1. Introduction
Fenitization is the metasomatism of wall rocks under the influence of fluids released from crystallizing carbonatite or alkaline intrusions [
1,
2]. Fenites are named after the Fen complex (southern Norway), where these rocks were first described in 1921 by Brögger [
1]. Initially, fenites were defined as rocks of originally granitic composition that had been metasomatically altered towards an alkali-syenitic composition by solutions sourced from ijolite–melteigite magma. Now the term ‘fenite’ has a more general meaning and includes a wide spectrum of alkaline alteration products developed near alkaline and carbonatite intrusions. The mineralogical and textural diversities of fenites are determined by a large number of parameters, such as compositions of alkaline melt and fluid, protolith mineralogy, temperature, and pressure [
3,
4,
5,
6,
7,
8].
Fenites are of great economic importance because they often contain elevated concentrations of rare earth elements (REE) as well as high field strength elements (HFSE). The reason is that fluids exsolved from the crystallizing intrusions of alkaline rocks and carbonatites contain Cl
−, F
−, SO
42−, PO
43− or CO
32− anions, which can form complexes with REE and HFSE [
9,
10,
11,
12]. As a result, the solubility of these elements increases significantly, and they can be transported into fenite aureoles and precipitated there [
7,
13,
14].
Generally, fenites associated with alkaline and carbonatite intrusions differ from each other in volume. Indeed, compared to carbonatites, alkaline intrusions produce comparatively narrow fenite aureoles. For example, fenitization extends 1–2 km from the central Sokli (Finland) carbonatite intrusion [
15], whereas fenitization surrounding large nepheline-syenite intrusions such as Khibiny (NW Russia) and Ilímaussaq (Greenland) does not typically exceed 100–120 m from the intrusion [
16,
17,
18,
19]. The reason for such differences is the high solubility of water in alkaline melts [
20]. Aqueous fluid gradually accumulates during magmatic crystallization, and at the late- to post-magmatic stage, this fluid causes intense autometasomatic reactions. As a result of such reactions, secondary assemblages of minerals are formed at the expense of primary magmatic minerals [
7]. Typical examples of autometasomatic reactions are the replacement of nepheline and sodalite by natrolite and gonnardite [
21,
22]. In other words, the main volume of fluids is spent on autometasomatic reactions occurring within the boundaries of the alkaline intrusion.
An excellent example of a case where a huge alkaline massif is surrounded by a surprisingly narrow fenite aureole is the Lovozero massif, Kola Peninsula, Russia. This massif was emplaced 360–370 Ma ago [
23,
24,
25] into Archean gneiss and gneissose granite and has a size of 20 × 30 km at the top, and about 12 × 16 km at a 5 km depth [
26]. According to Arzamastsev and colleagues [
27], the calculated volume of the Lovozero massif is 1600 ± 250 km
3. Depending on the initial water content in the melt, the volume of water fluid released during crystallization of the massif could reach 100 km
3. Considering such a huge volume of fluid, one should assume the presence of a wide fenite aureole. However, fenitization in the Lovozero massif is associated mainly with alkaline pegmatites and hydrothermal veins that have intruded into the exocontact [
17,
28], while at the contact of alkaline rocks and country rocks, the width of the fenite aureole does not exceed several meters. Thus, a relatively wide fenitization aureoles are observed only in areas where pegmatites and hydrothermal veins are present.
In this article, we present the results of petrological, geochemical and fluid inclusion studies of the contact influence of alkaline rocks of the Lovozero massif on country Archean gneiss. In order to study the direct effect of the alkaline melt on the surrounding rocks, samples for this research were taken from an area where pegmatites and hydrothermal veins are absent. Based on the results of the fluid inclusions study, as well as the chemical compositions of minerals and rocks, we made conclusions about the sequence of metasomatic alterations of the country rocks and the evolution of the fenitizing fluids.
2. Geological Background and Previous Research
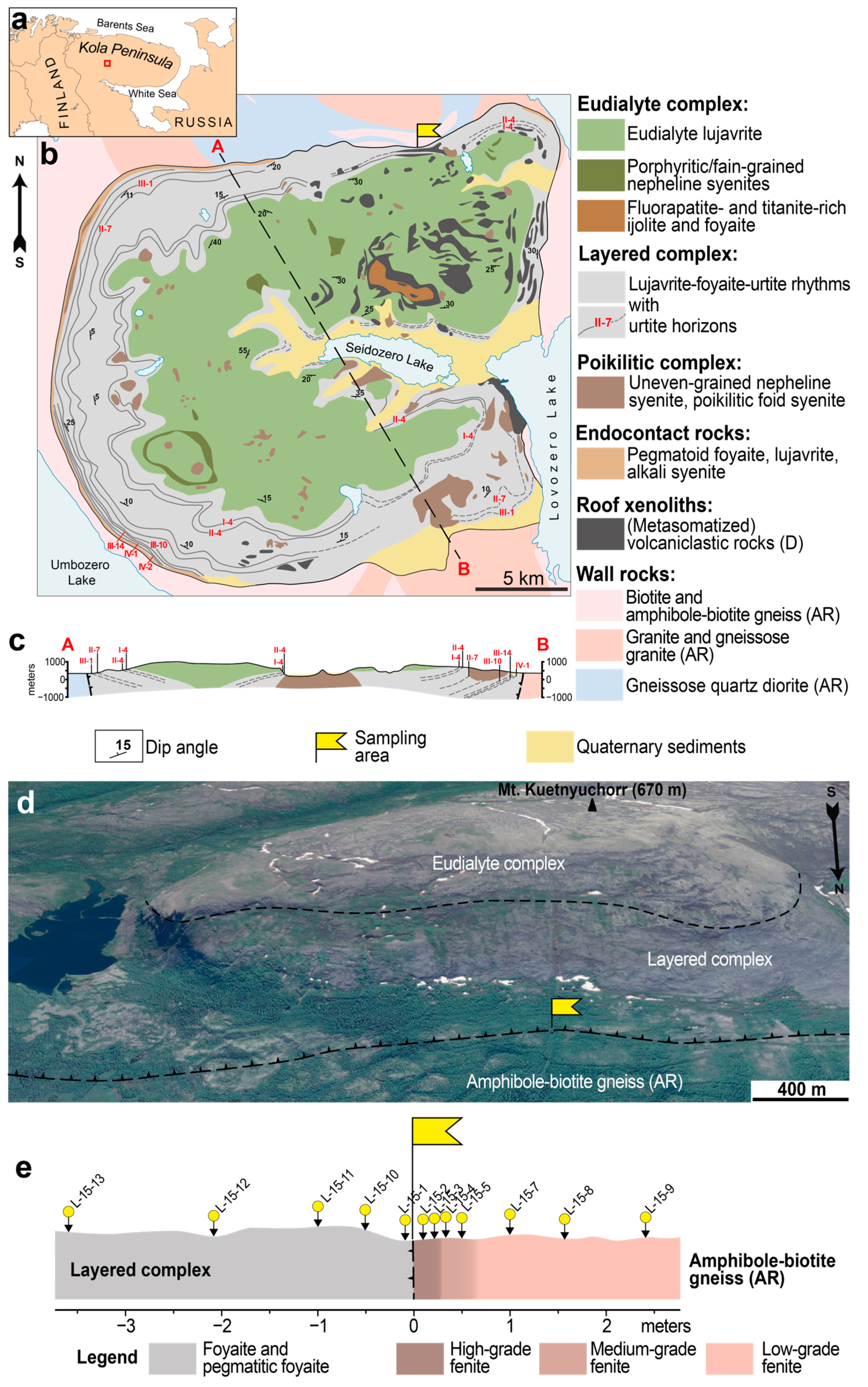
The Lovozero layered intrusion [
26,
29] is located on the Kola Peninsula, Russia (
Figure 1) and covers an area of 650 km
2. The main rock types of the Lovozero massif are foid syenites and foidolites, which compose three main units (or complexes;
Figure 1a–c). The most voluminous (77% of massif’s volume), the so-called Layered complex, comprises ofnumerous subhorizontal layers (or rhythms) [
26,
29,
30]. Each rhythm is a sequence of rocks: foidolite (urtite or ijolite; bottom of the rhythm)—leucocratic nepheline syenite (foyaite)—meso/melanocratic nepheline syenite (lujavrite). In some rhythms foidolite is absent and such rhythms consist only of foyaite (bottom of the rhythm) and lujavrite. The contact between underlying lujavrite and overlying urtite or foyaite is sharp. All rhythms of the Layered complex are grouped into seven series (I–VII from top to bottom). In each series, the urtite layers are additionally indicated by Arabian numerals.
Figure 1b,c shows only some of the urtite horizons, namely I-4, II-4, II-7, III-1, III-10, III-14, IV-1, IV-2.
The second, so-called Eudialyte complex (18% of massif’s volume; thickness varies from 0.1 to 0.8 km), overlies the Layered complex (
Figure 1c) and consists of lujavrite enriched in eudialyte-group minerals (EGM), so-called eudialyte lujavrite. In addition to eudialyte lujavrite, this complex also contains lenses and sheet-like bodies of foyaite, as well as fine-grained/porphyritic nepheline syenites (
Figure 1b).
The Poikilitic complex (5% of massif’s volume) consists of leucocratic feldspathoid syenites, in which grains of feldspathoids are poikilitically incorporated into large crystals of alkali feldspar. These rocks form lenses, or irregularly shaped bodies, which are located in both the Layered and Eudialyte complexes. In the Poikilitic complex, the following two main groups of rocks are recognized: uneven-grained nepheline syenite and poikilitic foid syenite. These rocks are connected by gradual transitions but differ in the content of poikilitic feldspar crystals. A large number of xenoliths of Devonian volcaniclastic rocks [
31,
32], both unaltered and intensely metasomatized, are found among the rocks of the Layered and the Eudialyte complexes.
Figure 1.
Geological background and sampling points. (
a) Location of the Lovozero massif (red square); (
b) geological scheme of the Lovozero alkaline massif and (
c) cross-section along the line A–B (after [
33] with simplifications); (
d) satellite image of the northern slope of the Mt. Kuetnyuchorr. The contact of the Eudialyte and Layered complexes (dashed line), the contact of the massif with country amphibole-biotite gneiss (dotted line with triangles), and the sampling area (yellow flag) are shown; (
e) sampling scheme; yellow circles with arrows show sampling points (see also
Table 1).
Figure 1.
Geological background and sampling points. (
a) Location of the Lovozero massif (red square); (
b) geological scheme of the Lovozero alkaline massif and (
c) cross-section along the line A–B (after [
33] with simplifications); (
d) satellite image of the northern slope of the Mt. Kuetnyuchorr. The contact of the Eudialyte and Layered complexes (dashed line), the contact of the massif with country amphibole-biotite gneiss (dotted line with triangles), and the sampling area (yellow flag) are shown; (
e) sampling scheme; yellow circles with arrows show sampling points (see also
Table 1).
The earliest studies of fenites of the Lovozero massif were carried out in the 60s of the last century [
34,
35]. The following features of the contact zone have been recognized: (1) the fenite aureole at the direct contact between alkaline rocks and Archean gneiss is very narrow; (2) pegmatites and hydrothermal veins surrounded by wide fenitization halos are widespread in the exocontact; (3) pegmatitic foyaite with rare-metal mineralization is widespread in the endocontact. Later, the contact interactions of the Lovozero massif with country rocks were studied by Arzamastsev and colleagues [
17,
28]. These researchers found that fenitization was associated predominantly with hydrothermal veins and occurred 359 ± 5 Ma ago, i.e., after crystallization of the bulk of the massif. According to Arzamastsev [
17], metasomatic alterations of gneiss occurred under the influence of an essentially aqueous fluid that also transported Nb, Ta, Zr, Hf, and REE in complexes with F
−, Cl
−, and SO
42− to the exocontact zone. In endocontact up to 200 m wide, nepheline syenites have a fine-grained or, conversely, pegmatitic texture and are enriched in calcium-bearing minerals, such as diopside, katophorite, Sr-rich apatite, titanite, pyrochlore-group minerals, and rinkite.
3. Materials and Methods
For this study, 12 rock samples were collected along a profile crossing the contact of the Lovozero massif with surrounding amphibole-biotite gneiss. This cross-section is located at the foot of Mt. Kuetnyuchorr in the north of the massif (
Figure 1b,d). The sampling scheme is shown in
Figure 1e, and a list of samples studied samples and their brief description is shown in
Table 1. Fenites were classified based on the intensity of textural or mineralogical change in accordance with the qualitative ‘grade’ classification of V. Morogan [
36]:
- (1)
low-grade fenites (textures and mineralogy inherited from the protolith largely unaltered);
- (2)
medium-grade fenites (relicts rare, new rock textures develop from fluid injection and by replacement reactions or recrystallization);
- (3)
high-grade fenites (protolith mineralogy almost fully altered).
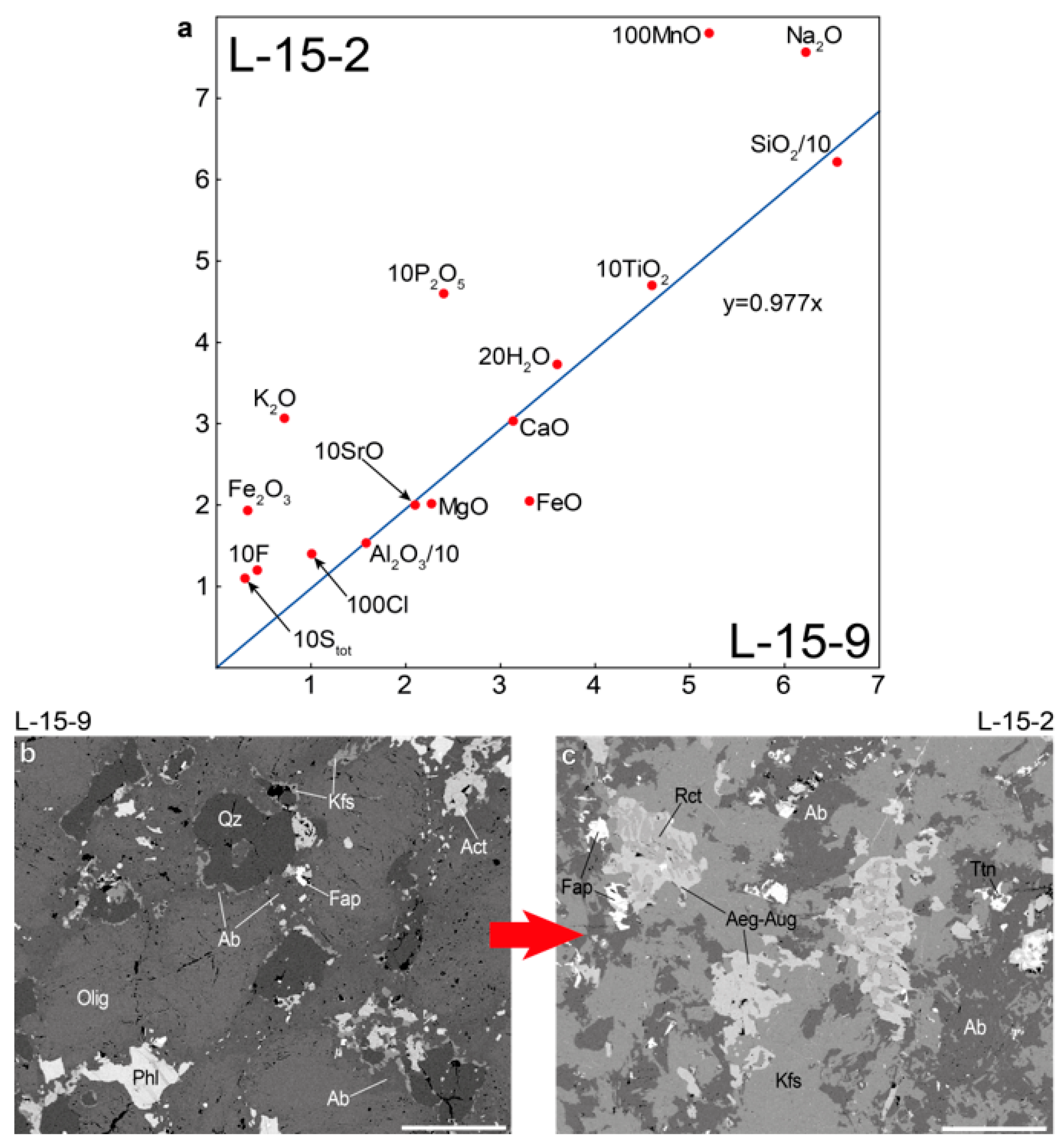
Endocontact alkaline rocks are leucocratic nepheline syenites that differ in grain size, so we subdivided these rocks into medium- to coarse-grained foyaite (hereinafter, we refer to this rock as foyaite) and pegmatoid foyaite (
Table 1).
The 12 doubly polished thin sections were prepared for fluid inclusion investigations and microprobe analyses. Back-scattered electron (BSE) images were obtained and minerals were diagnosed at the Geological Institute of the Kola Science Center of the Russian Academy of Sciences (GI KSC RAS, Apatity, Russia) using a scanning electron microscope LEO-1450 (Carl Zeiss Microscopy, Oberkochen, Germany) with the energy-dispersive system AZtec Ultimmax 100 (Oxford Instruments, Oxford, UK). The chemical composition of minerals was analyzed with the Cameca MS-46 electron microprobe (Cameca, Gennevilliers, France) operating in the WDS-mode at 22 kV with a beam diameter of 10 μm, a beam current of 20–40 nA, and counting times of 10 s (for a peak) and 10 s (for background before and after the peak), with 5–10 counts for every element in each point. Standards are shown in
Table A1. The analytical precision (reproducibility) of mineral analyses was 0.2–0.05 wt.% (2 standard deviations) for the major element and approximately 0.01 wt.% for impurities. The systematic errors were within the random errors.
Major elements in rocks (12 samples) were determined by wet chemical analysis at the GI KSC RAS. The accuracy limits for SiO2, TiO2, ZrO2, Fe2O3, Al2O3, CaO, SrO, MgO, MnO, Na2O, K2O, P2O5, REE2O3, Stot, F, Cl, H2O are 0.01 wt.%, and for FeO is 0.1 wt.%.
The Raman spectra were recorded with a Horiba Jobin–Yvon LabRAM HR800 spectrometer equipped with an Olympus BX-41 microscope in backscattering geometry (Saint-Petersburg State University). The 514.5 nm Ar + laser line was used for spectra excitation. The spectra were obtained in the range of 100–4200 cm−1 at a resolution of 1 cm−1 at room temperature. All spectra were processed using the algorithms implemented in the OriginPro 8.1 software package (Originlab Corporation, Northampton, MA, USA).
Heating and freezing experiments with fluid inclusions were carried out using a Linkam THMSG-600 stage with a measuring range from −196 to +600 °C. Cooling was carried out by supplying a stream of liquid nitrogen. The measurement errors of freezing and heating temperature are ±0.1 and ±1 °C, respectively. Temperature changes were controlled using the Linksys 32 software module. Visual control of phase transformations in inclusions was carried out on an Olympus BX53 microscope (Olympus Corp., Tokyo, Japan) equipped with an Olympus 50× long-focus objective and an Olympus EP50 digital camera.
Mineral abbreviations (
Table 2) are given in accordance with International Mineralogical Association (IMA)-approved mineral symbols [
37].
5. Discussion
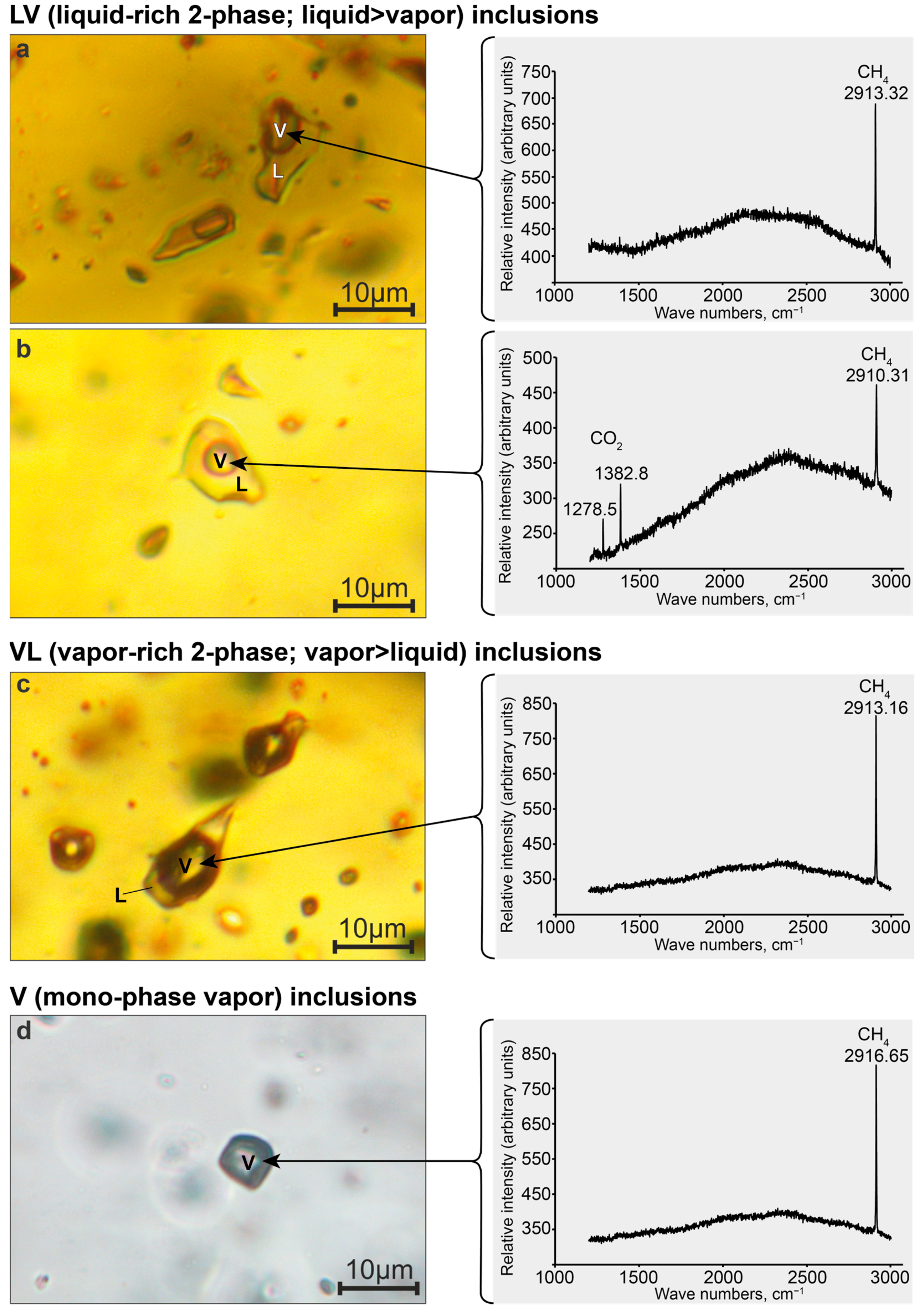
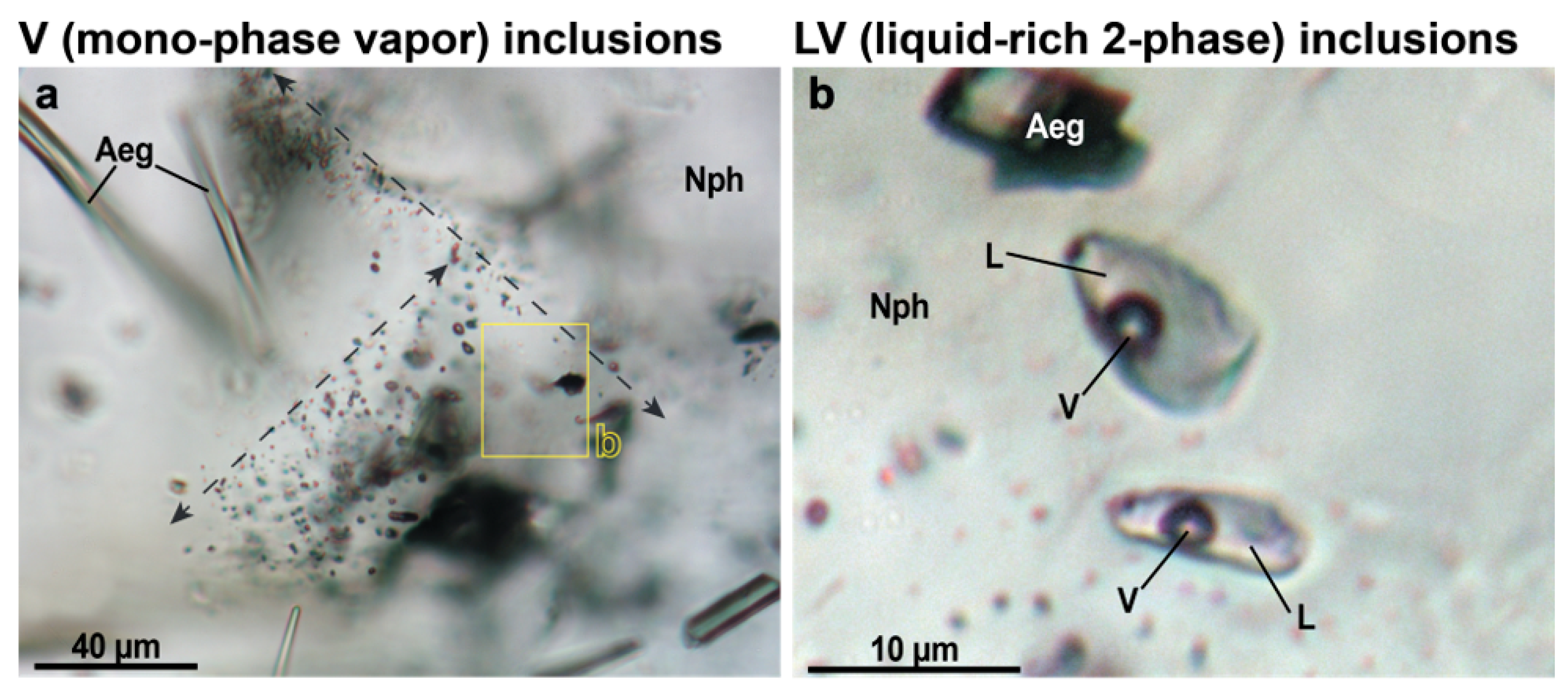
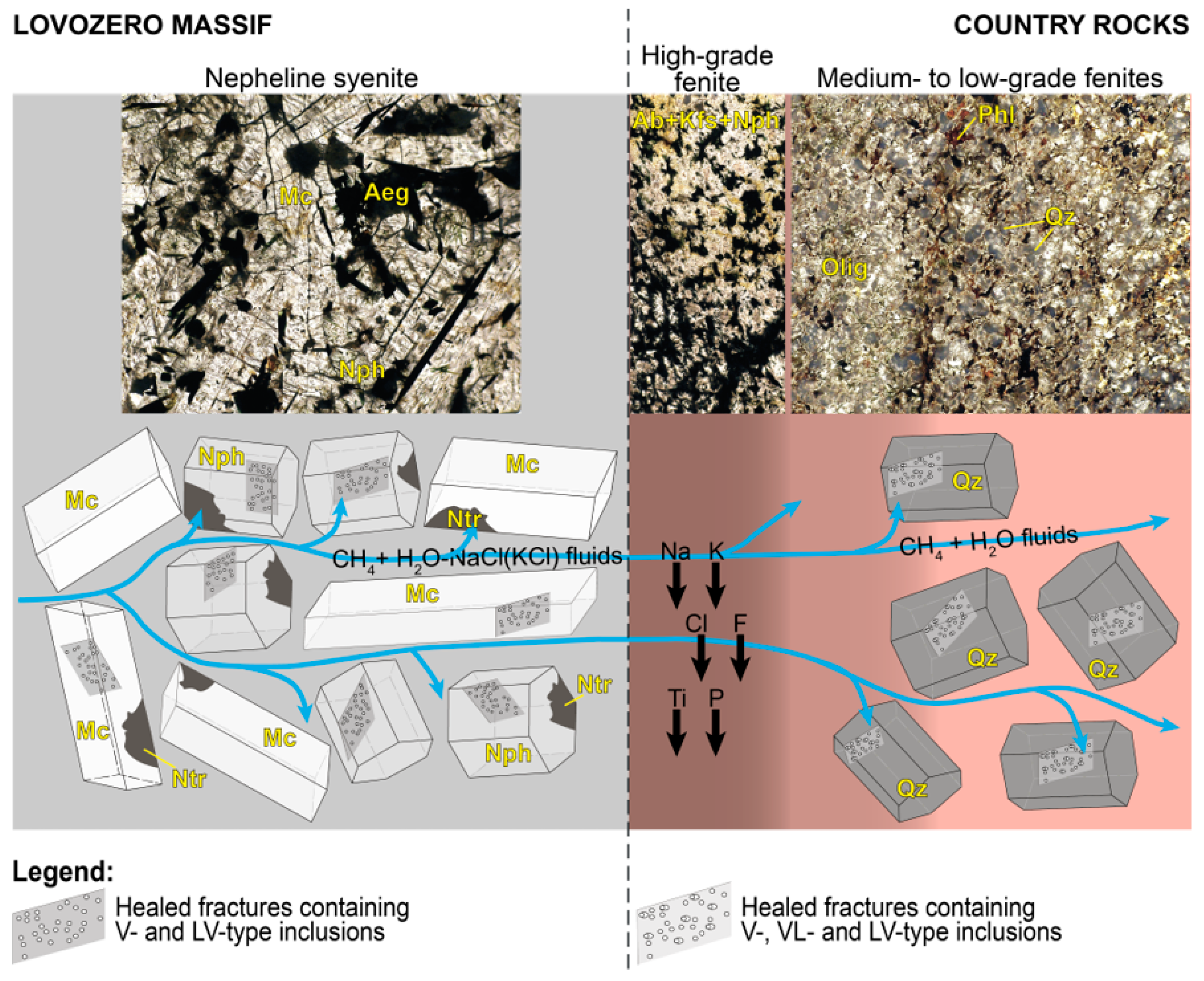
Our studies have shown that nepheline from endocontact foyaites contains secondary fluid inclusions of two types: mono-phase vapor (V = CH
4) and liquid-rich two-phase (LV; L = H
2O; V = CH
4). The salinity of LV-type inclusions is 8.6–15.1 eq. wt.% NaCl, and methane density in V- and LV-type inclusions varies from 0.09 to 0.10 g/cm
3. It is important to note that mono-phase vapor (V-type) inclusions absolutely predominate, while LV-type inclusions are very rare and are found along the same healed microfractures as V-type inclusions (
Figure 9). Such inclusions are typical of nepheline (and also for K-feldspar, sodalite and eudialyte-group minerals) from the rocks of the internal parts of the Lovozero massif and were previously studied in detail by J. Potter and colleagues [
46,
47,
48]. J. Potter in work [
46] stressed that the rarity of H
2O-rich two-phase inclusions and the coexistence of these inclusions in the same trails as the mono-phase vapor (CH
4) inclusions implies coeval, immiscible trapping of aqueous and methane fluids, at or below the CH
4–H
2O solvus at ~350 °C.
We assume that the reason for the predominance of mono-phase methane inclusions and the rarity of H
2O-rich two-phase inclusions in nepheline is that aqueous fluid is intensively consumed in autometasomatic mineral reactions. Apparently, these reactions occurred before (or simultaneously) with the entrap of fluid inclusions. In the Lovozero massif, post-magmatic autometasomatic alterations of previously crystallized magmatic minerals are extremely widespread [
21,
49,
50]. For example, rock-forming feldspathoids such as nepheline and sodalite are intensively replaced by zeolites (mainly natrolite) in accordance with the following water-consumed reactions [
22,
51,
52,
53]:
The autometasomatic reactions are in places so intense that urtite is almost completely transformed into natrolite rock [
50]. In the studied foyaites, intensive metasomatic alterations are also observed, including natrolitization of nepheline (
Figure 3a,b,e,f). Thus, if immiscible aqueous fluid and methane were present in the rock at the post-magmatic stage, then the bulk of the aqueous fluid was consumed in reactions similar to (1)–(4), while methane remained in the fluid. For this reason, methane inclusions predominate in nepheline, while H
2O-rich two-phase inclusions are very rare. Intense autometasomatic reactions are also responsible for the extremely narrow fenite aureole that we observed in this study.
Quartz from low- and medium-grade fenites contains numerous secondary inclusions with very different ratios of vapor and liquid phases: LV (liquid-rich 2-phase; liquid > vapor), VL (vapor-rich 2-phase; vapor > liquid), and V (mono-phase vapor). The coexistence of LV-, VL- and V-type inclusions along the same healed microfractures (
Figure 8) provides strong evidence that the fluids were entrapped under immiscible conditions at or below the CH
4–H
2O solvus. In fact, secondary inclusions in quartz have the following characteristics of heterogeneously trapped inclusions: (1) variable phase ratios at room temperature, ranging from gas-dominated to water-dominated compositions and (2) the gas-dominated inclusions homogenize towards the vapor-phase, and the water-dominated inclusions homogenize to liquid phase [
54,
55].
Thus, the phase composition and mechanism of formation of inclusions in exocontact quartz and inclusions in endocontact nepheline are similar. However, the salinity of liquid-bearing (LV and VL) inclusions in quartz is an order of magnitude lower than the salinity of LV inclusions in nepheline. Such low salinity is the result of the precipitation of hydrothermal solution components in the process of fenitization.
To identify the components added and lost during fenitization of the amphibole-biotite gneiss, we used the method proposed by J. Grant [
56,
57]. According to this method, the equation for composition–volume relations in metasomatic alteration was written as
where, C
i is the concentration of component “i”.
“O” and “A”—the original and altered rocks, respectively.
MO—the equivalent mass before alteration.
MA—the equivalent mass after alteration.
ΔCi—the change in the concentration of component “i”.
For each component, there is an equation of this form in which MO/MA is constant. If it is possible to identify immobile components, for which ΔCi = 0, MO/MA can be obtained by solving the set of simultaneous equations of the form CiA = (MO/MA)CiO. This procedure can be executed graphically by plotting the analytical data, in which case the immobile components define a straight line (isocon) through the origin. The isocon equation is CA = (MO/MA)CO. The slope of the isocon yields the overall change in mass relative to MO.
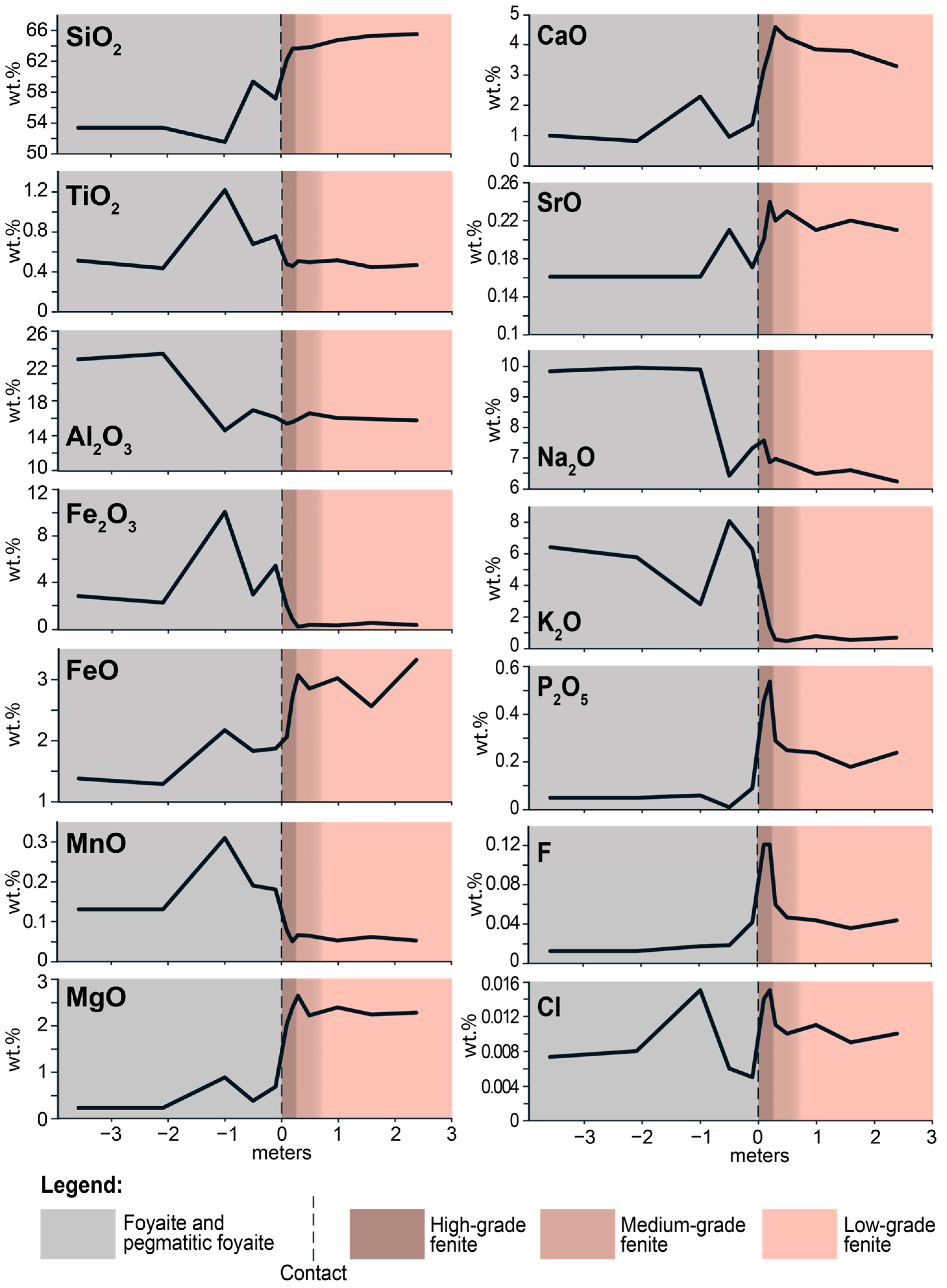
Figure 10 is an isocon diagram comparing the chemical composition of the low-grade fenite (sample L-15-9) and high-grade fenite (sample L-15-2). The slope of the isocon was determined from the a priori assumption that Al
2O
3 was immobile. Conclusions that can be drawn from this isocon diagram are that K
2O, Na
2O, P
2O
5, TiO
2, H
2O, F, Cl, and S were added in the process of fenitization, while SiO
2 was removed. In addition, iron was oxidized during fenitization. These data are consistent with the results obtained by A. Arzamastsev in the study of fenites associated with hydrothermal veins [
17].
When the immiscible aqueous and methane fluids infiltrated in amphibole-biotite gneiss, sodium and potassium precipitated from aqueous fluid in the form of albite, nepheline, and K-feldspar. The following zoning is observed in the fenite aureole: at a distance 0–10 cm from the contact there is K-rich fenite, consisting mainly of K-feldspar, and at a distance 10–20 cm from the contact there is Na-rich fenite, consisting mainly of albite and nepheline (
Figure 2e,h). According to [
58], this zoning may be associated with a decrease in the NaCl/KCl ratio during fluid-rock interaction at low fluid/rock ratios. The NaCl/KCl ratio decreases as a result of the crystallization of nepheline and albite, which leads to the subsequent crystallization of K-feldspar.
Figure 2e,f shows zonal segregations, the central parts of which consist of nepheline and albite, and the outer rim is composed of potassium feldspar. Nepheline and albite crystallized at a high NaCl/KCl ratio, while K-feldspar formed at a low NaCl/KCl ratio. Phosphorus, Ti, F, and Cl precipitate simultaneously with alkalis in the immediate vicinity of the contact and form minerals of the apatite group, titanite, and fluorine-rich Na- and Ca-Na-amphiboles. A low-salinity aqueous fluid, devoid of dissolved components as a result of the precipitation of albite, nepheline and other minerals, together with coexisting methane fluid, was entrapped in quartz under immiscible conditions at or below the CH
4–H
2O solvus.
Figure 11 shows the general scheme of fluid evolution during fenitization based on our data.
Petrographic observations (
Figure 2a,b) show that the fenitization at the contact of the massif with the country rocks was of a pervasive nature, i.e., finitizing fluids flowed along the grain boundaries. However, as we established in this work, the aqueous and methane fluids flowing through the country rocks were immiscible. If fluid forms a continuous film through the grain boundaries, it can flow in response to gradients, but the presence of another immiscible phase effectively constricts the apertures available for it to pass through, reducing permeability [
59]. Therefore, it can be assumed that such a narrow halo of fenitization is due not only to the intense autometasomatism of alkaline rocks but also to the immiscibility of the fluids.
6. Conclusions
At the post-magmatic stage, the alkaline rocks of the Lovozero massif contained a heterogenous mixture of two coexisting fluids, namely medium-salinity (8.6–15.1 eq. wt.% NaCl) aqueous {H2O-NaCl(KCl)} and methane (CH4) fluids. The coexistence of these two fluids indicates immiscibility conditions at or below CH4–H2O solvus. The aqueous fluid was mainly consumed in autometasomatic reactions, such as natrolitization of nepheline. The methane fluid and a small fraction of the aqueous fluid were entrapped in nepheline (and K-feldspar) as secondary mono-phase vapor (V = CH4) and liquid-rich two-phase (LV; L = H2O; V = CH4) fluid inclusions located along the same healed microfractures.
At the contact of the massif, the mixture of high saline aqueous and methane fluids flowed from endocontact alkaline rocks into country amphibole-biotite gneiss. The aqueous fluid transported K2O, Na2O, P2O5, TiO2, H2O, F, Cl, and S to the exocontact zone. These components were precipitated in the immediate vicinity of the contact of the massif, and the salinity of the aqueous fluid significantly decreased. The methane and low-salinity aqueous fluids were entrapped in quartz as secondary fluid inclusions with variable phase ratios at or below CH4–H2O solvus.