Pyrite Morphology and δ34S as Indicators of Deposition Environment in Organic-Rich Shales
Abstract
:1. Introduction
2. Geological Setting
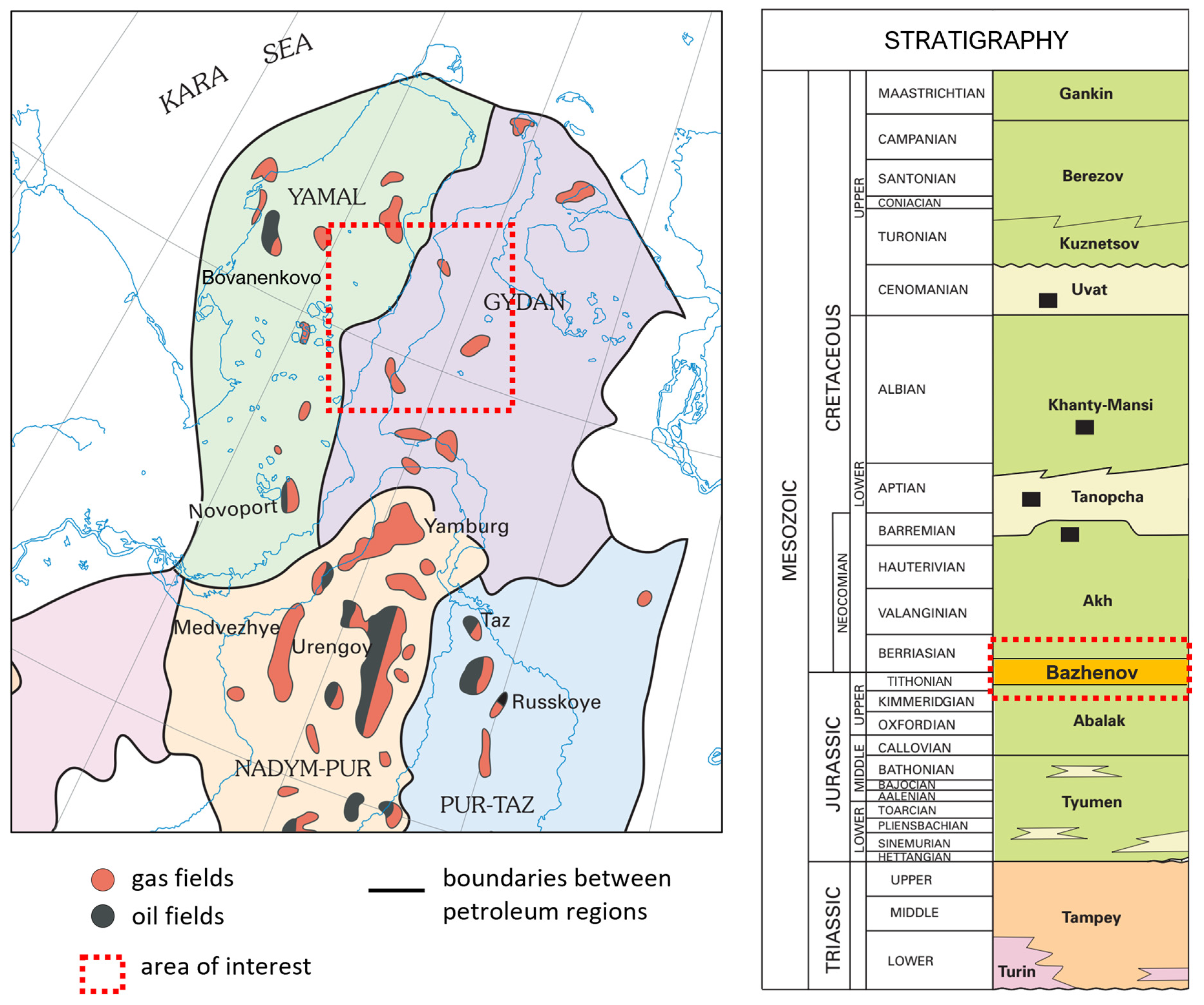
3. Materials and Methods
3.1. Characteristics of Texture and Organic Matter
3.2. Mass Spectrometry Analyses
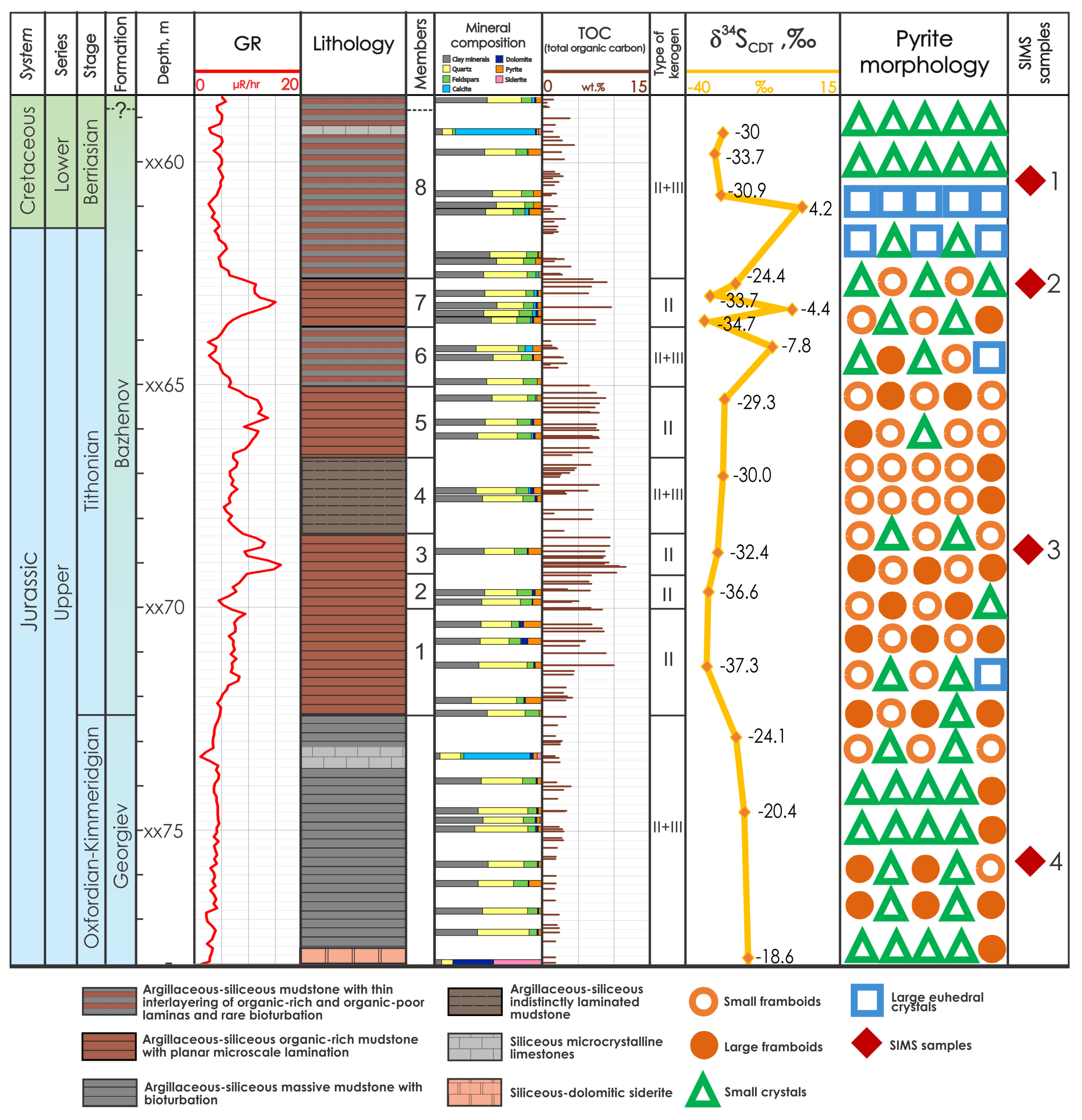
4. Results
4.1. Lithology and Organic Matter
- Argillaceous–siliceous mudstone with thin interlayering of organic-rich and organic-poor laminae and rare bioturbation
- Argillaceous–siliceous organic-rich mudstone with planar microscale lamination
- Argillaceous–siliceous massive mudstone with bioturbation
- Argillaceous–siliceous indistinctly laminated mudstone
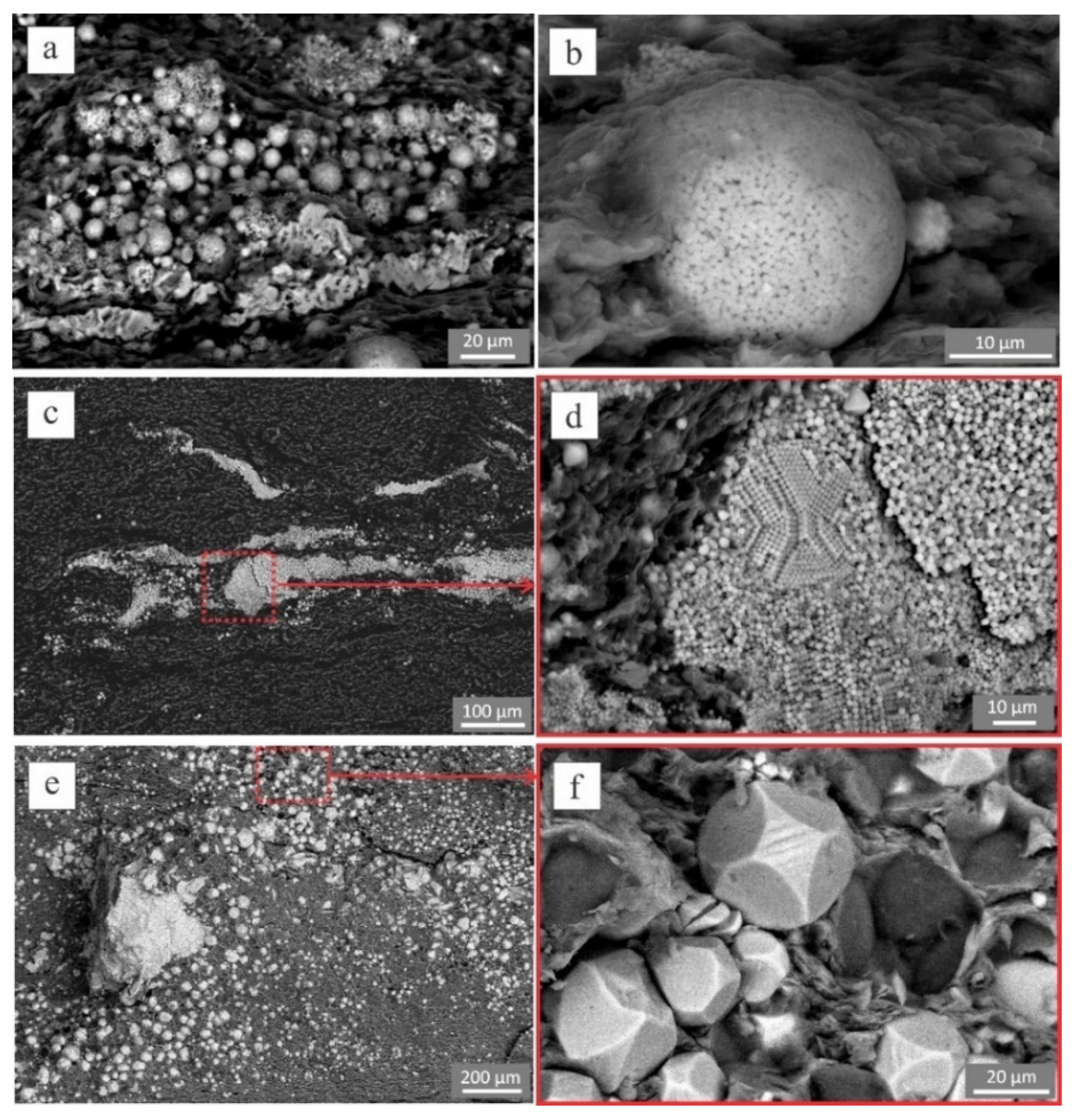
4.2. Pyrite Morphology
- Small framboids (diameter 5–10 µm) concentrations of sub-micron pyrite crystals 0.5–1 µm in size (Figure 3a). These framboids were porous, abundant in the sediment layers with high carbon content, and were often associated with organic matter.
- Large framboids were denser and less porous (without space between micro-crystals) than small framboids. Diameter of large framboids could reach 30 µm and consisted of microcrystals with the size range of 2–5 µm (Figure 3b).
- Small crystals (1–2 µm in size) were non-uniformly distributed in lenses and layers (Figure 3c,d). Small crystals were often abundant within bioturbation traces and organic remnants.
- Large euhedral crystals (15–30 µm in size) were non-uniformly distributed in layers and aggregates within the rocks (Figure 3e,f).
- Framboids predominant (members 1–5);
- Framboids alternated with small crystals, which replaced organic remnants (Georgiev Formation and members 6–8);
- Large crystals localized in the layers of the upper portion of the section (member 8).
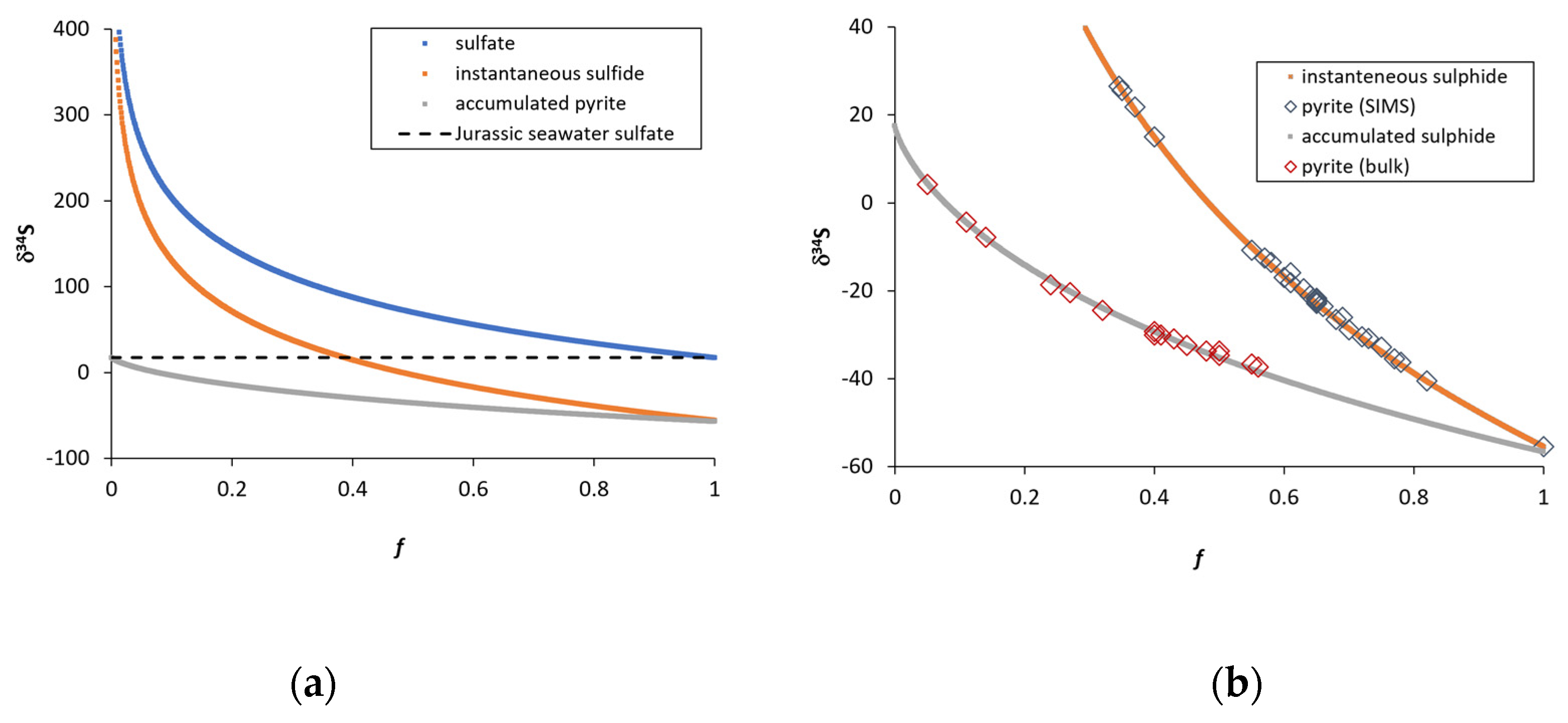
4.3. Sulfur Isotope Composition (Bulk IRMS)
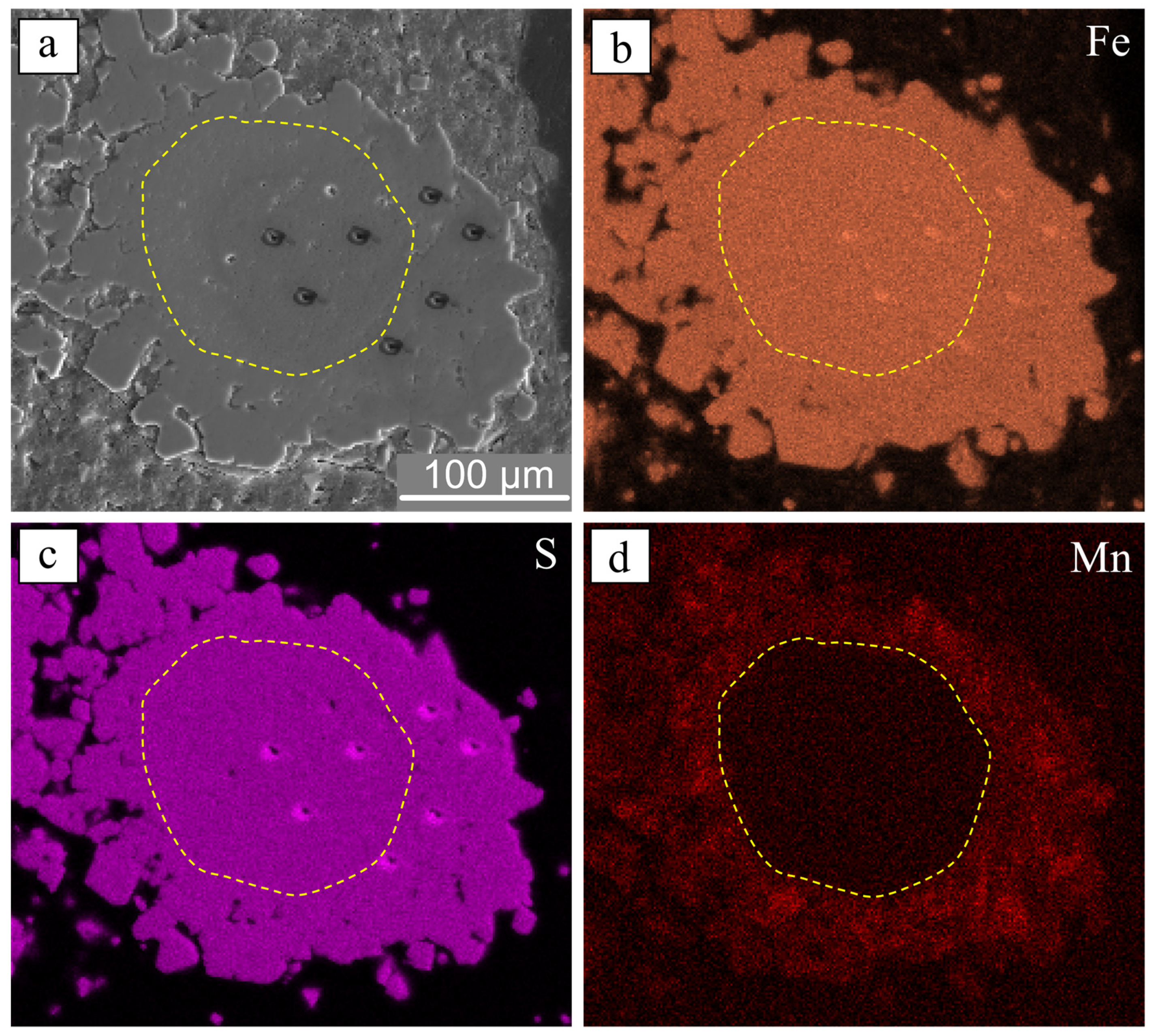
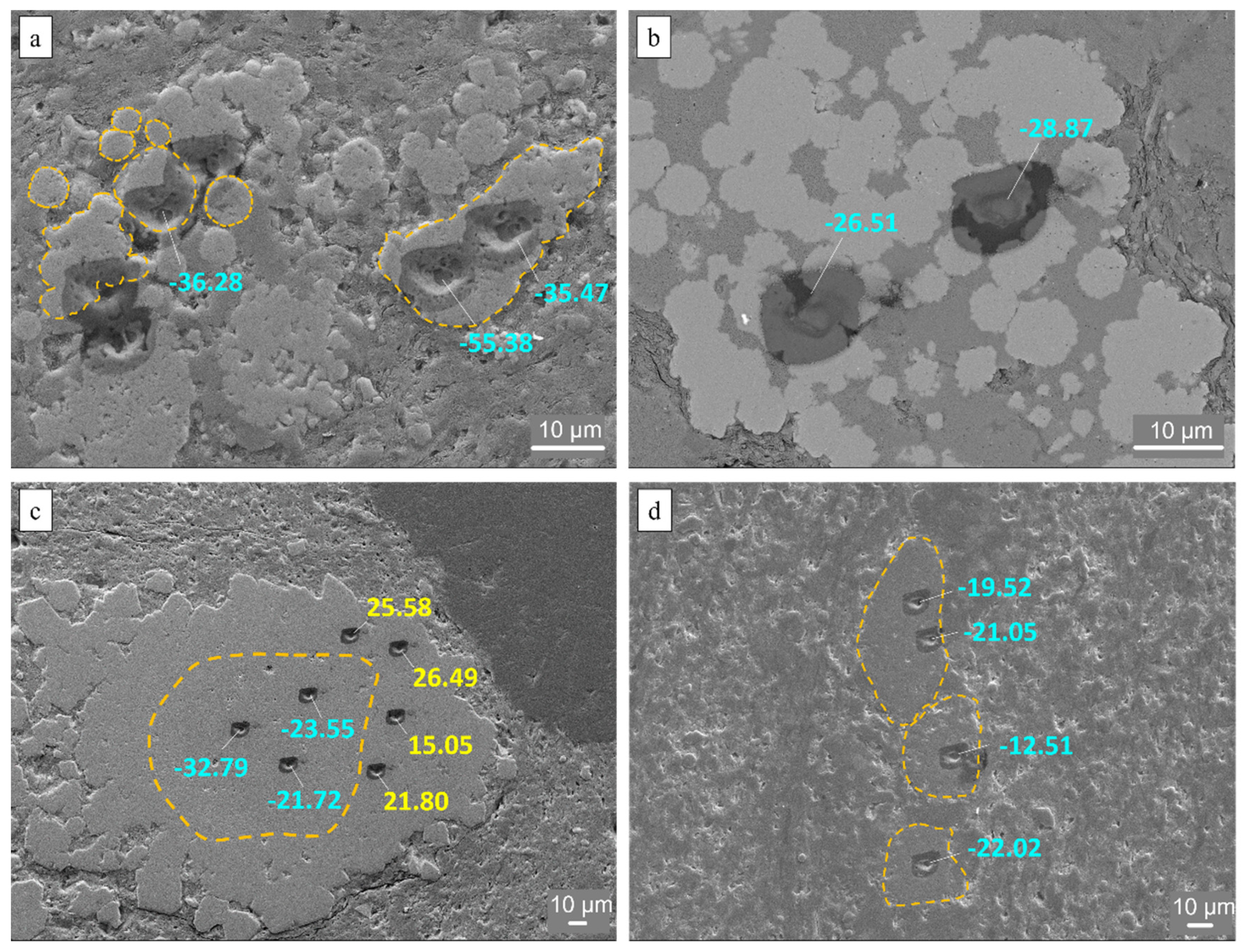
4.4. Sulfur Isotope Composition of Individual Pyrites (SIMS)
5. Discussion
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| № | Sample | Morphology | Size, µm | δ34S | Mineral and Element Composition (EDS) |
|---|---|---|---|---|---|
| 1 | 1 | Aggregate of euhedral crystals | 250 | 15.05 | FeS2 |
| 2 | 1 | Aggregate of euhedral crystals | 250 | 26.49 | FeS2 |
| 3 | 1 | Aggregate of euhedral crystals | 250 | 25.58 | FeS2 |
| 4 | 1 | Aggregate of euhedral crystals | 250 | 21.80 | FeS2 |
| 5 | 1 | Aggregate of euhedral crystals | 250 | −23.55 | FeS2 |
| 6 | 1 | Aggregate of euhedral crystals | 250 | −21.72 | FeS2 |
| 7 | 1 | Aggregate of euhedral crystals | 250 | −32.79 | FeS2 |
| 8 | 1 | Single crystal | 12 | −14.39 | CuFeS2 |
| 9 | 2 | Small framboid | 10 | −14.40 | FeS2 (contaminated Si = 5.08 at%) |
| 10 | 2 | Euhedral crystal | 40 | −18.08 | ZnS |
| 11 | 2 | Euhedral crystal | 40 | −18.56 | ZnS |
| 12 | 2 | Large framboid? | - | −13.51 | FeS2 |
| 13 | 2 | Large framboid? | - | −15.84 | FeS2 |
| 14 | 2 | - | 20 | −15.9 | ZnS (contaminated with Cu, Fe, Si, Al) |
| 15 | 2 | - | 10 | −10.72 | FeS2 |
| 16 | 2 | Framboid aggregate | 10 | −8.95 | FeS (contaminated with Zn, Si, Al) |
| 17 | 3 | Aggregate of small framboids | 10 | −22.49 | FeS |
| 18 | 3 | Small framboid | 10 | −36.28 | FeS2 |
| 19 | 3 | Aggregate of small framboids | 30 | −55.38 | FeS2 |
| 20 | 3 | Aggregate of small framboids | 30 | −35.47 | FeS2 |
| 21 | 3 | Consolidated aggregate | 72 | −19.52 | FeS2 |
| 22 | 3 | Consolidated aggregate | 72 | −21.05 | FeS2 |
| 23 | 3 | Consolidated aggregate | 27 | −12.51 | FeS2 |
| 24 | 3 | Consolidated aggregate | 27 | −22.02 | FeS2 |
| 25 | 4 | Aggregate of small framboids | 20 | −22.87 | - |
| 26 | 4 | Aggregate of small framboids | 20 | −30.39 | - |
| 27 | 4 | Aggregate of small framboids | - | −40.51 | - |
| 28 | 4 | Aggregate of small framboids | - | −26.02 | - |
| 29 | 4 | Aggregate of small framboids | - | −30.79 | - |
| 30 | 4 | Aggregate of small framboids | 7–10? | −16.96 | - |
| 31 | 4 | Aggregate of small framboids | 7 | −28.87 | FeS2 |
| 32 | 4 | Aggregate of small framboids | 10 | −26.51 | FeS |
| 33 | 4 | Small framboid | 7–10? | −18.11 | FeS |
| 34 | 4 | Small framboid | 5 | −20.59 | FeS (contaminated with Mn) |
| 35 | 4 | Small framboid | 7 | −14.35 | FeS (contaminated with Mn) |
| 36 | 4 | Small framboid | 7 | −31.12 | FeS (contaminated with Mn) |
References
- Jiang, Z.; Zhang, W.; Liang, C.; Wang, Y.; Liu, H.; Chen, X. Basic Characteristics and Evaluation of Shale Oil Reservoirs. Pet. Res. 2016, 1, 149–163. [Google Scholar] [CrossRef]
- Tyson, R.V. Organic Matter Preservation: The Effects of Oxygen Deficiency. In Sedimentary Organic Matter; Springer: Dordrecht, The Netherlands, 1995; pp. 119–149. ISBN 978-94-010-4318-2. [Google Scholar]
- Gorsline, D.S.; Tyson, R.V.; Pearson, T.H. Modern and Ancient Continental Shelf Anoxia. PALAIOS 1993, 8, 307. [Google Scholar] [CrossRef]
- Kristensen, E. Organic Matter Diagenesis at the Oxic/Anoxic Interface in Coastal Marine Sediments, with Emphasis on the Role of Burrowing Animals. Hydrobiologia 2000, 426, 1–24. [Google Scholar] [CrossRef]
- Fisher, I.S.J.; Hudson, J.D. Pyrite Geochemistry and Fossil Preservation in Shales. Philos. Trans. R. Soc. Lond. B 1985, 311, 167–169. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Barnes, H.L.; Brantley, S.L. The Size Distribution of Framboidal Pyrite in Modern Sediments: An Indicator of Redox Conditions. Geochim. Cosmochim. Acta 1996, 60, 3897–3912. [Google Scholar] [CrossRef]
- Wang, P.; Huang, Y.; Wang, C.; Feng, Z.; Huang, Q. Pyrite Morphology in the First Member of the Late Cretaceous Qingshankou Formation, Songliao Basin, Northeast China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 385, 125–136. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, D.; Zhang, J.; Lü, X.; Wang, Z.; Liao, W.; Shi, X.; Tang, J.; Xie, G. Pyrite Morphology as an Indicator of Paleoredox Conditions and Shale Gas Content of the Longmaxi and Wufeng Shales in the Middle Yangtze Area, South China. Minerals 2019, 9, 428. [Google Scholar] [CrossRef] [Green Version]
- Raiswell, R.; Berner, R.A. Pyrite and Organic Matter in Phanerozoic Normal Marine Shales. Geochim. Cosmochim. Acta 1986, 50, 1967–1976. [Google Scholar] [CrossRef]
- Wilkin, R.T.; Barnes, H.L. Formation Processes of Framboidal Pyrite. Geochim. Cosmochim. Acta 1997, 61, 323–339. [Google Scholar] [CrossRef]
- Soliman, M.F.; El Goresy, A. Framboidal and Idiomorphic Pyrite in the Upper Maastrichtian Sedimentary Rocks at Gabal Oweina, Nile Valley, Egypt: Formation Processes, Oxidation Products and Genetic Implications to the Origin of Framboidal Pyrite. Geochim. Cosmochim. Acta 2012, 90, 195–220. [Google Scholar] [CrossRef]
- Kozina, N.; Reykhard, L.; Dara, O.; Gordeev, V. Framboidal Pyrite Formation in the Bottom Sediments of the South Caspian Basin under Conditions of Hydrogen Sulfide Contamination. Russ. J. Earth Sci. 2018, 18, 1–10. [Google Scholar] [CrossRef]
- Berner, R.A. Sedimentary Pyrite Formation: An Update. Geochim. Cosmochim. Acta 1984, 48, 605–615. [Google Scholar] [CrossRef]
- Berner, R.A. Burial of Organic Carbon and Pyrite Sulfur in the Modern Ocean: Its Geochemical and Environmental Significance. Am. J. Sci. 1982. [Google Scholar] [CrossRef]
- Blood, D.R.; Lash, G.G. Dynamic redox conditions in the Marcellus Shale as recorded by pyrite framboid size distributions. In Geological Society of America Special Papers; Geological Society of America: McLean, VA, USA, 2015; Volume 515, pp. 153–168. ISBN 978-0-8137-2515-4. [Google Scholar]
- Wang, W.; Hu, Y.; Muscente, A.D.; Cui, H.; Guan, C.; Hao, J.; Zhou, C. Revisiting Ediacaran Sulfur Isotope Chemostratigraphy with in Situ NanoSIMS Analysis of Sedimentary Pyrite. Geology 2021, 49, 611–616. [Google Scholar] [CrossRef]
- Loka Bharathi, P.A. Sulfur Cycle. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 3424–3431. ISBN 978-0-08-045405-4. [Google Scholar]
- Parkes, R.J.; Sass, H. Deep Sub-Surface. In Encyclopedia of Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 64–79. ISBN 978-0-12-373944-5. [Google Scholar]
- Beaudoin, G.; Taylor, B.E.; Rumble, D.; Thiemens, M. Variations in the Sulfur Isotope Composition of Troilite from the Cañon Diablo Iron Meteorite. Geochim. Cosmochim. Acta 1994, 58, 4253–4255. [Google Scholar] [CrossRef]
- Seal, R.R. Sulfur Isotope Geochemistry of Sulfide Minerals. Rev. Mineral. Geochem. 2006, 61, 633–677. [Google Scholar] [CrossRef]
- Afanasiev, I.S.; Gavrilova, E.V.; Birun, E.M.; Kalmykov, G.A.; Balushkina, N.S. Bazhenov Formation. Overall Review, Unsolved Problems. Sci. Tech. Bull. MK Rosneft 2010, 4, 20–25. [Google Scholar]
- Gurova, T.I.; Kazarinov, V.P. Lithology and Paleogeography of the West Siberian Plain in Terms of Petroleum Potential; Gostoptekhizdat: Moscow, Russia, 1962. (In Russian) [Google Scholar]
- Golbert, A.V.; Markova, L.G.; Polyakova, I.D.; Saks, V.N.; Teslenko, Y.V. Paleolandscapes of West Siberia in the Jurassic, Cretaceous and Paleogene; Saks, V.N., Ed.; Nauka: Moscow, Russia, 1968. (In Russian) [Google Scholar]
- Zubkov, M.Y. Rocks of the Bazhenovo and Abalak Formations, Central Krasnoleninsk Arch, Western Siberia: Composition, Structure, and Formation Conditions. Lithol. Miner. Resour. 2001, 36, 33–42. [Google Scholar] [CrossRef]
- Kontorovich, A.E.; Yan, P.A.; Zamirailova, A.G.; Kostyreva, E.A.; Eder, V.G. Classification of Rocks of the Bazhenov Formation. Russ. Geol. Geophys. 2016, 57, 1606–1612. [Google Scholar] [CrossRef]
- Zakharov, V.A.; Zanin, Y.N.; Zamirailova, A.G. First Find of Trace Fossils in High-Carbon Black Shales of the Bazhenov Formation, West Siberia. Geol. Geofiz. 1998, 39, 402–405. [Google Scholar]
- Kontorovich, A.E.; Ershov, S.V.; Kazanenkov, V.A.; Karogodin, Y.N.; Kontorovich, V.A.; Lebedeva, N.K.; Nikitenko, B.L.; Popova, N.I.; Shurygin, B.N. Cretaceous Paleogeography of the West Siberian Sedimentary Basin. Russ. Geol. Geophys. 2014, 55, 582–609. [Google Scholar] [CrossRef]
- Ryzhkova, S.V.; Burshtein, L.M.; Ershov, S.V.; Kazanenkov, V.A.; Kontorovich, A.E.; Kontorovich, V.A.; Nekhaev, A.Y.; Nikitenko, B.L.; Fomin, M.A.; Shurygin, B.N.; et al. The Bazhenov Horizon of West Siberia: Structure, Correlation, and Thickness. Russ. Geol. Geophys. 2018, 59, 846–863. [Google Scholar] [CrossRef]
- Ulmishek, G.F. Petroleum Geology and Resources of the West Siberian Basin, Russia; US Geological Survey: Reston, VA, USA, 2003.
- Kozdon, R.; Williford, K.; Kita, N.; Huberty, J.; Fournelle, J.; Valley, J. In Situ Sulfur Isotope Analysis of Sphalerite and Other Sulfides by SIMS: Precision vs. Accuracy. In Proceedings of the Astrobiology Science Conference 2010: Evolution and Life: Surviving Catastrophes and Extremes on Earth and Beyond, League City, TX, USA, 26–29 April 2010. [Google Scholar]
- Thiel, J.; Byrne, J.M.; Kappler, A.; Schink, B.; Pester, M. Pyrite Formation from FeS and H2S Is Mediated through Microbial Redox Activity. Proc. Natl. Acad. Sci. USA 2019, 116, 6897–6902. [Google Scholar] [CrossRef] [Green Version]
- Rudnicki, M.D.; Elderfield, H.; Spiro, B. Fractionation of Sulfur Isotopes during Bacterial Sulfate Reduction in Deep Ocean Sediments at Elevated Temperatures. Geochim. Cosmochim. Acta 2001, 65, 777–789. [Google Scholar] [CrossRef]
- Brunner, B.; Bernasconi, S.M. A Revised Isotope Fractionation Model for Dissimilatory Sulfate Reduction in Sulfate Reducing Bacteria. Geochim. Cosmochim. Acta 2005, 69, 4759–4771. [Google Scholar] [CrossRef]
- Sawlowicz, Z. Pyrite Framboids and Their Development: A New Conceptual Mechanism. Geol. Rundsch. 1993, 82, 148–156. [Google Scholar] [CrossRef]
- Strauss, H. The Isotopic Composition of Sedimentary Sulfur through Time. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 132, 97–118. [Google Scholar] [CrossRef]
- Kampschulte, A.; Strauss, H. The Sulfur Isotopic Evolution of Phanerozoic Seawater Based on the Analysis of Structurally Substituted Sulfate in Carbonates. Chem. Geol. 2004, 204, 255–286. [Google Scholar] [CrossRef]
- Magnall, J.M.; Gleeson, S.A.; Stern, R.A.; Newton, R.J.; Poulton, S.W.; Paradis, S. Open System Sulphate Reduction in a Diagenetic Environment–Isotopic Analysis of Barite (Δ34S and Δ18O) and Pyrite (Δ34S) from the Tom and Jason Late Devonian Zn–Pb–Ba Deposits, Selwyn Basin, Canada. Geochim. Cosmochim. Acta 2016, 180, 146–163. [Google Scholar] [CrossRef] [Green Version]
- Fry, B.; Gest, H.; Hayes, J.M. 34S/32S Fractionation in Sulfur Cycles Catalyzed by Anaerobic Bacteria. Appl. Environ. Microbiol. 1988, 54, 250–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werne, J.P.; Lyons, T.W.; Hollander, D.J.; Formolo, M.J.; Sinninghe Damsté, J.S. Reduced Sulfur in Euxinic Sediments of the Cariaco Basin: Sulfur Isotope Constraints on Organic Sulfur Formation. Chem. Geol. 2003, 195, 159–179. [Google Scholar] [CrossRef]
- Claypool, G.E. Ventilation of marine sediments indicated by depth profiles of pore water sulfate and δ34S. In The Geochemical Society Special Publications; Elsevier: Amsterdam, The Netherlands, 2004; Volume 9, pp. 59–65. ISBN 978-0-444-51647-3. [Google Scholar]
- Sim, M.S.; Bosak, T.; Ono, S. Large Sulfur Isotope Fractionation Does Not Require Disproportionation. Science 2011, 333, 74–77. [Google Scholar] [CrossRef]
- Canfield, D.E.; Farquhar, J.; Zerkle, A.L. High Isotope Fractionations during Sulfate Reduction in a Low-Sulfate Euxinic Ocean Analog. Geology 2010, 38, 415–418. [Google Scholar] [CrossRef]
- Otake, T.; Lasaga, A.C.; Ohmoto, H. Ab Initio Calculations for Equilibrium Fractionations in Multiple Sulfur Isotope Systems. Chem. Geol. 2008, 249, 357–376. [Google Scholar] [CrossRef]
- Eaton, A. The Impact of Anoxia on Mn Fluxes in the Chesapeake Bay. Geochim. Cosmochim. Acta 1979, 43, 429–432. [Google Scholar] [CrossRef]
- De Baar, H.J.W.; German, C.R.; Elderfield, H.; van Gaans, P. Rare Earth Element Distributions in Anoxic Waters of the Cariaco Trench. Geochim. Cosmochim. Acta 1988, 52, 1203–1219. [Google Scholar] [CrossRef]
- Nabhan, S.; Wiedenbeck, M.; Milke, R.; Heubeck, C. Biogenic Overgrowth on Detrital Pyrite in ca. 3.2 Ga Archean Paleosols. Geology 2016, 44, 763–766. [Google Scholar] [CrossRef]

| Sample | Number of Points | Morphology | Size, µm | δ34SCDT, ‰ |
|---|---|---|---|---|
| 1 | 7 | Aggregate of euhedral crystals | 250*150 | (−32)–(+26) |
| 2 | 2 | Large framboids | 10–15 | (−14)–(−16) |
| 3 | 3 | Aggregate of small framboids | 30 | (−55)–(−35) |
| 3 | 4 | Consolidated aggregate of small crystals or framboids | 30–70 | (−22)–(−12) |
| 4 | 5 | Aggregate of small crystals or framboids | 15–20 | (−40)–(−22) |
| 4 | 3 | Aggregate of small framboids | 7–10 | (−17)–(−29) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idrisova, E.; Gabitov, R.; Karamov, T.; Voropaev, A.; Liu, M.-C.; Bogdanovich, N.; Spasennykh, M. Pyrite Morphology and δ34S as Indicators of Deposition Environment in Organic-Rich Shales. Geosciences 2021, 11, 355. https://doi.org/10.3390/geosciences11090355
Idrisova E, Gabitov R, Karamov T, Voropaev A, Liu M-C, Bogdanovich N, Spasennykh M. Pyrite Morphology and δ34S as Indicators of Deposition Environment in Organic-Rich Shales. Geosciences. 2021; 11(9):355. https://doi.org/10.3390/geosciences11090355
Chicago/Turabian StyleIdrisova, Elizaveta, Rinat Gabitov, Tagir Karamov, Andrey Voropaev, Ming-Chang Liu, Natalia Bogdanovich, and Mikhail Spasennykh. 2021. "Pyrite Morphology and δ34S as Indicators of Deposition Environment in Organic-Rich Shales" Geosciences 11, no. 9: 355. https://doi.org/10.3390/geosciences11090355
APA StyleIdrisova, E., Gabitov, R., Karamov, T., Voropaev, A., Liu, M.-C., Bogdanovich, N., & Spasennykh, M. (2021). Pyrite Morphology and δ34S as Indicators of Deposition Environment in Organic-Rich Shales. Geosciences, 11(9), 355. https://doi.org/10.3390/geosciences11090355






