scholar]
- Chuvilin, E.; Bukhanov, B. Effect of hydrate formation conditions on thermal conductivity of gas-saturated sediments. Energy Fuels 2017, 31, 5246–5254. [Google Scholar] [CrossRef]
- Chuvilin, E.; Bukhanov, B. Thermal conductivity of frozen sediments containing self-preserved pore gas hydrates at atmospheric pressure: An experimental study. Geosciences 2019, 9, 65. [Google Scholar] [CrossRef]
- Chuvilin, E.; Tipenko, G.; Bukhanov, B.; Istomin, V.; Pissarenko, D. Simulating thermal interaction of gas production wells with relict gas hydrate-bearing permafrost. Geosciences 2022, 12, 115. [Google Scholar] [CrossRef]
- Vasil’eva, Z.A.; Yakushev, V.S. Influence of gas well thermal insulation parameters on thawing intensity of permafrost and intrapermafrost gas hydrates. Earth’s Cryosphere 2017, 21, 92–98. [Google Scholar]
- Yakushev, V.S. Difficulties for Gas-Bearing Permafrost. Oil Gas J. Russ. 2017, 1, 66–70. [Google Scholar] [CrossRef]
- Yakushev, V.S. Natural gas liberations around production wells at Russian Arctic gas fields. Geosciences 2020, 10, 184. [Google Scholar] [CrossRef]
- Yakushev, V. Environmental and technological problems for natural gas production in permafrost regions. Energies 2023, 16, 4522. [Google Scholar] [CrossRef]
- Bristow, K.L.; White, R.D.; Kluitenberg, G.J. Comparison of single and dual probes for measuring soil thermal properties with transient heating. Aust. J. Soil Res. 1994, 32, 447–464. [Google Scholar] [CrossRef]
- Chuvilin, E.; Bukhanov, B.; Davletshina, D.; Grebenkin, S.; Istomin, V. Dissociation and self-preservation of gas hydrates in permafrost. Geosciences 2018, 8, 431. [Google Scholar] [CrossRef]
- Staykova, D.K.; Kuhs, W.F.; Salamatin, A.; Hansen, T. Formation of porous gas hydrates from ice powder: Diffraction experiments and multi-stage model. Phys. Chem. B 2003, 107, 10299–10311. [Google Scholar] [CrossRef]
- Chuvilin, E.M.; Davletshina, D.A.; Lupachik, M.V. Hydrate formation in frozen and thawing methane saturated sediments. Earth’s Cryosphere 2019, 23, 50–61. [Google Scholar]


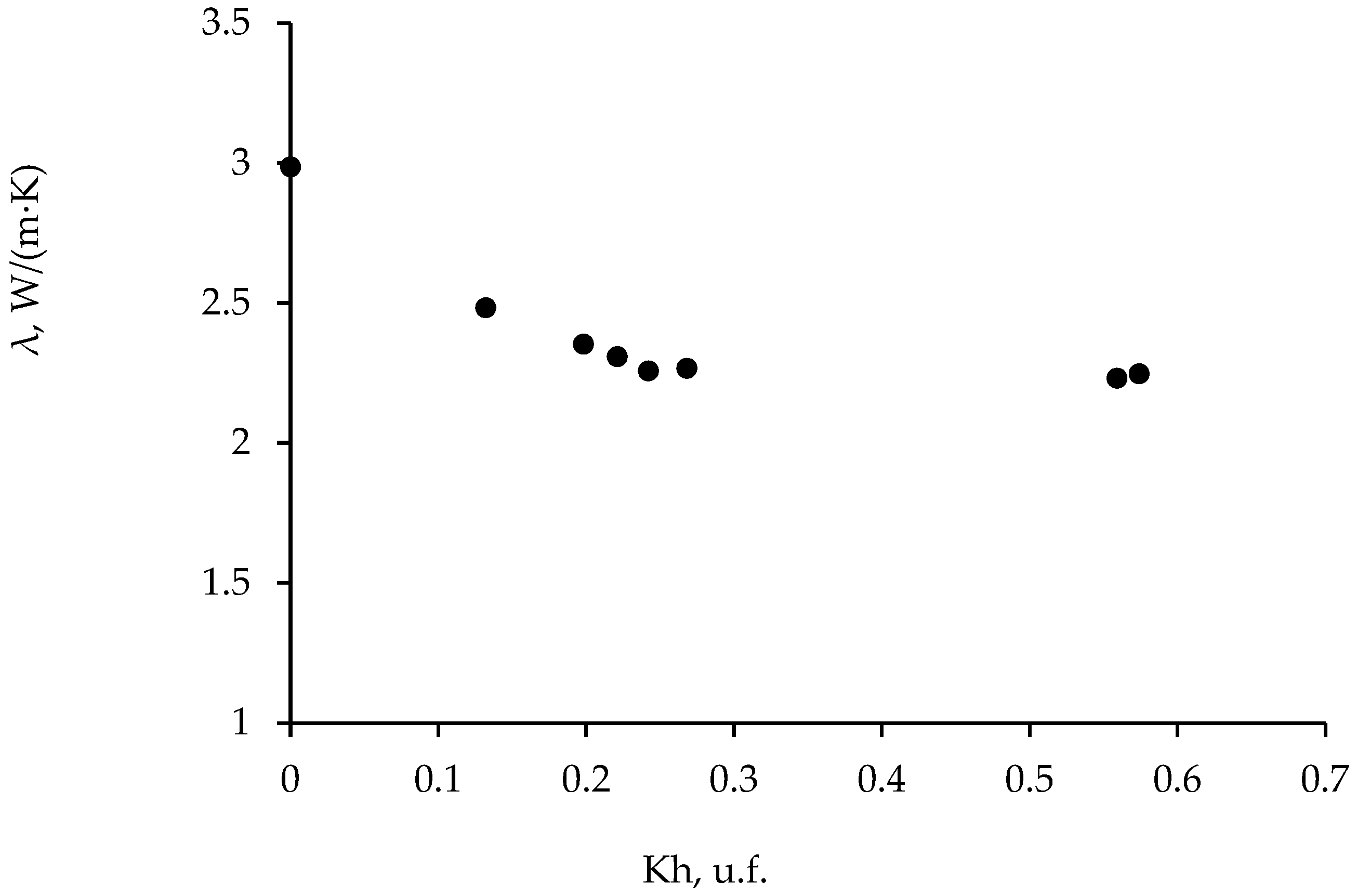
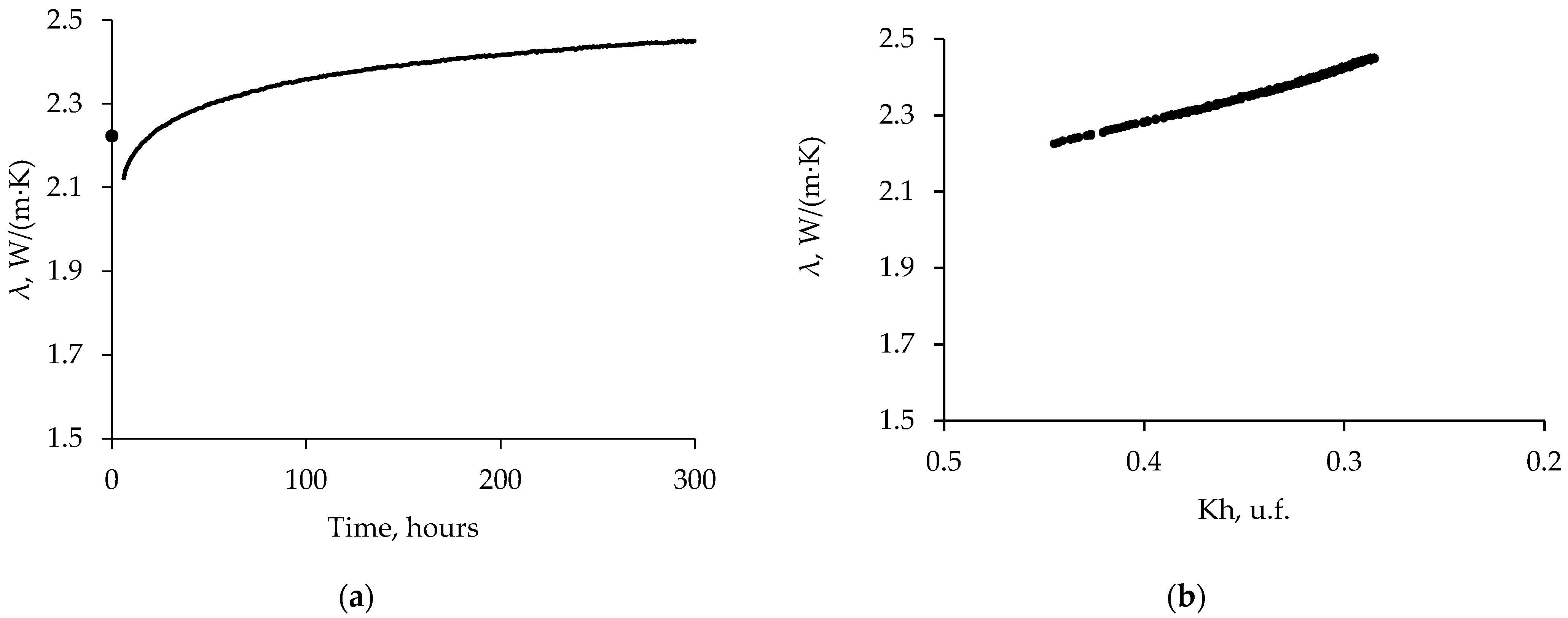
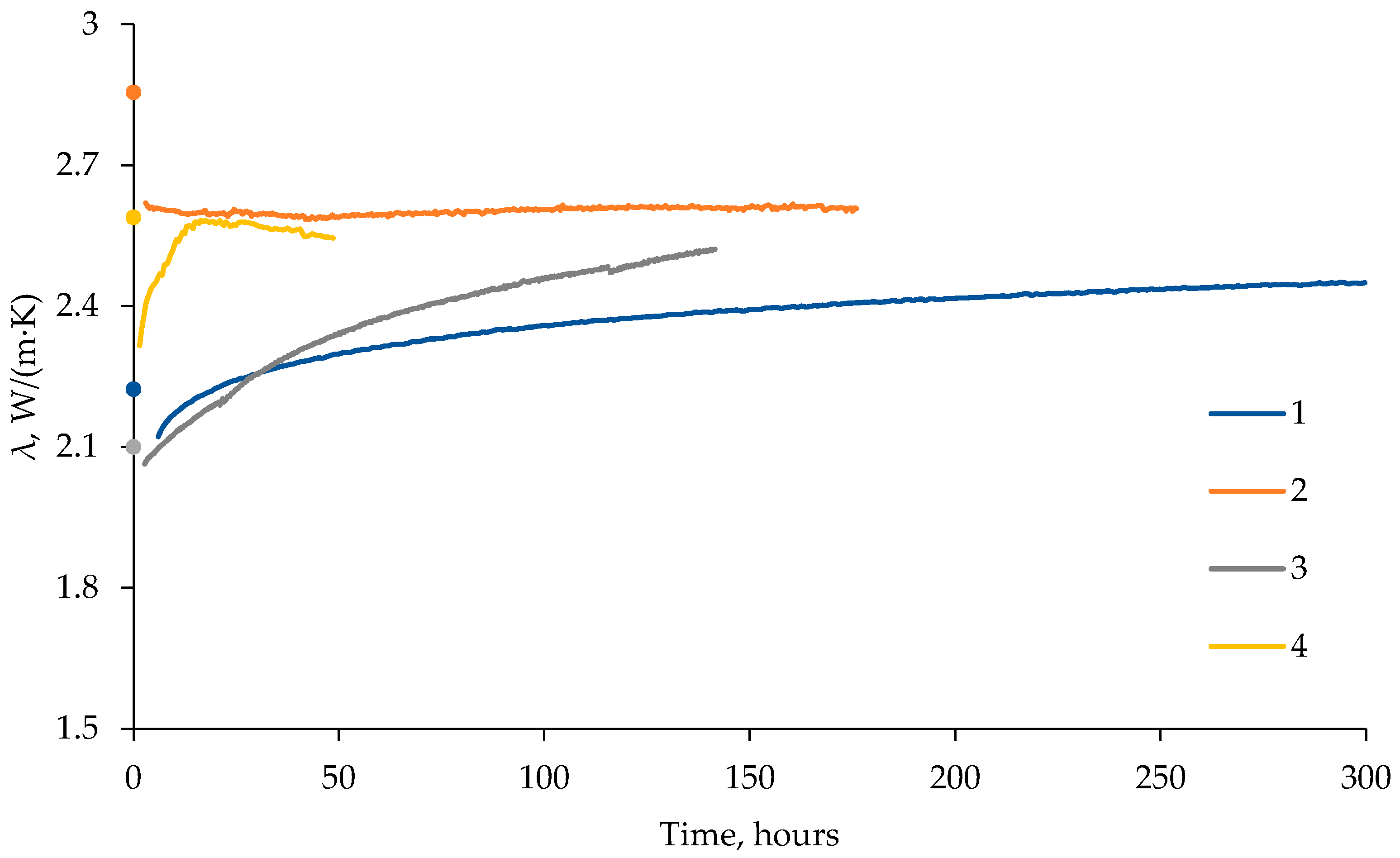
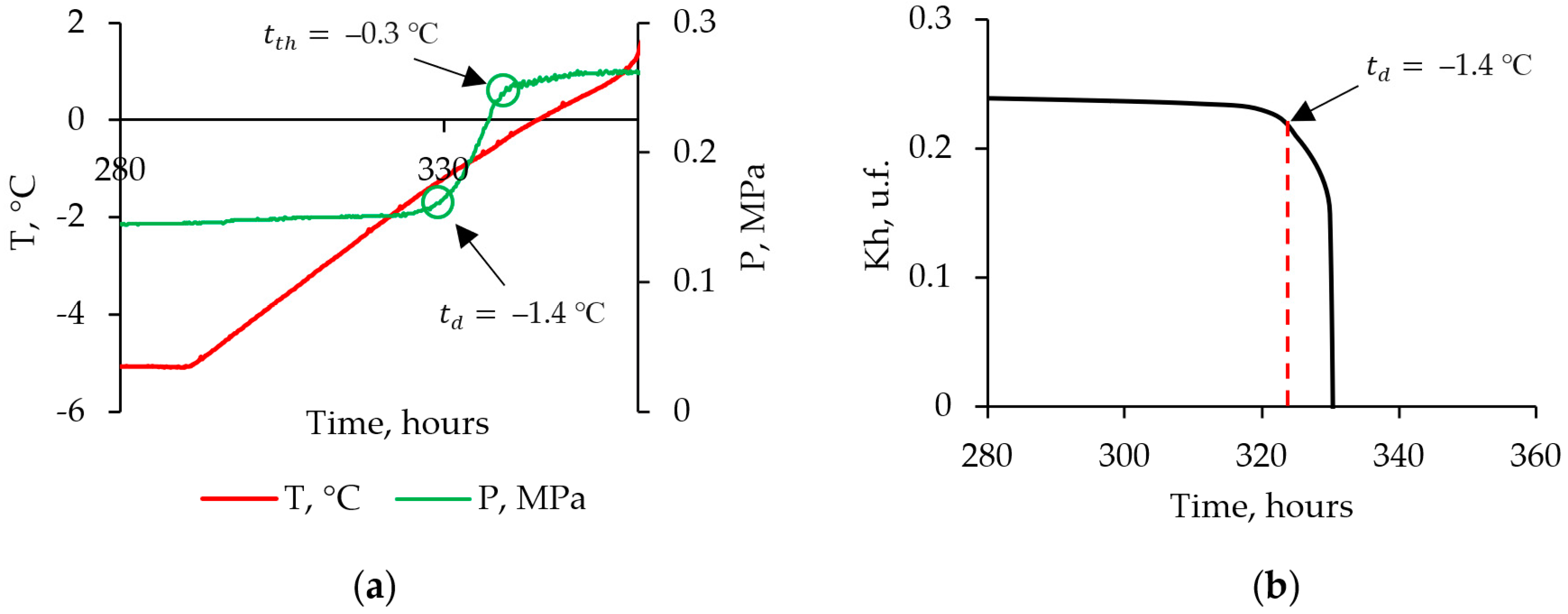
| Run | Moisture Content, % | Dry Density, g/cm3 | Porosity, u.f. | , % |
|---|---|---|---|---|
| 1 | 16 | 1.64 | 0.37 | 78 |
| 2 | 16 | 1.65 | 0.37 | 78 |
| 3 | 14 | 1.68 | 0.35 | 73 |
| 4 | 16 | 1.69 | 0.35 | 85 |
| Run | Hydrate Saturation | |||||
|---|---|---|---|---|---|---|
| Equilibrium Pressure | Below-Equilibrium Pressure | |||||
| , % | , u.f | , % | , u.f | , u.f | , Hours | |
| 1 | 58 | 0.57 | 32 | 0.31 | 53 | 300 |
| 2 | 48 | 0.46 | 40 | 0.39 | 84 | 175 |
| 3 | 46 | 0.48 | 21 | 0.21 | 44 | 140 |
| 4 | 50 | 0.44 | 0 | 0 | 0 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuvilin, E.; Davletshina, D.; Bukhanov, B.; Grebenkin, S.; Pankratova, E. Thermal Conductivity Variations in Frozen Hydrate-Bearing Sand upon Heating and Dissociation of Pore Gas Hydrate. Geosciences 2023, 13, 316. https://doi.org/10.3390/geosciences13100316
Chuvilin E, Davletshina D, Bukhanov B, Grebenkin S, Pankratova E. Thermal Conductivity Variations in Frozen Hydrate-Bearing Sand upon Heating and Dissociation of Pore Gas Hydrate. Geosciences. 2023; 13(10):316. https://doi.org/10.3390/geosciences13100316
Chicago/Turabian StyleChuvilin, Evgeny, Dinara Davletshina, Boris Bukhanov, Sergey Grebenkin, and Elena Pankratova. 2023. "Thermal Conductivity Variations in Frozen Hydrate-Bearing Sand upon Heating and Dissociation of Pore Gas Hydrate" Geosciences 13, no. 10: 316. https://doi.org/10.3390/geosciences13100316
APA StyleChuvilin, E., Davletshina, D., Bukhanov, B., Grebenkin, S., & Pankratova, E. (2023). Thermal Conductivity Variations in Frozen Hydrate-Bearing Sand upon Heating and Dissociation of Pore Gas Hydrate. Geosciences, 13(10), 316. https://doi.org/10.3390/geosciences13100316










