Air Quality Monitoring for Preventive Conservation of the Built Heritage Deteriorated by Salt Crystallization
Abstract
1. Introduction
2. Materials and Methods
2.1. Site and Geological Setting
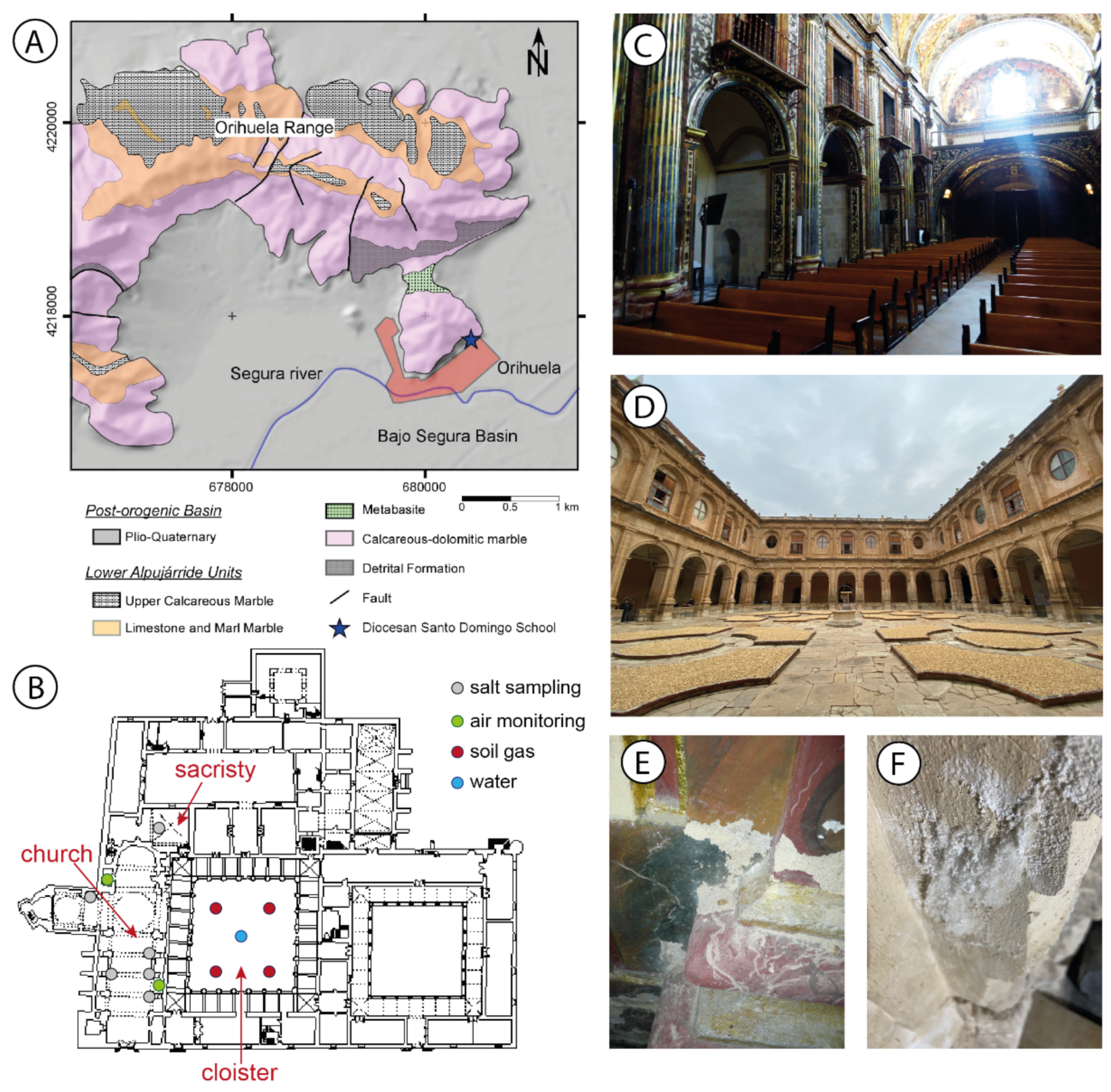
2.2. Soil, Water, and Indoor Radon Activity
2.3. Environment Monitoring System
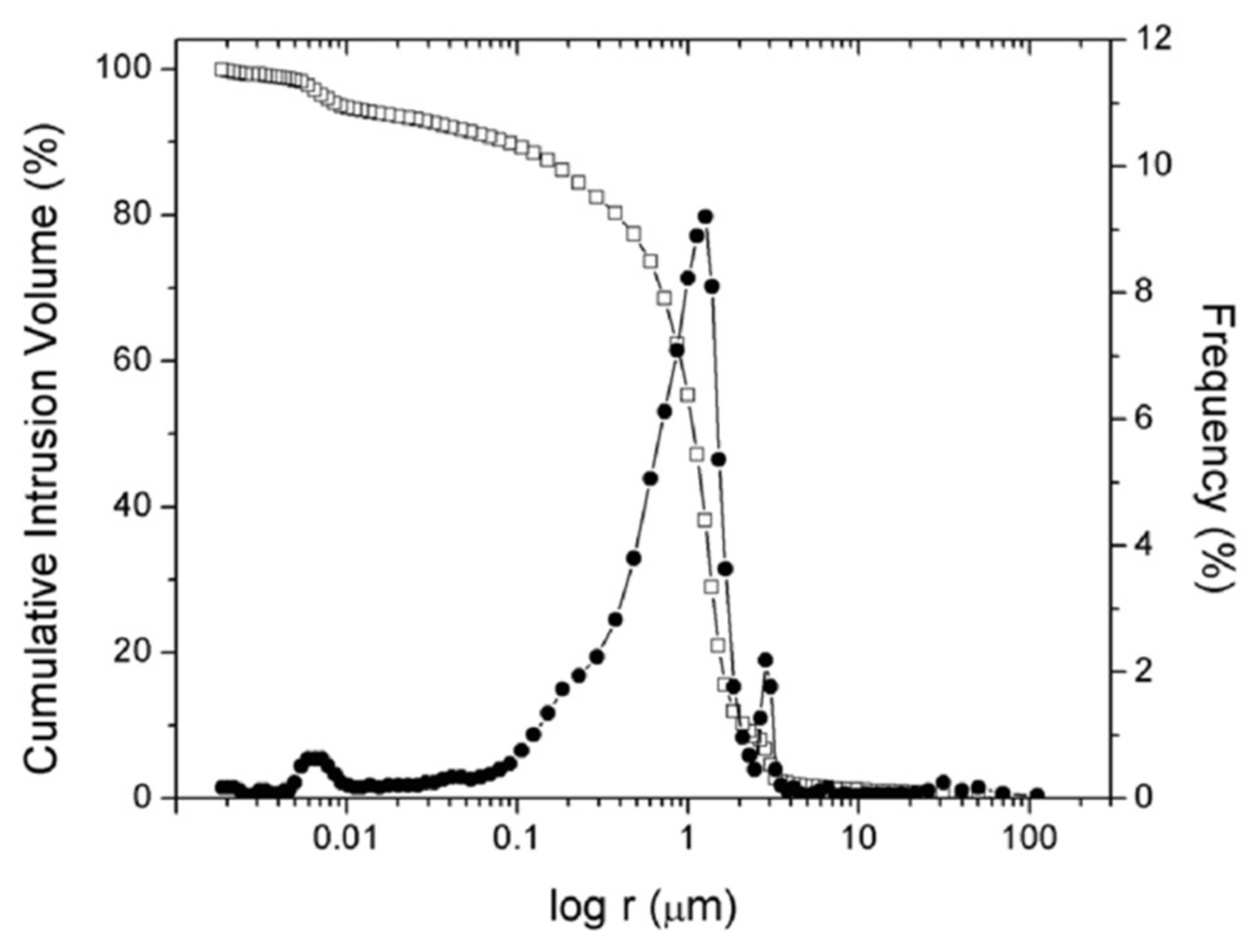
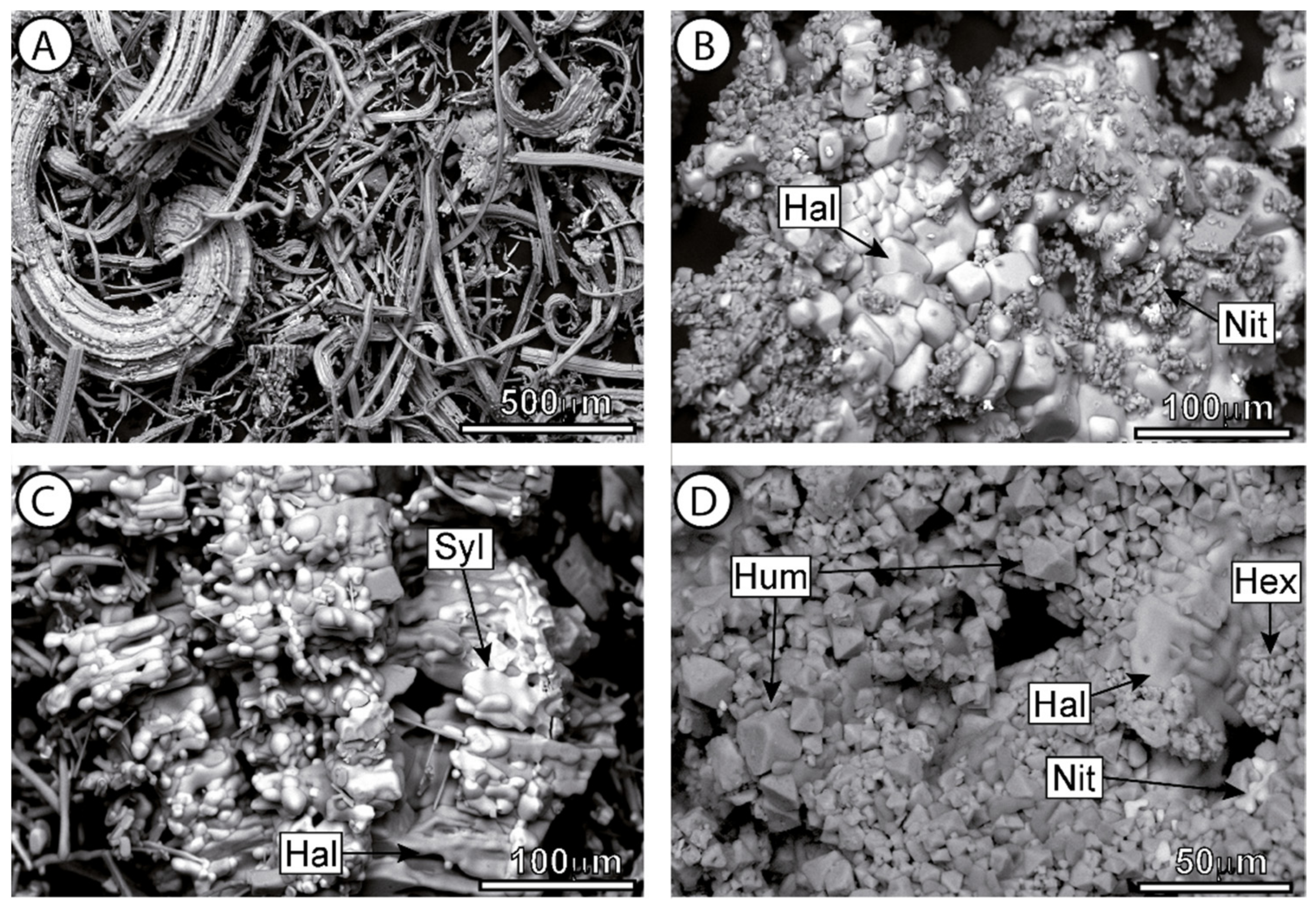
2.4. Stone Properties and Salt Characterization
2.5. Geochemical Modelling
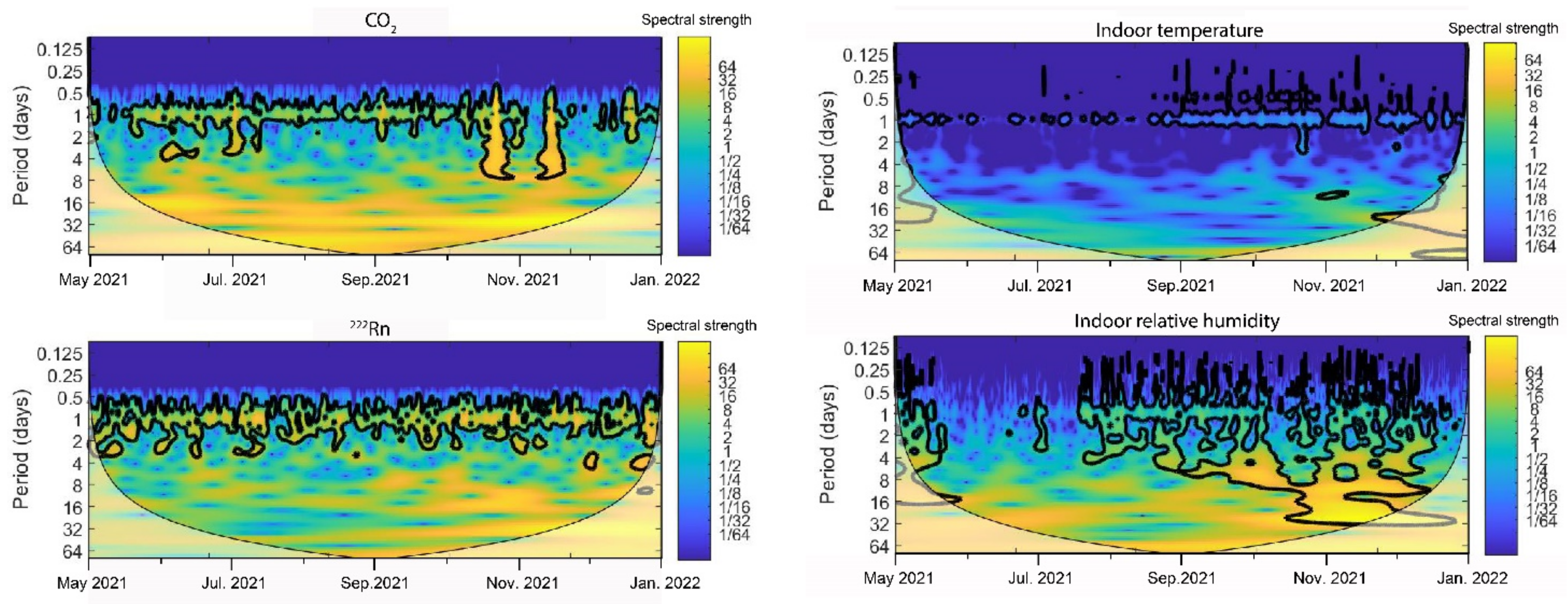
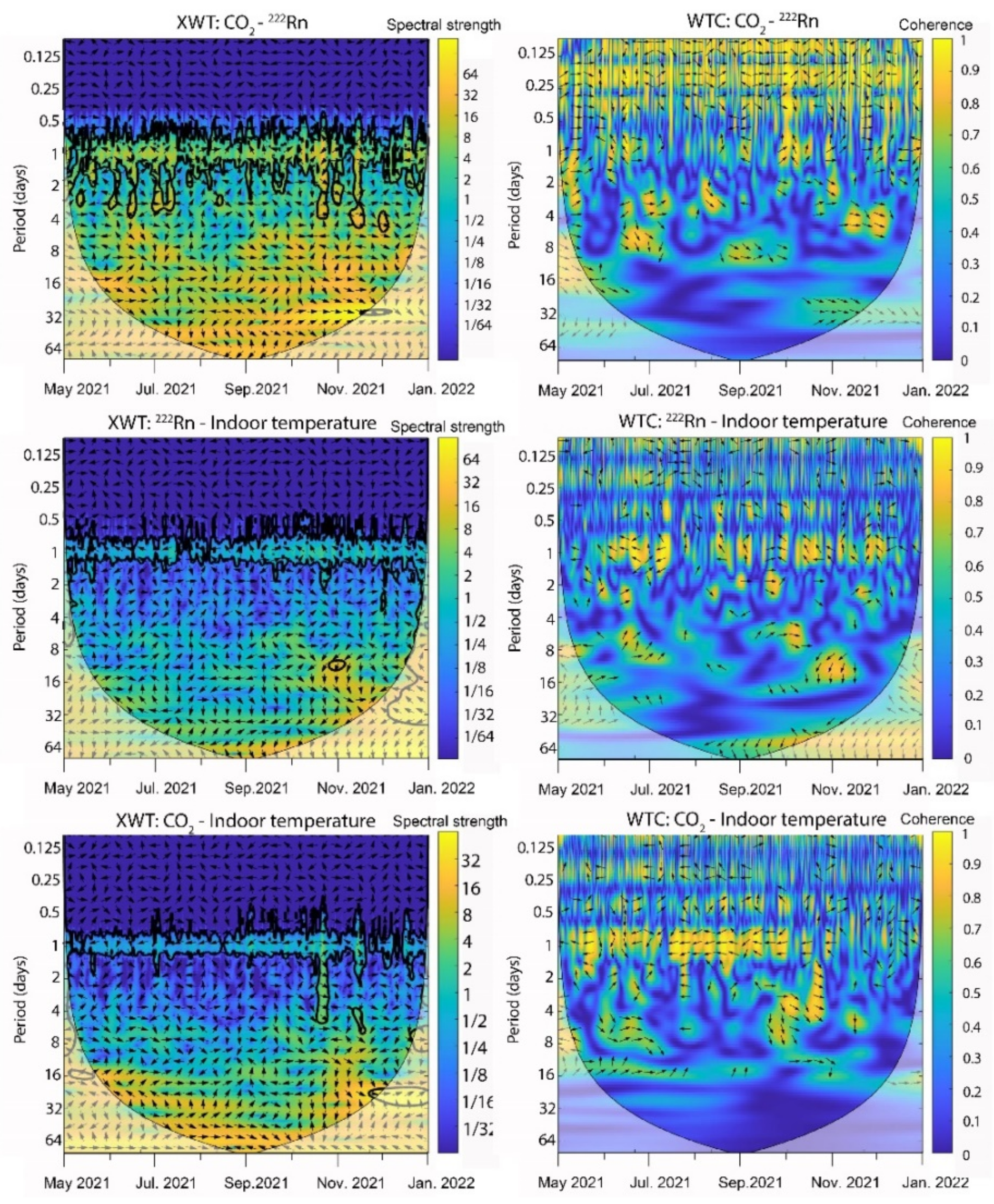
2.6. Time Series Frequency Analysis
3. Results and Discussion
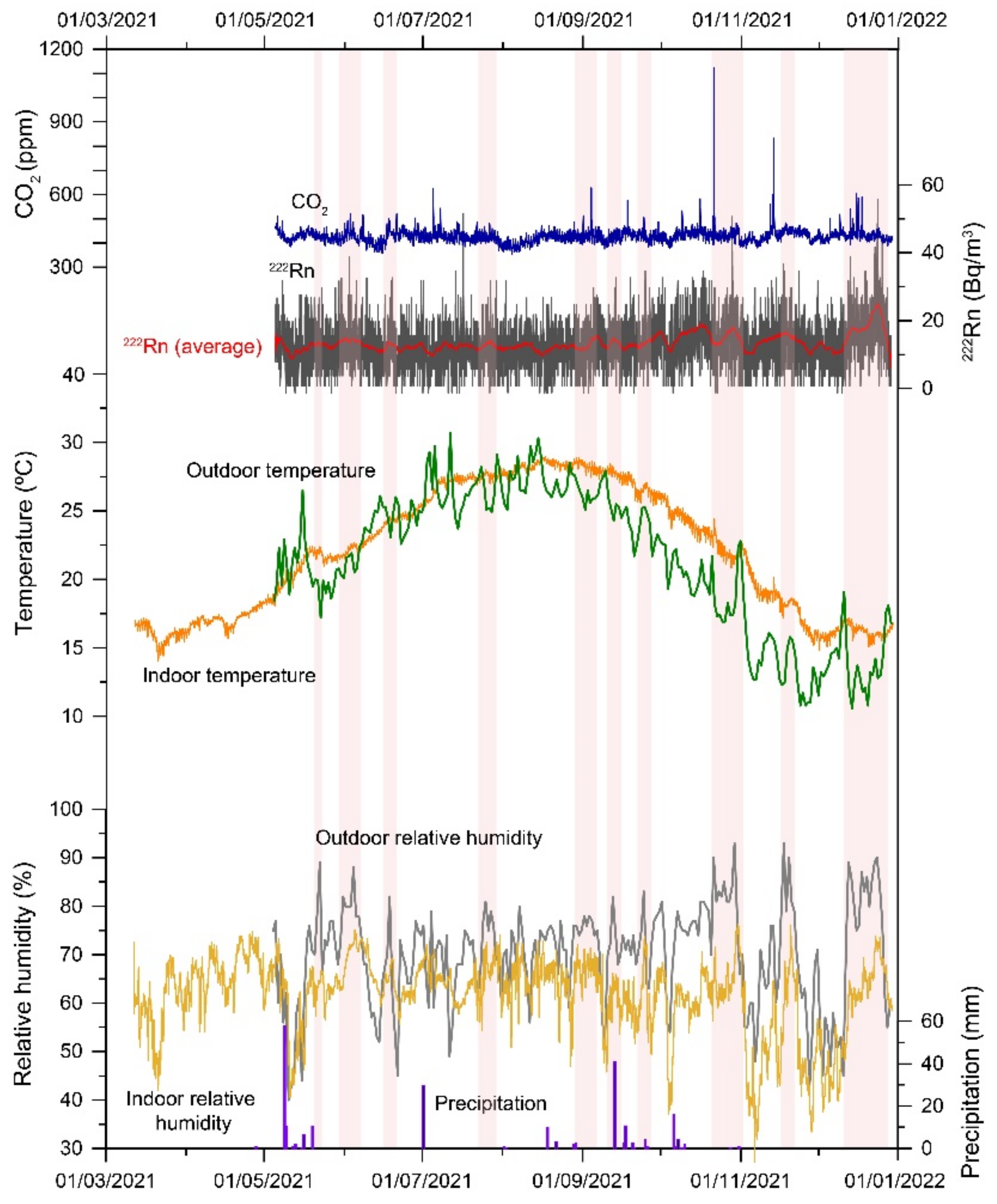
3.1. Radon Activity
3.2. Indoor Environment Characterization
3.3. Rock Properties and Decay Pattern
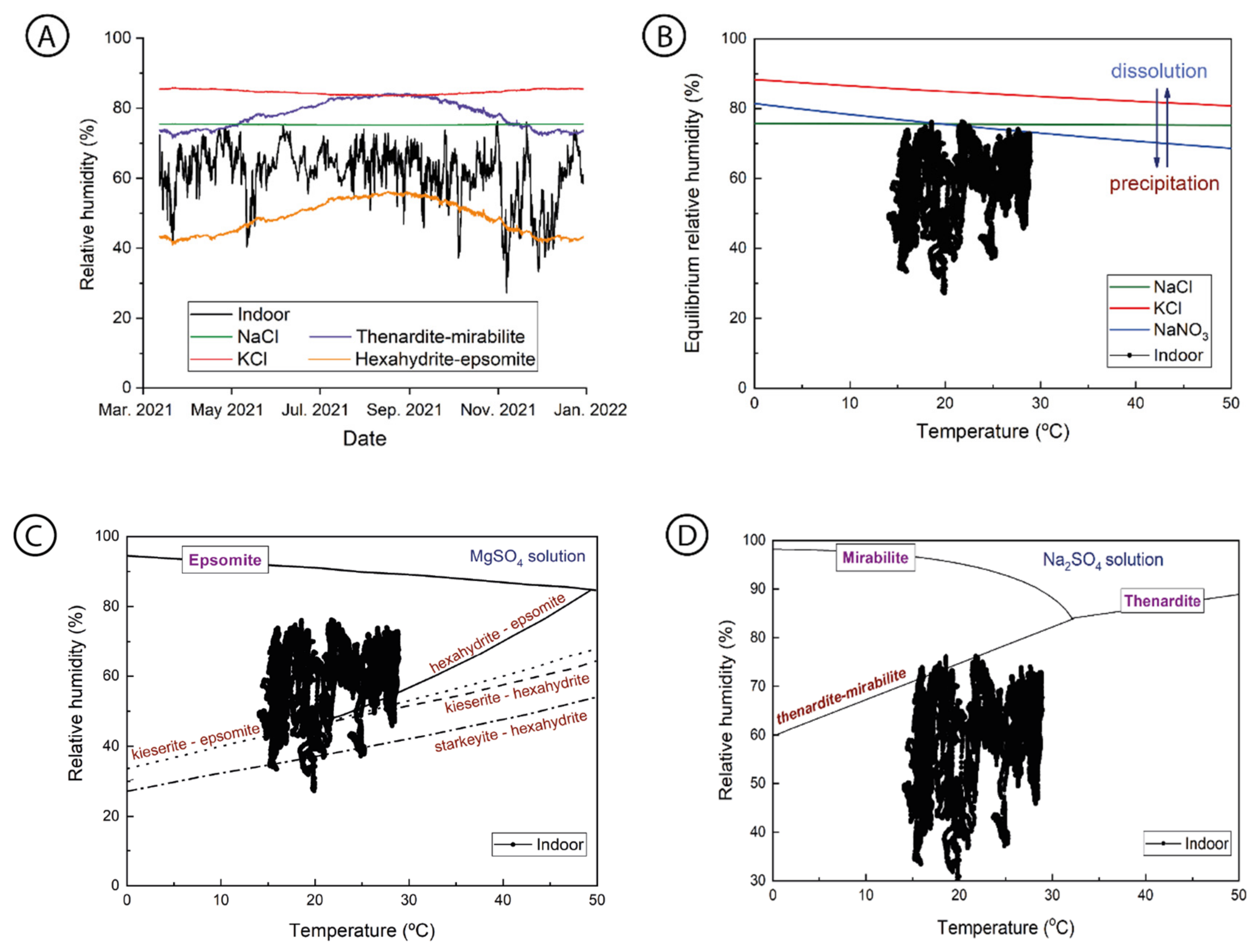
3.4. Salt Transitions by Changes in the Indoor Thermo-Hygrometric Conditions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dovjak, M.; Vene, O.; Vaupotič, J. Analysis of Ventilation Efficiency as Simultaneous Control of Radon and Carbon Dioxide Levels in Indoor Air Applying Transient Modelling. Int. J. Environ. Res. Public Health 2022, 19, 2125. [Google Scholar] [CrossRef] [PubMed]
- Küçükhüseyin, Ö. CO2 monitoring and indoor air quality. REHVA J. 2021, 1, 45–59. [Google Scholar]
- Pla, C.; Fernandez-Cortes, A.; Cuezva, S.; Galiana-Merino, J.J.; Cañaveras, J.C.; Sanchez-Moral, S.; Benavente, D. Insights on Climate-Driven Fluctuations of Cave 222Rn and CO2 Concentrations Using Statistical and Wavelet Analyses. Geofluids 2020, 2020, 8858295. [Google Scholar] [CrossRef]
- Eff-Darwich, A.; Vinas, R.; Soler, V.; de la Nuez, J.; Quesada, M.L. Natural air ventilation in underground galleries as a tool to increase radon sampling volumes for geologic monitoring. Radiat. Meas. 2008, 43, 1429–1436. [Google Scholar] [CrossRef]
- Fernández-Cortés, A.; Benavente, D.; Cuezva, S.; Cañaveras, J.C.; Alvarez-Gallego, M.; Garcia-Anton, E.; Soler, V.; Sanchez-Moral, S. Effect of water vapour condensation on the radon content in subsurface air in a hypogeal inactive-volcanic environment in Galdar Cave, Spain. Atmos. Environ. 2013, 75, 15–23. [Google Scholar] [CrossRef]
- Wang, S.G.; Niu, X.B.; Chen, Q.; Yang, W. Radon Exhalation Patterns in A Dead-end Tunnel. In Proceedings of the 2016 International Conference on Mechatronics, Manufacturing and Materials Engineering, Hong Kong, China, 11–12 June 2016; Volume 63. [Google Scholar]
- Skubacz, K.; Wysocka, M.; Michalik, B.; Dziurzynski, W.; Krach, A.; Krawczyk, J.; Palka, T. Modelling of radon hazards in underground mine workings. Sci. Total. Environ. 2019, 695, 133853. [Google Scholar] [CrossRef]
- Ajayi, K.; Shahbazi, K.; Tukkaraja, P.; Katzenstein, K. Numerical investigation of the effectiveness of radon control measures in cave mines. Int. J. Min. Sci. Technol. 2019, 29, 469–475. [Google Scholar] [CrossRef]
- REHVA (Federation of European Heating, Ventilation and Air Conditioning Associations). REHVA COVID-19 Guidance Document How to Operate HVAC and Other Building Service Systems to Prevent the Spread of the Coronavirus (SARS-CoV-2) Disease (COVID-19) in Workplaces; REHVA: Brussels, Belgium, 2021. [Google Scholar]
- Camuffo, D. Microclimate for Cultural Heritage—Conservation, Restoration and Maintenance of Indoor and Outdoor Monuments; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Brimblecombe, P. Heritage climatology. In Climate Change and Cultural Heritage; Lefevre, R.A., Sabbioni, C., Eds.; Edipuglia: Bari, Italy, 2010; pp. 54–57. [Google Scholar]
- Díaz-Arellano, I.; Zarzo, M.; García-Diego, F.-J.; Perles, A. A Methodology for the Multi-Point Characterization of Short-Term Temperature Fluctuations in Complex Microclimates Based on the European Standard EN 15757:2010: Application to the Archaeological Museum of l’Almoina (Valencia, Spain). Sensors 2021, 21, 7754. [Google Scholar] [CrossRef]
- West, G. Effect of suction on the strength of rock. Q. J. Eng. Geol. Hydrogeol. 1994, 27, 51–56. [Google Scholar] [CrossRef]
- Sanchez-Moral, S.; Luque, L.; Cuezva, S.; Soler, V.; Benavente, D.; Laiz, L.; Gonzalez, J.M.; Saiz-Jimenez, C. Deterioration of building materials in Roman catacombs: The influence of visitors. Sci. Total Environ. 2005, 349, 260–276. [Google Scholar] [CrossRef]
- Benavente, D.; Sanchez-Moral, S.; Fernandez-Cortes, A.; Canaveras, J.C.; Elez, J.; Saiz-Jimenez, C. Salt damage and microclimate in the Postumius Tomb, Roman Necropolis of Carmona, Spain. Environ. Earth Sci. 2011, 63, 1529–1543. [Google Scholar] [CrossRef]
- Lopez-Doncel, R.; Wedekind, W.; Dohrmann, R.; Siegesmund, S. Moisture expansion associated to secondary porosity: An example of the Loseros Tuff of Guanajuato, Mexico. Environ. Earth Sci. 2013, 69, 1189–1201. [Google Scholar] [CrossRef][Green Version]
- Cañaveras, J.C.; Fernandez-Cortes, A.; Elez, J.; Cuezva, S.; Jurado, V.; Miller, A.Z.; Rogerio-Candelera, M.A.; Benavente, D.; Hernandez-Marine, M.; Saiz-Jimenez, C.; et al. The deterioration of Circular Mausoleum, Roman Necropolis of Carmona, Spain. Sci. Total Environ. 2015, 518, 65–77. [Google Scholar] [CrossRef]
- Arnold, A. Rising Damp and Saline Minerals, Proceedings of the 4th International Congress on the Deterioration and Preservation of Stone Objects, Louisville, KY, USA, 7–8 July 1982; Gauri, K.L., Gwinn, J.A., Eds.; University of Louisville: Louisville, KY, USA, 1982. [Google Scholar]
- Arnold, A.; Zehnder, K. Salt weathering on monuments. In First International Symposium on the Conservation of Monuments in the Mediterranean Basin; Zezza, F., Ed.; Grafo: Bari, Italy, 1990; pp. 31–58. [Google Scholar]
- Lopez-Arce, P.; Doehne, E.; Greenshields, J.; Benavente, D.; Young, D. Treatment of rising damp and salt decay: The historic masonry buildings of Adelaide, South Australia. Mater. Struct. 2009, 41, 827–848. [Google Scholar] [CrossRef]
- Sawdy, A.; Price, C.A. Salt damage at Cleeve Abbey, England. Part I: A comparison of theoretical predictions and practical observations. J. Cult. Herit. 2005, 6, 125–135. [Google Scholar] [CrossRef]
- Köppen, W. Das geographische System der Klimate. In Handbuch der Klimatologie; Koppen, W., Geiger, R., Eds.; Gebrüder Borntraeger Verlagsbuchhandlung: Berlin, Germany, 1936; Volume I, Part C. [Google Scholar]
- Beck, H.E.; Zimmermann, N.E.; Mcvicar, T.R.; Vergopolan, N.; Berg, A.; Wood, E.F. Data Descriptor: Present and future Köppen-Geiger climate classification maps at 1-km resolution. Sci. Data. 2018, 5, 180214. [Google Scholar] [CrossRef]
- De Boer, A.; Egeler, C.G.; Kampschuur, W.; Montenat, C.H.; Rondeel, H.E.; Simon, O.J.; van Winkoop, A.A. Mapa Geológico y Memoria de la Hoja Geológica nº 913 (Orihuela). Mapa Geológico de España. 1:50.000, 1st ed.; MAGNA 2; IGME: Madrid, Spain, 1951; 40p. [Google Scholar]
- Martín-Rojas, I.; Estévez, A.; Martín-Martín, M.; Delgado, F.; García-Tortosa, F.J. New data from Orihuela and Callosa Mountains (Betic Internal Zone, Alicante, SE Spain). Implications for the “Almágride Complex” controversy. J. Iber. Geol. 2007, 33, 311–318. [Google Scholar]
- IGME. Las Aguas Subterráneas en la Comunidad Valenciana. Uso, Calidad y Perspectivas de Utilización; Instituto Geológico y Minero de España: Madrid, Spain, 1988. [Google Scholar]
- Benavente, D.; Bernabeu, A.; Fort, R.; Martinez-Martinez, J.; Garcia-del-Cura, M.A. The decolouration of brecciated black marbles used in heritage monuments of Alicante. In Heritage, Weathering and Conservation; Fort, R., Alvarez de Buergo, M., Gomez-Heras, M., Vazquez-Calvo, C., Eds.; Balkema: Rotterdam, The Netherlands, 2006; pp. 205–210. [Google Scholar]
- Parkhurst, D.L.; Appelo, C.A.J. Description of input and examples for PHREEQC version 3—A computer program for speciation, batch-reaction, one-dimensional transport, and inverse geochemical calculations. In U.S. Geological Survey Techniques and Methods; United States Geological Survey: Reston, VA, USA, 2013. [Google Scholar]
- Langmuir, D. Aqueous Environmental Geochemistry; Prentice Hall: Sacramento, CA, USA, 1997. [Google Scholar]
- Benavente, D.; Brimblecome, P.; Grossi, C.M. Thermodynamic calculations for the salt crystallisation damage in porous built heritage using PHREEQC. Environ. Earth Sci. 2015, 74, 2297–2313. [Google Scholar] [CrossRef]
- Daubechies, I. Ten Lectures on Wavelets; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1992. [Google Scholar]
- Wickerhauser, M.V. Adapted Wavelet Analysis from Theory to Software; A K Peters: Natick, MA, USA; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Galiana-Merino, J.J. EnvironmentalWaveletTool: Continuous and discrete wavelet analysis and filtering for environmental time series. Comput. Phys. Commun. 2014, 185, 2758–2770. [Google Scholar] [CrossRef]
- Grinsted, A.; Moore, J.C.; Jevrejeva, S. Application of the cross wavelet transform and wavelet coherence to geophysical time series. Nonlinear Process. Geophys. 2004, 11, 561–566. [Google Scholar] [CrossRef]
- Benavente, D.; Valdes-Abellan, J.; Pla, C.; Sanz-Rubio, E. Estimation of soil gas permeability for assessing radon risk using Rosetta pedotransfer function based on soil texture and water content. J. Environ. Radioact. 2019, 208–209, 105992. [Google Scholar] [CrossRef] [PubMed]
- Neznal, M.; Matolín, M.; Barnet, I.; Mikšová, J. New method for assessing the radon risk of building sites. In Czech Geological Survey Special Papers 1; CGS: Prague, Czech Republic, 2004; p. 47. [Google Scholar]
- European Union Law. Commission Recommendation of 20 December 2001 on the protection of the public against exposure to radon in drinking water supplies (notified under document number C (2001) 4580). Off. J. L 2001, 344, 85–88. [Google Scholar]
- Benavente, D. Why pore size is important in the deterioration of porous stones used in the built heritage? Macla 2011, 15, 41–42. [Google Scholar]
- Benavente, D.; Martinez-Martinez, J.; Cueto, N.; Ordonez, S.; Garcia-del-Cura, M.A. Impact of salt and frost weathering on the physical and durability properties of travertines and carbonate tufas used as building material. Environ. Earth Sci. 2018, 77, 147. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Dohene, E. Salt weathering: Influence of evaporation rate, supersaturation and crystallisation pattern. Earth Surf. Process. Landf. 1999, 24, 191–209. [Google Scholar] [CrossRef]
- Sunagawa, I. Crystals: Growth, Morphology and Perfection; Cambridge University Press: Cambridge, UK, 2005. [Google Scholar]
- Benavente, D.; de Jongh, M.; Cañaveras, J.C. Weathering Processes and Mechanisms Caused by Capillary Waters and Pigeon Droppings on Porous Limestones. Minerals 2021, 11, 18. [Google Scholar] [CrossRef]
- Steiger, M.; Linnow, K.; Ehrhardt, D.; Rohde, M. Decomposition reactions of magnesium sulfate hydrates and phase equilibria in the MgSO4–H2O and Na+–Mg2+–Cl−–SO42−–H2O systems with implications for Mars. Geochim. Cosmochim. Acta 2011, 75, 3600–3626. [Google Scholar] [CrossRef]
- Lasaga, A.C. Kinetic Theory in the Earth Sciences; Princeton University Press: Chichester, UK; Princeton, NJ, USA, 1998. [Google Scholar]
- Correns, C.W. Growth and dissolution of crystals under linear pressure. Disc. Faraday Soc. 1949, 5, 267–271. [Google Scholar] [CrossRef]
| CE | pH | Na+ | K+ | Mg2+ | Ca2+ | Cl− | SO42− | HCO3− | NO3− | 222Rn |
|---|---|---|---|---|---|---|---|---|---|---|
| [mS/cm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [ppm] | [Bq/L] | |
| 3.31 | 7.65 | 353.52 | 42.38 | 113.12 | 221.55 | 550.90 | 759.37 | 280.60 | 41.96 | 13.6 |
| Group | Mineral Name | Chemical Formula | Saturation Index |
|---|---|---|---|
| Sulfates | thenardite | Na2SO4 | −6.05 |
| mirabilite | Na2SO4·10H2O | −5.14 | |
| aphthitalite | K3Na(SO4)2 | −12.27 | |
| gypsum | CaSO4·2H2O | −0.68 | |
| epsomite | MgSO4·7H2O | −3.48 | |
| hexahydrite | MgSO4·6H2O | −3.76 | |
| Sulfate nitrate | humberstonite | K3Na7Mg2(SO4)6(NO3)2·6H2O | −40.60 |
| Nitrates | niter | KNO3 | −5.43 |
| Chlorides | halite | NaCl | −5.36 |
| sylvite | KCl | −5.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benavente, D.; Pla, C.; Gil-Oncina, S.; Ruiz, M.C.; Blanco-Quintero, I.F.; Huesca-Tortosa, J.A.; Spairani-Berrio, Y.; Sanchez-Moral, S. Air Quality Monitoring for Preventive Conservation of the Built Heritage Deteriorated by Salt Crystallization. Geosciences 2022, 12, 325. https://doi.org/10.3390/geosciences12090325
Benavente D, Pla C, Gil-Oncina S, Ruiz MC, Blanco-Quintero IF, Huesca-Tortosa JA, Spairani-Berrio Y, Sanchez-Moral S. Air Quality Monitoring for Preventive Conservation of the Built Heritage Deteriorated by Salt Crystallization. Geosciences. 2022; 12(9):325. https://doi.org/10.3390/geosciences12090325
Chicago/Turabian StyleBenavente, David, Concepción Pla, Sara Gil-Oncina, Maria Candela Ruiz, Idael Francisco Blanco-Quintero, Jose Antonio Huesca-Tortosa, Yolanda Spairani-Berrio, and Sergio Sanchez-Moral. 2022. "Air Quality Monitoring for Preventive Conservation of the Built Heritage Deteriorated by Salt Crystallization" Geosciences 12, no. 9: 325. https://doi.org/10.3390/geosciences12090325
APA StyleBenavente, D., Pla, C., Gil-Oncina, S., Ruiz, M. C., Blanco-Quintero, I. F., Huesca-Tortosa, J. A., Spairani-Berrio, Y., & Sanchez-Moral, S. (2022). Air Quality Monitoring for Preventive Conservation of the Built Heritage Deteriorated by Salt Crystallization. Geosciences, 12(9), 325. https://doi.org/10.3390/geosciences12090325








