Abstract
Analyses of benthic foraminiferal assemblages were carried out on sediment samples collected in the Gulf of Patti (NE Sicily, Tyrrhenian Sea), where high sedimentation rates in front of the Mazzarrà River led to the growth of a prodelta. The frequency of riverine sedimentary fluxes is testified by the widespread occurrence of erosional and depositional bedforms indicative of high-energy processes on the prodelta slope.The frequency of riverine sedimentary fluxes suggests the widespread occurrence of erosional and depositional bedforms indicative of high-energy processes on the prodelta slope. The study aimed to assess the spatial distribution of benthic foraminiferal assemblages and sediment grain size along different sectors of this prodelta to define any relationship between the foraminiferal assemblages, the environmental gradients and the sedimentary processes. In particular, we focused on the role of the highly energetic impulsive torrential inputs that dominate the depositional environment and likely affect food supply and its control on the foraminiferal density and biodiversity. The dominance of opportunistic agglutinated taxa associated with hyaline eutrophic species is a distinctive character likely related to organic matter enrichment and physical disturbance associated with inputs from torrential rivers.
1. Introduction
The continental shelf represents a sediment transfer zone connecting terrestrial source areas and deep-sea basins. This environment may receive a large amount of nutrients and organic carbon directly from rivers or other processes, including the decomposition of organic matter in surface sediments and the benthic primary production from seaweed and algae [1,2]. On river-dominated continental shelves, large sediment supply and high primary production rates, combined with shallow water depth, enhance organic matter both in the water column and in surficial sediments [3,4,5,6,7]. Moreover, the high variability of riverine discharge leads to diversified environmental conditions at the seafloor, strongly influencing benthic communities’ spatial and temporal distribution [8,9,10].
Benthic foraminifera are an important component of the marine meiofaunal community at every depth [11,12,13,14,15], locally contributing up to ~80% of the meiofaunal biomass [16,17]. Nevertheless, the distribution of foraminiferal assemblages in modern Mediterranean prodelta environments has received poor attention. The different approaches followed in the few studies (e.g., based on living, dead or fossil assemblages) further hamper a comparative analysis. Most of the studies dealing with the distribution of foraminiferal assemblages in Mediterranean prodeltas are focused on large river deltas, such as those associated with the Ebro, Rhone and Nile rivers [10,18,19,20]. Along the Italian coasts, microfaunal studies were conducted at the Ombrone, Tiber and Po deltas [21,22,23,24,25]. All these were mainly focused on the paleoenvironmental evolution of the Late Quaternary succession since these large deltas contain among the most complete record of the Mediterranean basin [24,26]. Few studies link living foraminiferal distribution and sedimentary processes in deltaic areas characterized by short rivers with torrential regimes [27].
Available studies on living foraminiferal assemblages from river-dominated environments suggest a complex interplay between physical and geochemical processes [3,4], resulting in both positive and negative effects on benthic communities. While the high organic input may enhance the benthic biomass [8,9,28], rapid organic matter enrichment and high sedimentation rates may determine eutrophic and oxygen-depleted conditions at the seafloor and near-bottom waters, resulting in a massive reduction or mortality of benthic fauna [29,30,31]. Furthermore, the hydrodynamic and sedimentary processes associated with river outflow can represent additional stress for the benthic fauna, leading to communities dominated by few opportunistic species [32,33,34,35,36]. Given all of the above, benthic foraminifera, with their rapid response to ecosystem changes, represent a useful indicator of high-energy sedimentary process and a proxy of paleoenvironmental changes in river-dominated settings. In this view, benthic foraminifera are widely used to study and monitor present shallow marine ecosystems and reconstruct the past environmental conditions, e.g., [24,37,38,39].
This study investigates an area in the Gulf of Patti, offshore NE Sicily, where the abundant sediment funneled to the sea by the Mazzarrà River led to the growth of a deltaic system, showing different erosional and depositional bedforms indicative of high-energy processes on the prodelta slope [40]. The main goal is to define the varied response of living foraminiferal assemblages to the sedimentary fluxes, variably impacting different sectors of the prodelta. In addition, the dead foraminiferal assemblage is investigated at all sites to identify reworked or displaced foraminifera, possibly representing allochthonous individuals. These data may suggest important indications about the shelf and slope sedimentary dynamics and the sediment source area. These results provide a basis for investigating similar paleoecological relationships during longer (e.g., pre-late Holocene) intervals, highlighting major climate-driven (glacio-eustatic) environmental changes, or even on the shorter, historical time scale, during which also anthropogenic impact becomes relevant. More generally, our study contributes to the analytical and comparative studies of living foraminiferal assemblages in a combined sedimentary and ecological approach.
Environmental Setting
The study area includes a 6 km wide shelf sector and the upper continental slope, extending in the depth range from 40 to 160 m offshore the Mazzarrà River in the Gulf of Patti (NE Sicily, Figure 1). The shelf environment is characterized by a microtidal regime (maximum amplitude 0.6 m) and storm waves with a significant maximum height of 3–4 m; longshore currents show a generally predominant eastward flow [41]. The tectonic setting of the Gulf of Patti is characterized by regional uplift at rates in the order of 1–2 mm/a since the Pleistocene [42,43]) and frequent seismicity (over 2000 earthquakes recorded in the last few decades [44]). Because of the rapid uplift, several short and steep course streams, locally known as “fiumara”, deeply incise the coastal highlands backing the Gulf of Patti [40]. These rivers are typically dominated by a torrential regime, with longer intervals of reduced discharge during dry seasons (encompassing spring to fall), followed by abrupt pulses of increased discharge (during the rainy winters) that may result in flash-flood events. During the flash-flood events, a large volume of sediment is transported into the sea at high concentrations in a very short time (within few days), resulting in hyperpycnal flows [45].
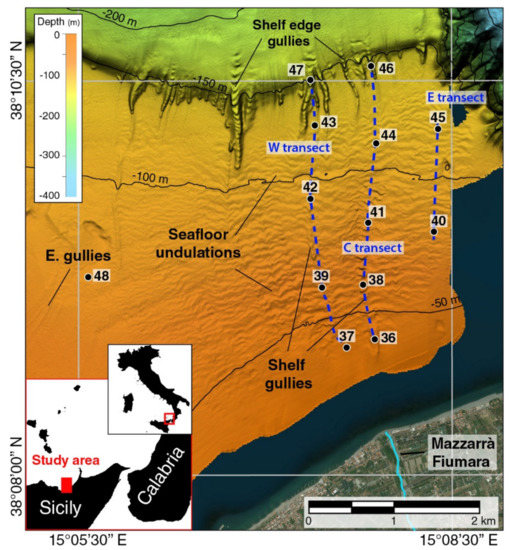
Figure 1.
Multibeam shaded relief map of the prodelta of the Mazzarrà Fiumara and location of samples (numbered black dots) along three transects (dashed blue lines indicated as western (W), central (C) and eastern (E)). Bedforms along the shelf in correspondence of the prodelta consist of shallow gullies and sediment undulations. “E. gullies” indicates part of a small gully field associated with the Elicona Fiumara (not shown in the figure).
In the study area, the Mazzarrà River is the main sediment source. A 5 km wide prodelta has been identified at its mouth [40]. The prodeltaic deposits are part of a stratigraphic unit up to 50 m thick that developed during the post-glacial (late Pleistocene-Holocene) sea-level rise and highstand. The seafloor in the area of the prodelta is shaped by sediment undulations with their crests oriented roughly parallel to the bathymetric contours and cut cross-strike by arrays of variable length gullies. More in detail, gullies are few tens of meters wide and a few meters deep in the inner-middle shelf, whereas they increase their width and depth at the shelf edge. Down-slope, trains of coaxial and crescent-shaped bedforms resemble those recently interpreted, in similar settings, as the expression of upper-flow regime bedforms by [46]. Based on overall low-quality multibeam backscatter and sparse seafloor samples, the sediment composition of the area is mostly dominated by fine-grained sediments with a silty component [40]. High backscatter values associated with sandy sediments are found only within outer shelf gullies.
2. Materials and Methods
2.1. Sampling Area and Strategy
Surface sediment samples (Table 1) in the study area were sampled using a 30 L Van Veen grab during the EPICA cruise, carried out in the Southern Tyrrhenian from 16 December 2015 to 3 January 2016 onboard the R/V Minerva Uno (Italian National Research Council).

Table 1.
Location and water depth of sediment samples: (g) from gullies; (u) from seafloor undulations.
Thirteen samples (numbered 36 to 48) were collected from the prodelta deposits along three transects extending at depth range from 40 to 160 m, referred to as western (37,39,42,43,47), central (36,38,41,44,46) and eastern (40,45) transect (Figure 1). In addition, an isolated sample (48) was collected on the middle shelf immediately outside the Mazzarà prodelta area (Figure 1; Table 1), where small gullies are likely related to the Elicona Fiumara (not showing in Figure 1), 6 km west of Mazzarrà Fiumara. The samples were positioned according to different targets indicative of sediment deposition and erosion in the prodelta environment. Samples 36 and 37 were recovered in the inner shelf, respectively, inside and outside of a larger gully facing the present-day mouth of Mazzarà River, which is the largest incision except for those forming along the shelf edge (Figure 1). Samples 38 to 42 were recovered within or close to the thalweg of the smaller gullies in the middle shelf; as an exception, sample 40 is from the top of a sediment undulation. Samples 43 to 47 were collected on the outer shelf from the distal part of the prodelta (43–45) and within the thalweg of the shelf edge incisions (46 and 47; Figure 1).
2.2. Grain-Size Analysis
Grain-size analyses on grab samples were performed using dry sieving and laser particle sizer. All samples were treated with hydrogen peroxide and distilled water to remove organic matter and salts before drying in a convection oven at ca. 40 °C. The grain size of samples with <5% of fine fractions (<63 μm) was determined only by dry-sieving (from +4 through −4.5 φ; ASTM series); for heterogeneous samples, wet sieving was used to separate coarse and fine (i.e., clay and silt) fractions. The fine fraction was treated with 500 mL of distilled water and a 50 mL solution of sodium hexametaphosphate (NaPO3) before analyzing with a laser particle sizer. The descriptive statistics of grain-size distribution were calculated using the logarithmic (original) Folk and Ward formulas [47]. In contrast, grain-size classification was performed based on the Folk classification scheme [48,49] and plotted on ternary diagrams using the USGS software Sedplot [50].
2.3. Foraminiferal Assemblages
The micropaleontological analyses were focused on the characterization of benthic foraminiferal assemblages. Specifically, small cores (about 15 cm long and 4 cm in diameter) were collected by subsampling the central part of the grab samples, which was assumed as relatively undisturbed. Since it is known that the grab sampling method does not guarantee the full integrity of the undisturbed sediments [51], the total living microfaunal content was considered in the topmost 5 cm of the cores. This approach does not significantly impact our results since literature data indicate that, in similar environmental contexts, the uppermost sediment (0–2 cm) records the highest faunal density and diversity [20,34,52,53,54].
The response of the living benthic community to the maximum fluvial input, related to rainy winter seasons, was assessed by considering only the living fauna (rose bengal-stained individuals). For this purpose, all subsamples were stained and preserved in a solution of 90% ethanol with 2 g/L of rose bengal [51,55,56]. After 15 days, the samples were wet-sieved through a 63 μm sieve and then dried at 40 °C. For each sample, rose bengal-stained foraminifera (>63 μm) with well-preserved tests were counted, hand-picked, and identified using a binocular microscope. Nontransparent agglutinated and porcelaneous tests were broken to inspect the interior. The rose bengal staining method has been widely used in ecological studies for distinguishing living from dead foraminifera [5,57,58]. However, under specific conditions (i.e., anoxic environments), the accuracy of this method may be affected by the presence of undecayed protoplasm, which can persist for weeks or months after death [57,59,60,61]. While the staining criteria are confidently applied to the superficial samples, ambiguities may arise in the case of deeper intervals [62], commonly consisting of a slight overestimation of the living assemblages [63].
To avoid an overestimation of the abundance of tubular agglutinated taxa like Rhabdammina, Hyperammina (their test fragments were frequently found in the samples), only specimens reaching at least 0.5 cm in length were counted. Benthic foraminiferal density expressed as individuals per grams of dry sediment (ind/g) and diversity indexes were calculated for each sample (5 cm top layer). Species diversity was quantified considering the number of taxa occurring in the samples (S) [5], while Shannon–Weaver (H) [64] and Fisher (α) [65] indexes were calculated using the Paleontological Statistics (PAST) version 1.38 data analysis package [66].
To highlight the occurrence of the maximum concentration of living foraminifera inside the sediments, we calculated the average living depth (ALDx) for the total assemblage in the first 5 cm of the sediments based on the following equation [67]:
where ALDX is the average living depth (cm) of the fauna in a core of x centimeters length; ni is the number of specimens in the sediment interval i; Di is the midpoint of the sediment interval i (cm); N is the total number of individuals for all layers.
ALDX = ∑i=1,x (ni × Di)/N
Charts of species density (relative abundance of single species per g of dry sediment) at each station were also derived.
The foraminifera were identified according to the generic classification of [68]. Identification of species was mainly based on previous studies from shelf and canyon systems in Mediterranean and extra-Mediterranean settings [69,70,71,72,73].
The dead assemblages were analyzed as well to identify possible allochthonous foraminiferal tests. Following [74], a minimum of 100 individuals was counted in the top 5 cm for each station. When necessary, the samples were divided into subfractions using an Otto microsplitter. Based on the state of preservation (broken, badly preserved specimens) and ecological characteristics of the taxa, reworked or displaced taxa were considered and counted to quantify allochthonous taxa and better estimate sedimentary dynamics. In fact, because of their small size, foraminifera can be easily transported by sedimentary processes [5,75].
Finally, energy-dispersive spectrometry (EDS) analysis on sulfur crystals collected in sample 42 was performed with FEI-QUANTA 400 scanning electron microscope (SEM Laboratory of Earth Sciences Department Sapienza University of Rome).
2.4. Statistical Analyses
A two-way hierarchical cluster analysis (HCA) was applied on the matrices of living and dead specimens to recognize groups of samples with homogeneous foraminiferal content, likely indicating uniform ecological conditions. For both living and dead data, simplified matrices of species abundance occurring in the 0–5 cm sediment interval were used only for those species showing relative abundances greater than 5% in at least one station [74]. Samples 42, recording an insufficient number of benthic foraminiferal individuals, was not considered for the statistical analysis. The HCA was carried out with the statistical package Paleontological Statistics (PAST) [76,77], using the group average method on a Bray–Curtis similarity matrix derived from square-root transformation of the data.
3. Results
3.1. Grain Size Analysis
The samples analyzed in this work consist mainly of sediment with silt fraction between 57% and 75% (e.g., [48,49]; see Figure 2 and Table 2) and clay and sand content between 10 and 22% and 4–30%, respectively; five samples (39, 40, 46, 47 and 48) also have a minimum gravel content, with percentages of 0.1–11%. The mean size ranges between 4.9 φ and 6.7φ. The sediment is generally poor with an overall symmetric distribution, except for samples 46, 47 and 48, which are very poorly sorted and coarse/very coarse skewed, and samples 36, 37 and 38 with a fine skewness. A platykurtic or slightly mesokurtic sediment distribution is common, again except for samples 46, 47 and 48, for which the distribution curve is leptokurtic. By considering the distribution of the samples (Figure 2c), these results indicate an overall downslope decrease in sand content, except for samples 46 and 47 (recovered within the upper slope gullies), which also yield the maximum gravel content.
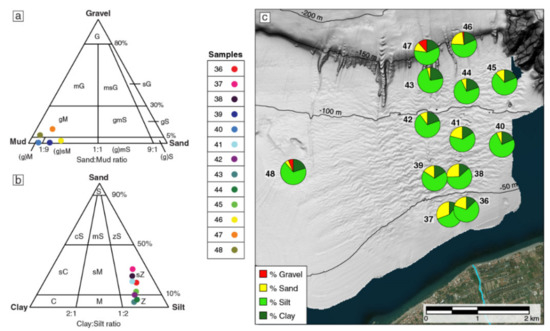
Figure 2.
Ternary diagrams for the sediment samples collected in the study area. Sediment classification scheme from [48,49]. (a) Samples with >0.1% gravel; (b) samples with no gravel; (c) pie charts showing the proportion of gravel, sand, silt and clay for each sample. G—gravel; g—gravelly; (g)—slightly gravelly; S—sand; s—sandy; M—mud; m—muddy; Z—silt; z—silty; C—clay; c—clayey.

Table 2.
Granulometric characteristics of sediment samples collected during Epica cruise. (s) slightly.
3.2. General Features of Living Foraminiferal Assemblages
A total of 102 species of living benthic foraminifera were identified in the study area, of which 69 were hyaline, 5 were porcelaneous, and 28 were agglutinated (Table S1). Agglutinated species are dominant in most samples (especially down to −110 m, Figure 3a), largely represented by Ammoglobigerina globigeriniformis, Eggerelloides scaber, Reophax scorpiurus, Lagenammina spp. (L. atlantica and L. fusiformis); only some deeper samples (41,43,45,47) show prevailing hyaline taxa with the dominance of Bolivina spathulata and Bulimina marginata followed by Cassidulina carinata, Globocassidulina subglobosa, Globobulimina pyrula and Uvigerina mediterranea. The porcelaneous taxa are absent or very scarce, their occurrence resulting appreciable only in the shallowest (36,37) and in the deepest samples (46,47), where genera Adelosina, Quinqueloculina, Triloculina and Pyrgo are present (although with frequencies <1%).
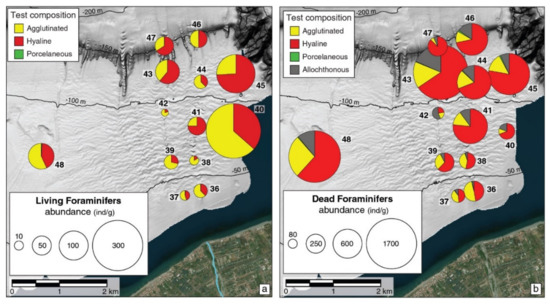
Figure 3.
Test composition of living (a) and dead (b) foraminifera in the study area. Each pie chart is proportional to foraminiferal relative abundance. The size of the pie charts is based on average density values from the 0–5 cm sampled sediment interval.
Overall, the mean values of living foraminiferal abundance increase from the inner to the outer sectors of the prodelta (Figure 3a and Table 3) and in the eastern transect (45 and 40) of the study area except for sample 42 (western transect), where the lowest value was found (6.06 ind/g). The lower values (<24 ind/g) were detected in front of the river mouth, between 35 and 112 m depth (Figure 3a and Table 3); intermediate values (between 35 and 79 ind/g) were found on the outer shelf between 120 and 160 m water depth (43,46,47) and also outside (to the west) of the prodelta (48). The maximum abundances (from 175 up to 353 ind/g) were recorded in two samples from the eastern transect (40 at −80 m, and 45 at −113 m; Figure 3a and Table 3).

Table 3.
Summary of data for living foraminifera in the analyzed samples. Average living depth (ALD5) for the total fauna in the first 5 cm; (g) gullies; (u) seafloor undulations. The total number of specimens, proportion of agglutinated (Aggl.), hyaline (Hyal.) and porcelanaceous (Porc.) taxa (expressed as ind/g) and the diversity indices (α-Fisher and H) are reported for each sample. (W) western transect, (C) central transect, (E) eastern transect.
The mean values of diversity in the topmost 0–5 cm clearly show low values (Table 3) along the western transect (stations 37,39,42,43,47) with α-Fisher index ranging from 4.53 to 9.18 and Shannon (H) index <2. The highest values are from station 44 (−112 m; α-Fisher index: 10.97 and H index: 2.29) and 48 (−180 m; α-Fisher index: 16.63 and H index: 2.48). At all stations, the ALD5 values show that most living foraminifera occurs in the superficial sediment layers (topmost 2 cm, Table 3). In samples 39 and 46, the average density of living foraminifera is recorded in the topmost 1 cm. In all samples, the faunal composition consists mainly of infaunal groups (Ammoscalaria spp., Eggerelloides scaber, Bolivina spp., B. marginata and Globobulimina spp., U. mediterranea). In contrast, epifaunal taxa (mainly L. lobatula, Epistominella vitrea, Textularia spp., Valvulineria bradyana) are very scarce.
Following the bathymetric gradient, the foraminiferal distribution shows the dominance of A. globigeriniformis and E. scaber down to 50 m depth; at greater depth, R. scorpiurus strongly increases, together with bolivinids and buliminids. The presence of Globobulimina spp. is documented starting from 80 m water depth. In comparison, U. mediterranea and Chilostomella oolina are recorded starting from 120 m water depth.
3.3. Living Foraminiferal Assemblages Distribution
The HCA discriminates four main clusters corresponding to as many living assemblages, labeled a, b, c and d (Figure 4a; distribution shown in Figure 5a).
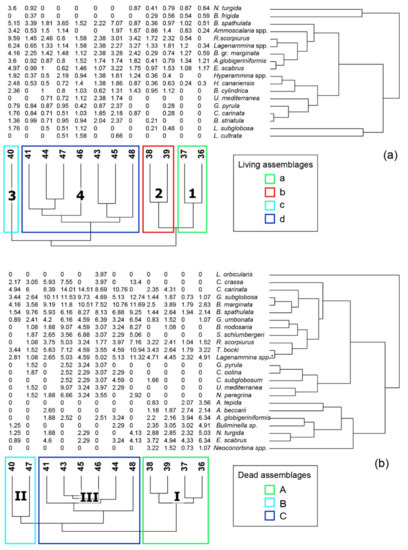
Figure 4.
Two-way hierarchical cluster analysis applied on living (a) and dead (b) commonly occurring species (>5%) in the Gulf of Patti. The number of clusters and the corresponding assemblage ID are also shown.
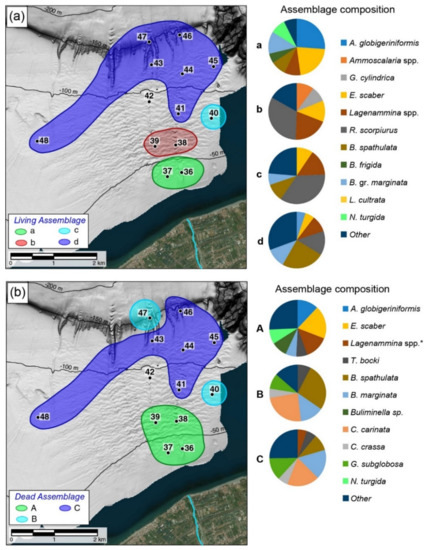
Figure 5.
Spatial distribution of the foraminiferal assemblages identified from the cluster analysis and related pie charts representing the composition (>5%) of each assemblage: (a) living assemblages; (b) dead assemblages.
Cluster 1 groups the inner shelf samples (36 and 37) collected at depths shallower than 50 m (Figure 5a), characterized by species of assemblage a, mainly including agglutinated taxa, such as A. globigeriniformis (26%), E. scaber (22%), Lagenammina spp. (9%) together with the hyaline Bulimina gr. marginata (B. marginata and B. fusiformis, 13%). Nonionella turgida and B. spathulata are present with mean values of 9%, while Buccella frigida follows with frequencies of 5%.
Cluster 2 includes two samples from the mid-shelf collected at about 60 m water depth, in correspondence with seafloor incisions (38, 39 in Figure 1 and Figure 5a). The resulting assemblage b is mainly characterized by agglutinated taxa like R. scorpiurus (33%), Lagenammina spp. (20%) and E. scaber (12%). These taxa are followed by Ammoscalaria spp. and Goesella cylindrica with mean values of 10 and 9%, respectively. Hyaline taxa display low values of less than 4% and are represented mainly by B. spathulata, B. gr. marginata, N. turgida and B. frigida.
Cluster 3 includes only sample 40 characterized by assemblage c. This is similar to assemblage b, from which it differs for the high frequencies of hyaline taxa like B. spathulata (10%), B. marginata (7%), and N. turgida (5%).
Cluster 4 groups the deepest samples recovered from the outer shelf (43–47) with samples from the middle shelf (41 and 48). The resulting assemblage d is more diversified than the previous ones and is mainly composed of B. spathulata (25%), R. scorpiurus (14%), B.gr. marginata (11%) and Lagenammina spp. (9%). This assemblage is also characterized by typical circalittoral species like C. carinata (3%), G. pyrula (2%) and U. mediterranea (3%).
3.4. General Features of Dead Foraminiferal Assemblages
A total of 113 species of benthic foraminifera were identified in the dead assemblages, of which 76 were hyaline, 13 were porcelaneous, and 24 were agglutinated (Table S2). Hyaline individuals are dominant in most samples except for sample 36, where similar frequencies of hyaline and agglutinated individuals are recorded (Figure 3b). The agglutinated group shows the highest frequencies (30–49%) closer to the coast and decreases (27–6%) at water depths greater than 80 m (Figure 3b), with the most abundant species being the same as that of the living assemblages (A. globigeriniformis, E. scaber, Lagenammina spp., Table S2). Downward 60 m depth, we observe an increase of Textularia spp. associated with Lagenammina spp. and, occasionally, with E. scaber, Bigenerina nodosaria and Sigmoilopsis schlumbergeri. The composition of the hyaline group is similar to that of the living assemblages, characterized by the abundance of typical shallow-water taxa like Ammonia spp. (36,37), associated with Buliminella sp., N. turgida and Haynesina depressula. An increase of V. bradyana and C. carinata is recorded between 60 to 80 m water depth, accompanied by higher frequencies of Buliminidae and Bolivinidae in sample 40. Downward 80 m depth, V. bradyana decreases, while C. carinata is associated with high frequencies of G. subglobosa, B. marginata, B. spathulata and typical circalittoral species (Melonis spp., Gyroidina umbonata, Uvigerina spp.).
Unlike the living assemblage, the porcelaneous taxa are absent or very scarce (0–3%) except for the distal samples (Figure 3b), where they are represented by reworked or displaced specimens or deep-water taxa like Pyrgo and Biloculinella spp. Overall, the dead microfauna density increases from the inner to the outer sectors of the prodelta. However, minimum values are recorded by samples 42, similarly to the living assemblage (Figure 3b and Table 4). It is to note that in this sample, native sulfur minerals were recorded (Figure 6).

Table 4.
Summary of dead foraminiferal data for the analyzed samples in the first 5 cm. (g) gullies; (u) seafloor undulations; (s) slightly. Density (ind/g), proportion of agglutinated (Aggl.), hyaline (Hyal.), porcelanaceous (Porc.) and allochthonous (Allocht.) taxa (expressed as ind/g) are reported for each sample.
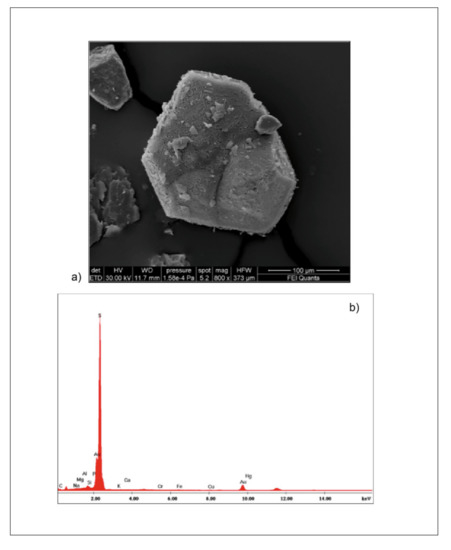
Figure 6.
(a) SEM micrographs (scale bar = 100 μm) and (b) EDS analysis of sulfur crystals collected in sample 42.
Reworked or displaced taxa show frequencies ranging from 3 (36) to 56% (42) at water depths below 100 m (Figure 3b). Apart from samples 42 and 40 (19%), showing the highest allochthonous content, in the shallower sectors (up to 80 m water depth), reworked or displaced taxa are represented by broken or poorly preserved tests with frequencies ranging between 2 and 9%. Downward 100 m depth, the allochthonous fraction increases (12–14%, Figure 3b) and is mainly represented by typically shallow-water taxa most likely displaced, as for instance,: Ammonia spp., H. depressula, Elphidium spp., Quinqueloculina spp. and Adelosina spp., e.g., [5,32,70,78,79,80,81] (Table S3).
3.5. Dead Foraminiferal Assemblages Distribution
In the dendrogram obtained from the HCA, three main clusters related to assemblages A, B and C may be recognized (Figure 4b).
Cluster I groups the shallowest samples (36–39) from the depth range 35–60 m (Figure 5b). The assemblage A is characterized by high frequencies of agglutinated taxa (E. scaber 19%, Lagenammina 14%, A. globigeriniformis 12%, T. bocki 6%) associated with hyaline taxa like Buliminella spp. (9%) and B. marginata (6%).
Cluster II includes two samples (40 and 47) located in the eastern part of the study area and at the slope edge, respectively (Figure 5b). The assemblage B is dominated by B. spathulata (27%), C. carinata (24%), and B. marginata (14%). Globocassidulina subglobosa (9%) and T. bocki (8%) follow with lower frequencies.
Cluster III is represented by the distal samples (41, 43–46, 47) located in a depth interval between 82 and 160 m depth (Figure 5b). The assemblage C shows an increase of B. marginata (17%) and G. subglobosa (13%) concerning C. carinata (18%) and B. spathulata (9%), which in turn display a slight decrease compared to the previous assemblage (B).
4. Discussion
4.1. Response of Living Foraminiferal Assemblages to Sedimentary Disturbance
The environmental interpretation of the living foraminiferal assemblages in the Gulf of Patti reflects the interplay of variable trophic conditions, high-energy sedimentary processes and diverse abiotic factors, the most relevant being depth gradient, sediment grain size and seasonal climate regime. The studied samples were collected during the winter season, when rainfall and river discharges are more intense, therefore, representing the most affecting disturbance for benthic fauna. During these highly energetic hydrodynamic conditions, the distribution of the benthic foraminifera results primarily controlled by two factors: sedimentation rates (depending on sediment input from rivers) and water depth (Figure 5). The low values of average living depth (ALD5 < 2 cm) recorded in all sites of the study area could be due to the combined effect of scarcely oxygenated seafloor, with a redox front located very close to the water–sediment surface, and the physical disturbance associated with frequent sedimentary transport processes. Both these factors could prevent the colonization of deeper sediment layers. These environmental conditions are testified by the prevalence of low diversity assemblages dominated by opportunistic species. However, some differences along the prodelta area can be envisaged. In the central part of the prodelta, down to 100 m depth (Figure 3a), where gullies and the larger scale sediment undulations mostly concentrate, agglutinated taxa reach more than 80% of the total assemblage. Two agglutinated opportunistic species dominate the assemblages a and b, respectively: A. globigeriniformis at shallow depths (<50 m) and R. scorpiurus from 50 to 96 m depth [71,82]. These findings are consistent with literature data reporting similar distributions in other Mediterranean and extra-Mediterranean sites and relating them to stressful conditions at the seafloor [31,71,82,83,84,85,86]. Although both taxa live in a wide depth range and are tolerant to substrate disturbance caused by rapid sediment deposition or by strong currents [33,87,88], the abrupt replacement of A. globigeriniformis (assemblage a) with R. scorpiurus (assemblage b), observed at 50 m depth in our study area (Figure 5), is in contrast with literature data, which report an overlap of the ecological niches of the two species. In the Gulf of Patti, the distance of the river mouth might represent a possible cause of the observed distribution. This fact could imply differences in the distribution of the organic carbon content and grain size and the outreach of sediment fluxes significantly impacting the seafloor [85,89,90,91]. A stronger influence of the fiumara discharge can be envisaged in the inner shelf, also suggested by the higher sand content preferred by A. globigeriniformis [31,86,87]. The occurrence of euryhaline taxa like E. scaber would be favored by the freshwater input [39,92]. The high abundance of E. scaber can be due to its capability to rapidly colonize new areas [93,94] in shallow, highly energetic, and organic matter-rich environments [5,32,84,95]. In assemblage a, the agglutinated taxa are associated with typical infaunal eutrophic species feeding on low-quality organic matter, like Bulimina gr. marginata, B. spathulata and N. turgida confirm the high availability of nutrients linked to enhanced river input during the winter season [29,32,52,80,92,96]. Moreover, the presence of typical infralittoral taxa like B. frigida, which generally live in environments where factors like grain size, organic matter contents and salinity are highly variable [5,97], agrees with intense erosive-depositional dynamics expected in the prodelta area of the Mazzarrà Fiumara.
In the outer sector of the prodelta (at depths greater than 100 m), where the impact of the sedimentary flows seems to decrease [40], the foraminiferal distribution follows an overall depth gradient. Higher abundance values are observed (48 to the west, 43 in the central part and 45 to the east, Figure 3a). However, sedimentary gravity flows can sometimes reach the shelf edge and accelerate due to the increase of slope gradients, resulting in deeper and larger incisions hosting upper-flow regime bedforms [46] and coarser sediment (i.e., samples 46 and 47 in Figure 2). In this sector (assemblage d), the hyaline taxa increase with depth due to reducing the opportunistic agglutinated taxa (R. scorpiurus, A. globigeriniformis, E. scaber). Concurrently, typical circalittoral species like Cassidulina spp., Globobulimina spp. and Uvigerina mediterranea make their appearance. Buliminids and bolivinids indicate persisting eutrophic conditions at the seafloor, although they occur along with shallow infauna typical of mesotrophic environments, as for instance, U. mediterranea [54,96,98,99,100]. This may be interpreted due to the gradual decrease of organic flux towards deeper areas [62].
At the easternmost site (40), the high frequencies of typical eutrophic and dysoxic taxa (assemblage c) like Bolivina spp., B. gr. marginata, and N. turgida, e.g., [21,62,79,81,101,102,103], and the high frequencies of allochthonous taxa (19%), suggest a possible deviation of river fluxes towards this sector of the prodelta, in agreement both with the progressive deflection in the same direction of the crest lines of sediment undulations [40], and the main direction of along-shelf currents [41]. Moreover, the presence of a canyon head scarp on this side of the prodelta could influence the direction of sediment transport.
4.2. Dead Foraminiferal Assemblages and Allochthonous Taxa
The dead assemblages can be very useful to understand and reconstruct the population dynamics, especially in areas subject to environmental instability (i.e., related to sediment gravity flows). Whereas the living fauna provides environmental information related to their lifetime, the dead assemblage provides insights into the relatively longer-term depositional and taphonomic processes.
The comparison between living and dead assemblages highlights a major faunal homogenization for the shallower sectors (assemblage A) with a reduction of agglutinated taxa in the dead assemblages of all samples, indicative of a poor preservation potential. The dead assemblages (assemblage A and C) confirm a distribution controlled by sediment and organic matter input along a depth gradient. The most abundant species are represented by eutrophic taxa (N. turgida, Bolivina spp. and B. marginata ) associated with taxa tolerating intense bottom currents, like C. carinata and G. subglobosa [34,104,105,106]. Moreover, the dead assemblages reveal a high concentration of B. spathulata (assemblage B) in the eastern and in the deepest sectors of the study area, suggesting low oxygen condition at the seafloor probably due to the persistent accumulation of organic matter through time; this is also in agreement with that which has been observed for the living fauna.
The highest frequencies of the allochthonous taxa are recorded in deeper shelf sectors in the study area (from about 100 m depth, 43, 44, 46 samples) and correlate with a predominance of fine-grained sediment. They are mainly represented by infralittoral taxa that are resumed and transported post-mortem into deeper water. In this view, sample 42 is peculiar for the low-density of living foraminifera (dominated by agglutinated taxa) and the higher frequency of the allochthonous component (56%). The decrease of living foraminifera is probably due to a stressed local condition at seafloor inhibiting benthic communities. In this sample, the finding of sulfur native crystals, totally rose-bengal stained and clearly showing bioerosion signs, suggests a possible presence of bacteria, such as Thiothrix or Beggiatoa, reduce sulfur compounds present in local fluid/gas emissions to elemental sulfur [107,108]. The high values of allochthonous content may be due to the ubication of the sample located fully well within a gully.
4.3. Comparison with Other Mediterranean Prodeltas
The Mazzarrà prodelta is characterized by surficial sediments with an overall silty composition, indicating the main influence of river-derived sediment plumes, as observed on other shelf settings supplied by steep and short rivers, as, for instance, on the northern shelf of the Alboran Sea [109]. The prevailing sediments and associated values of mean grain-size (around 5–6.6 φ) are generally lower than the values found in larger prodeltaic settings, such as off the Tiber (around 5–7.8 φ; [110]) or the Ebro (8.5–10.5 φ; [111]) rivers. This evidence can be associated with differences in the hydraulic regime and sedimentary load carried out by short- and medium-size rivers during floods. The more frequent generation of hyperpycnal flows concerning larger rivers [112].
In the Mediterranean basin, the relationship between foraminiferal distribution and sedimentary processes in deltaic areas linked to short rivers with the torrential regime was carried out by [27] in Guadalfeo and Adra submarine deltas off the northern coast of the Alboran Sea. In these areas, a hydrodynamic and climatic regime similar to those found in the Mazzarrà River prodelta (strong climatic seasonality determining torrential floods alternated with periods of low rainfall or dry periods) greatly influences the benthic microfauna distribution, resulting in foraminiferal assemblages with compositional features comparable to those reported in this study. Like our study area, opportunistic taxa prevails in the Spanish prodeltas, mainly represented by agglutinated species feeding on organic matter and fresh phytodetritus, capable of rapidly recolonizing the frequently reworked surficial sediments of high-energy shelf environments.
A qualitative comparison between the Gulf of Patti and the microfaunal distribution found in larger river deltas (Ebro, Rhone, Ombrone, Tiber deltas) shows that despite all assemblages are dominated by eutrophic species like Bulimina spp., Bolivina spp., V. bradyana, a lower abundance of agglutinated taxa occurs [24,81]. In these deltas, the effects of seasonality and consequently the hydrodynamic regime are buffered by the length of the rivers, the geomorphological setting of their catchments, and the numerous anthropogenic alterations (dams, defense works, etc.) of their course. These factors influence the foraminiferal assemblages resulting in a minor abundance of agglutinated opportunistic taxa like Reophax spp., Eggerelloides spp., A. globigeriniformis and L. scottii, that are concentrated close to the river mouth, where a more severe seafloor disturbance induced by massive sediment inputs occurs [10,53]. On the contrary, a higher similarity is highlighted in the assemblages developing on the continental margins characterized by short rivers with torrential regimes associated with the developing of shelf-indenting canyons. For instance, in the cases of canyons in the Ligurian and Gioia basins, despite marked differences between canyon and open shelf domains, the foraminiferal assemblages are substantially similar to those of Mazzarrà prodelta in terms of distribution, composition, density and diversity [34,54]. Like in the Gulf of Patti, we found the dominance of agglutinated taxa and poorly differentiated assemblages (in terms of species composition) due to the narrow and steep continental margin favoring the dispersal of riverine input throughout the shelf, which could be one of the most discriminating factors concerning the large river deltas. The record of similar assemblages in other sites along the narrow Tyrrhenian margin, characterized by similar geological, hydrodynamic and sedimentary settings [34,54], confirms the peculiarity of these associations.
5. Conclusions
Analyses of benthic foraminiferal assemblages and sedimentological data carried out on sediments samples collected in the Gulf of Patti allowed us to better understand the influence of sedimentary processes driven by riverine input on the foraminiferal community. Specifically, the following consideration can be highlighted:
- In highly energetic hydrodynamic settings, physical disturbance related to sediment transport processes and food supply represents the most significant environmental factors controlling the foraminiferal assemblages in faunal density, biodiversity, and taxonomics compositions.
- The dominance of opportunistic agglutinated taxa (mainly Reophax spp., Eggerelloides spp.) associated with hyaline eutrophic species (Bolivina spp., Bulimina spp., N. turgida) represents a distinctive character of the living benthic foraminiferal community in this sector of the southern Tyrrhenian margin. A clear decrease of these taxa is recorded in the marginal areas of the prodelta, confirming their correlation with river input. At depth >100 m, more oligotrophic conditions due to greater distance from the coastline favor developing typical deep-sea assemblages characterized by the occurrence of shallow infaunal species. The dead assemblage confirms that the distribution is controlled by sedimentary and organic matter input along a depth gradient.
- Compared to other sites of the Tyrrhenian margin characterized by similar geological, hydrodynamic and sedimentary settings confirms the strong correlation between the assemblages and the organic matter enrichment and the concurrent physical disturbance by fluvial flows.
- The study provides insights for paleoenvironmental reconstructions in similar environments during the Holocene.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/geosciences11050220/s1, Table S1: Results of the quantitative data. Number of the living taxa found in the sediment depth interval 0–5 cm for each sample site. Table S2: Results of the quantitative data. Number of the dead taxa found in the sediment depth interval 0–5 cm for each sample site. Table S3: List of taxa considered allochthonous and their living depth distribution.
Author Contributions
L.D.B., M.P., D.C., D.R. collected the samples. L.D.B., C.B., M.P. prepared the samples and performed the analyses. M.P., D.C. and D.R. made the analyses of multibeam bathymetry and map images. M.P., C.B. carried out the grain size analysis. L.D.B., C.B., contributed to the classification and quantitative micropaleontological analyses. L.D.B. and M.P. contributed to the editing of present versions of the manuscript. All authors participated in the writing of the present versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the “Progetto di Ricerca di Università 2019—La risposta adattativa delle faune bentoniche in ambienti estremi attuali e fossili” (L. Di Bella).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to acknowledge the captains and crews of R/V Minerva for their critical assistance in the collection of data.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Mopper, K.; Degens, E.T. Organic carbon in the ocean: Nature and cycling. Glob. Carb. Cycle 1979, 13, 293–316. [Google Scholar]
- Bauer, J.E.; Druffel, E.R. Ocean margins as a significant source of organic matter to the deep open ocean. Nature 1998, 392, 482. [Google Scholar]
- Lohrenz, S.E.; Dagg, M.J.; Whitledge, T.E. Enhanced primary production at the plume/oceanic interface of the Mississippi River. Cont. Shelf Res. 1990, 10, 639–664. [Google Scholar]
- McKee, B.A.; Aller, R.C.; Allison, M.A.; Bianchi, T.S.; Kineke, G.C. Transport and transformation of dissolved and particulate materials on continental margins influenced by major rivers: Benthic boundary layer and seabed processes. Cont. Shelf Res. 2004, 24, 899–926. [Google Scholar]
- Murray, J.W. Ecology and Applications of Benthic Foraminifera; Cambridge University Press: Cambridge, UK, 2006; p. 426. [Google Scholar]
- Lohrenz, S.E.; Fahnenstiel, G.L.; Redalje, D.G.; Lang, G.A.; Chen, X.; Dagg, M.J. Variations in primary production of northern Gulf of Mexico continental shelf waters linked to nutrient inputs from the Mississippi River. Mar. Ecol. Prog. Ser. 1997, 155, 45–54. [Google Scholar]
- Dagg, M.J.; Breed, G.A. Biological effects of Mississippi River nitrogen on the northern Gulf of Mexico—A review and synthesis. J. Mar. Syst. 2003, 43, 133–152. [Google Scholar]
- Danovaro, R.; Gambi, C.; Manini, E.; Fabiano, M. Meiofauna response to a dynamic river plume front. Mar. Biol. 2000, 137, 359–370. [Google Scholar]
- Salen-Picard, C.; Arlhac, D.; Alliot, E. Responses of a Mediterranean soft bottom community to short-term (1993–1996) hydrological changes in the Rhone river. Mar. Environ. Res. 2003, 55, 409–427. [Google Scholar]
- Goineau, A.; Fontanier, C.; Jorissen, F.; Buscail, R.; Kerhervé, P.; Cathalot, C.; Pruski, A.M.; Lantoine, F.; Bourgeois, S.; Metzger, E.; et al. Temporal variability of live (stained) benthic foraminiferal faunas in a river-dominated shelf–Faunal response to rapid changes of the river influence (Rhône prodelta, NW Mediterranean). Biogeosciences 2012, 9, 1367–1388. [Google Scholar]
- Gooday, A.; Levin, L.A.; Linke, P.; Heeger, T. The role of benthic foraminifera in deep-sea food webs and carbon cycling. In Deep-Sea Food Chains and the Global Carbon Cycle; Rowe, G.T., Patiente, V., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992; pp. 63–69. [Google Scholar]
- Moodley, L.; van der Zwaan, G.J.; Herman, P.M.J.; Kempers, L.; van Breugel, P. Differential response of benthic meiofauna to anoxia with special reference to the Foraminifera (Protista: Sarcodina). Mar. Ecol. Prog. Ser. 1997, 158, 151–163. [Google Scholar]
- Debenay, J.P.; Fernandez, J.M. Benthic foraminifera records of complex anthropogenic environmental changes combined with geochemical data in a tropical bay of New Caledonia (SW Pacific). Mar. Pollut. Bull. 2009, 59, 311–322. [Google Scholar] [PubMed]
- Danovaro, R. Methods for the Study of Deep-Sea Sediments, Their Functioning and Biodiversity; CRC Press: Boca Raton, FL, USA, 2009; p. 428. [Google Scholar]
- Balsamo, M.; Semprucci, F.; Frontalini, F.; Coccioni, R. Meiofauna as a tool for marine ecosystem biomonitoring marine ecosystems. In Marine Ecosystems; Antonio, C., Ed.; In Tech: Rijeka, Hrvatska, 2012; pp. 77–710. [Google Scholar]
- Snider, L.J.; Burnett, B.R.; Hessler, R.R. The composition and distribution of meiofauna and nanobiota in a central North Pacific deep-sea are. Deep Sea Res. 1984, 31, 1225–1249. [Google Scholar]
- Van Der Zwaan, G.J.; Duijnstee, I.A.P.; Den Dulk, M.; Ernst, S.R.; Jannink, N.T.; Kouwenhoven, T.J. Benthic foraminifers: Proxies or problems? A review of paleocological concepts. Earth Sc. Rev. 1999, 46, 213–236. [Google Scholar]
- Cearreta, A.; Benito, X.; Ibáñez, C.; Trobajo, R.; Giosan, L. Holocene palaeoenvironmental evolution of the Ebro Delta (Western Mediterranean): Evidence for an early construction based on the benthic foraminiferal record. Holocene 2016, 26, 1438–1456. [Google Scholar] [CrossRef]
- Schmiedl, G.; de Bovée, F.; Buscail, R.; Charrière, B.; Hemleben, C.; Medernach, L.; Picon, P. Trophic control of benthic foraminiferal abundance and microhabitat in the bathyal Gulf of Lions, western Mediterranean Sea. Mar. Micropaleont. 2000, 40, 167–188. [Google Scholar]
- Hyams-Kaphzan, O.; Almogi-Labin, A.; Sivan, D.; Benjamini, C. Benthic foraminifera assemblage change along the southeastern Mediterranean inner shelf due to fall-off of Nile-derived siliciclastics. Neues Jahr. Geol. Paläontol. Abh. 2008, 248, 315–344. [Google Scholar]
- Jorissen, F.J. Benthic foraminifera from the Adriatic Sea; principles of phenotypic variations. Utrecht Micropaleontol. Bull. 1988, 37, 1–176. [Google Scholar]
- Asioli, A.; Trincardi, F.; Lowe, J.J.; Ariztegui, D.; Langone, L.; Oldfield, F. Sub-millennial scale climatic oscillations in the central Adriatic during the Lateglacial: Palaeoceanographic implications. Quat. Sci. Rev. 2001, 20, 1201–1221. [Google Scholar]
- Amorosi, A.; Colalongo, M.L.; Fiorini, F.; Fusco, F.; Pasini, G.; Vaiani, S.C.; Sarti, G. Palaeogeographic and palaeoclimatic evolution of the Po Plain from 150-ky core records. Glob. Planet. Chang. 2004, 40, 55–78. [Google Scholar]
- Di Bella, L.; Bellotti, P.; Milli, S. The role of foraminifera as indicators of the Late Pleistocene-Holocene palaeoclimatic fluctuations on the deltaic environment: The example of Tiber delta succession (Tyrrhenian margin, Italy). Quat. Int. 2013, 303, 191–209. [Google Scholar]
- Rossi, V.; Vaiani, S.C. Benthic foraminiferal evidence of sediment supply changes and fluvial drainage reorganization in Holocene deposits of the Po Delta, Italy. Mar. Micropaleontol. 2008, 69, 106–118. [Google Scholar]
- Milli, S.; D’Ambrogi, C.; Bellotti, P.; Calderoni, G.; Carboni, M.G.; Celant, A.; Di Bella, L.; Di Rita, F.; Frezza, V.; Magri, D.; et al. The transition from wave-dominated estuary to wave-dominated delta: The Late Quaternary stratigraphic architecture of Tiber River deltaic succession (Italy). Sediment. Geol. 2013, 284–285, 159–180. [Google Scholar]
- Mendes, F.J.; Lobo, L.M.; Fernández-Salas, N.; López-González, P.; Bárcenas, J.; Schönfeld, J.; Ferreira, O. Multi-proxy evidence of rainfall variability recorded in subaqueous deltaic deposits off the Adra River, southeast Iberian Peninsula Estuarine Coast. Shelf Sci. 2015, 167, 300–312. [Google Scholar]
- Manini, E.; Danovaro, R.; Fabiano, M. 2002. Benthic-pelagic coupling in frontal system areas of the northern Adriatic Sea: Analysis of the carbon budgets. Chem. Ecol. 2015, 18, 155–160. [Google Scholar]
- Jorissen, F.J.; Barmawidjaja, D.M.; Puskaric, S.; Van der Zwaan, G.J. Vertical distribution of benthic foraminifera in the northern Adriatic Sea: The relation with the organic flux. Mar. Micropaleontol. 1992, 19, 131–146. [Google Scholar]
- McAllen, R.; Davenport, J.; Bredendieck, K.; Dunne, D. Seasonal structuring of a benthic community exposed to regular hypoxic events. J. Experim. Mar. Biol. Ecol. 2009, 368, 67–74. [Google Scholar]
- Eichler, P.B.; McGann, M.; Rodrigues, A.R.; Mendonça, A.; Amorim, A.; Bonetti, C.; Cordeiro de Farias, C.; Mello e Sousa, S.H.; Vital, H.; Gomes, M.P. The occurrence of the invasive foraminifera Trochammina hadai Uchio in Flamengo Inlet, Ubatuba, São Paulo State, Brazil. Micropaleontology 2018, 64, 391–402. [Google Scholar]
- Goineau, A.; Fontanier, C.; Jorissen, F.J.; Lansard, B.; Buscail, R.; Mouret, A.; Kerhervé, P.; Zaragosi, S.; Ernoult, E.; Artero, C.; et al. Live (stained) benthic foraminifera from the Rhône prodelta (Gulf of Lion, NW Mediterranean): Environmental controls on a river-dominated shelf. J. Sea Res. 2011, 65, 58–75. [Google Scholar]
- Yamashita, C.; Omachi, C.; Aoki Santarosa, A.C.; Sayuri Iwai, F.; Dias Araujo, B.; Trevisan Disaró, S.; Martins, M.V.A.; Vicente, T.M.; Taniguchi, N.; Burone, L.; et al. Living benthic foraminifera of Santos continental shelf, southeastern Brazilian continental margin (SW Atlantic): Chlorophyll-a and particulate organic matter approach. J. Sediment. Environ. 2019, 5, 17–34. [Google Scholar]
- Di Bella, L.; Pierdomenico, M.; Porretta, R.; Chiocci, F.L.; Martorelli, E. Living and dead foraminiferal assemblages from an active submarine canyon and surrounding sectors: The Gioia Canyon system (Tyrrhenian Sea, Southern Italy). Deep Sea Res. Part I 2017, 123, 129–146. [Google Scholar]
- Pierdomenico, M.; Martorelli, E.; Dominguez-Carrió, C.; Gili, J.M.; Chiocci, F.L. Seafloor characterization and benthic megafaunal distribution of an active submarine canyon and surrounding sectors: The case of Gioia Canyon (Southern Tyrrhenian Sea). J. Mar. Syst. 2016, 157, 101–117. [Google Scholar]
- Pierdomenico, M.; Cardone, F.; Carluccio, A.; Casalbore, D.; Chiocci, F.; Maiorano, P.; D’Onghia, G. Megafauna distribution along active submarine canyons of the central Mediterranean: Relationships with environmental variables. Prog. Ocean. 2019, 171, 49–69. [Google Scholar]
- Martins, M.V.; Jouanneau, J.-M.; Weber, O.; Rocha, F. Tracing the late Holocene evolution of the NW Iberian upwelling system. Mar. Micropaleont. 2006, 59, 35–55. [Google Scholar]
- Bartels-Jonsdottir, H.B.; Voelker, A.H.L.; Knudsen, L.; Abrantes, F. Twentieth century warming and hydrographical changes in the Tagus prodelta, eastern North Atlantic. Holocene 2009, 19, 369–380. [Google Scholar]
- Mendes, I.; Dias, J.A.; Schonfeld, J.; Ferreira, O. Distribution of living benthic foraminifera on the northern Gulf of Cadiz continental shelf. J. Foraminifer. Res. 2012, 42, 18–38. [Google Scholar]
- Casalbore, D.; Ridente, D.; Bosman, A.; Chiocci, F.L. Depositional and erosional bedforms in Late Pleistocene-Holocene pro-delta deposits of the Gulf of Patti (southern Tyrrhenian margin, Italy). Mar. Geol. 2017, 385, 216–227. [Google Scholar]
- Istituto Idrografico della Marina. Atlante delle Correnti Superficiali dei Mari d’Italia; Istituto Idrografico della Marina Publ.: Genova, Italy, 1982. [Google Scholar]
- Ferranti, L.; Antonioli, F.; Mauz, B.; Amorosi, A.; Dai Pra, G.; Mastronuzzi, G.; Monaco, C.; Orrù, P.; Pappalardo, M.; Radtke, U.; et al. Markers of the last interglacial sea-level high stand along the coast of Italy: Tectonic implications. Quat. Int. 2006, 145, 30–54. [Google Scholar]
- Sulli, A.; Lo Presti, V.; Morticelli, M.G.; Antonioli, F. Vertical movements in NE Sicily and its offshore: Outcome of tectonic uplift during the last 125 ky. Quat. Int. 2013, 288, 168–182. [Google Scholar]
- CPTI Working Group. Catalogo Parametrico dei Terremoti Italiani, Version 2004. (CPTI04); INGV: Bologna, Italy, 2004; Available online: http://emidius.mi.ingv.it/CPTI (accessed on 1 March 2021).
- Casalbore, D.; Chiocci, F.L.; Mugnozza, G.S.; Tommasi, P.; Sposato, A. Flash-flood hyperpycnal flows generating shallow-water landslides at Fiumara mouths in Western Messina Strait (Italy). Mar. Geophys. Res. 2011, 32, 257. [Google Scholar]
- Kostic, S.; Casalbore, D.; Chiocci, F.; Lang, J.; Winsemann, J. Role of upper-flow-regime bedforms emplaced by sediment gravity flows in the evolution of deltas. J. Mar. Sci. Eng. 2019, 7, 5. [Google Scholar]
- Folk, R.L.; Ward, W.M. Brazos River bar: A study in the significance of grain size parameters. J. Sediment. Petrol. 1957, 27, 3–26. [Google Scholar]
- Folk, R.L. The distinction between grain size and mineral composition in sedimentary-rock nomenclature. J. Geol. 1954, 62, 344–359. [Google Scholar]
- Folk, R.L. Petrology of Sedimentary Rocks; Hemphill Publishing Co.: Austin, TX, USA, 1974; p. 182. [Google Scholar]
- Poppe, L.; Eliason, A. A Visual Basic program to plot sediment grain-size data on ternary diagrams. Comp. Geosci. 2008, 34, 561–565. [Google Scholar]
- Schönfeld, J.; Alve, E.; Geslin, E.; Jorissen, F.; Korsun, S.; Spezzaferri, S. Members of The Fobimo. The Fobimo (FOraminiferal BIo-MOnitoring) initiative—Towards a standardized protocol for soft-bottom benthic foraminiferal monitoring studies. Mar. Micropaleontol. 2012, 94–95, 1–13. [Google Scholar]
- Mojtahid, M.; Jorissen, F.; Lansard, B.; Fontanier, C.; Bombled, B.; Rabouille, C. Spatial distribution of live benthic foraminifera in the Rhône prodelta: Faunal response to a continental–marine organic matter gradient. Mar. Micropaleontol. 2009, 70, 177–200. [Google Scholar]
- Benito, X.; Trobajo, R.; Cearreta, A.; Ibanez, C. Benthic foraminifera as indicators of habitat in a Mediterranean delta: Implications for ecological and palaeoenvironmental studies Estuar. Coast. Shelf Sci. 2016, 180, 97–113. [Google Scholar]
- Di Bella, L.; Sabbatini, A.; Carugati, L.; Lo Martire, M.; Luna, G.M.; Pierdomenico, M.; Danovaro, R.A.; Negri, A. Living foraminiferal assemblages in two submarine canyons (Polcevera and Bisagno) of the Ligurian basin (Mediterranean Sea). Prog. Oceanogr. 2019, 173, 114–133. [Google Scholar]
- Walton, W.R. Tecniques for recognition of living foraminifera. Contrib. Cushman Found. Foraminifer. Res. 3, 56–60. Found. Foraminifer. Res. 1952, 3, 56–60. [Google Scholar]
- Lutze, G.F.; Altenbach, A. Technik und Signifikanz der Lebendfarbung benthischer Foraminiferen mit Bengalrot. Geol. Jahrb. A 1991, 128, 251–265. [Google Scholar]
- Bernhard, J.M. Distinguishing live from dead foraminifera: Methods review and proper applications. Micropaleontology 2000, 46, 38–46. [Google Scholar]
- Scott, D.B.; Medioli, F.S.; Schafer, C.T. Monitoring of Coastal Environments Using Foraminifera and Thecamoebian Indicators; Cambridge University Press: Cambridge, UK, 2001. [Google Scholar]
- Bernhard, J.M. Postmortem vital staining in benthic foraminifera: Duration and importance in population and distributional studies. J. Foraminifer. Res. 1988, 18, 143–146. [Google Scholar]
- Hannah, F.; Rogerson, A. The temporal and spatial distribution of foraminiferans in marine benthic sediments of the Clyde Sea, Scotland. Estuar. Coast. Shelf Sci. 1997, 44, 377–383. [Google Scholar]
- Murray, J.W.; Bowser, S.S. Mortality, protoplasm decay rate, and reliability of staining techniques to recognize ‘living’ foraminifera: A review. J. Foraminifer. Res. 2000, 30, 66–77. [Google Scholar]
- Fontanier, C.; Jorissen, F.J.; Licari, L.; Alexandre, A.; Anschutz, P.; Carbonel, P. Live benthic foraminiferal faunas from the Bay of Biscay: Faunal density, composition, and microhabitats. Deep Sea Res. I 2002, 49, 751–785. [Google Scholar]
- Frontalini, F.; Semprucci, F.; Di Bella, L.; Caruso, A.; Cosentino, C.; Maccotta, A.; Scopelliti, G.; Sbrocca, C.; Bucci, C.; Balsamo, M.; et al. The Response of Cultured Meiofaunal and Benthic Foraminiferal Communities to Lead Exposure: Results from Mesocosm Experiments. Environ. Toxicol. Chem. 2018, 37, 2439–2447. [Google Scholar] [PubMed]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar]
- Fisher, R.A.; Corbet, A.S.; Williams, C.B. The relationship between the number of species and the number of individuals in random samples of an animal population. J. Anim. Ecol. 1943, 12, 42–58. [Google Scholar]
- Hammer, Ø.; Harper, D.A.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Jorissen, F.J.; de Stigter, H.C.; Widmark, J.G.V. A conceptual model explaining benthic foraminiferal microhabitats. Mar. Micropaleontol. 1995, 26, 3–15. [Google Scholar]
- Loeblich, R.; Tappan, H. Foraminiferal Genera and Their Classification; Van Nostrand Reinhold Company: New York, NY, USA, 1987; p. 970. [Google Scholar]
- Cimerman, F.; Langer, M.R. Mediterranean Foraminifera. Acad. Scient. Artium Sloven. 1991, 30, 1–118. [Google Scholar]
- Sgarrella, F.; Moncharmont Zei, M. Benthic foraminifera of the Gulf of Naples (Italy): Systematics and autoecology. Boll. Soc. Paleontol. Ital. 1993, 32, 145–264. [Google Scholar]
- Koho, K.A.; Kouwenhoven, T.J.; de Stigter, H.C.; van der Zwaan, G.J. Benthic foraminifera in the Nazaré Canyon, Portuguese continental margin: Sedimentary environments and disturbance. Mar. Micropaleontol. 2007, 66, 27–51. [Google Scholar]
- Sen Gupta, B.; Lobegeier, M.; Smith, L. Foraminiferal Communites of Bathyal Hydrocarbon Seeps, Northen Gulf of Mexico: A taxonomic, Ecologic and Geologic Study; Louisiana State University: Baton Rouge, LA, USA, 2009; p. 213. [Google Scholar]
- Milker, Y.; Schmiedl, G. A taxonomic guide to modern benthic shelf foraminifera of the western Mediterranean Sea. Palaeontol. Electron. 2012, 15, 134. [Google Scholar]
- Fatela, F.; Taborda, R. Confidence limits of species proportions in microfossil assemblages. Mar. Micropaleontol. 2002, 45, 169–174. [Google Scholar]
- De Stigter, H.C.; van der Zwaan, G.J.; Langone, L. Differential rates of benthic foraminiferal test production in surface and subsurface sediment habitats in the southern Adriatic Sea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1999, 149, 67–88. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T. Paleontological Data Analysis. Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T. Paleontological Data Analysis. Blackwell Publishing: Oxford, UK, 2008. [Google Scholar]
- Langer, M.R. Epiphytic foraminifera. Mar. Micropaleontol. 1993, 20, 235–265. [Google Scholar]
- Donnici, S.; Barbero, R.S. The benthic foraminiferal communities of the northern Adriatic continental shelf. Mar. Micropaleontol. 2002, 44, 93–123. [Google Scholar]
- Frezza, V.; Carboni, M.G.; Matteucci, R. Recent foraminiferal assemblages near Ponza Island (Central Tyrrhenian Sea, Italy). Boll. Soc. Paleontol. Ital. 2005, 44, 155–173. [Google Scholar]
- Frezza, V.; Carboni, M.G. Distribution of recent foraminiferal assemblages nearthe Ombrone River mouth (Northern Tyrrhenian Sea, Italy). Rev. Micropaleontol. 2009, 52, 43–66. [Google Scholar]
- Hess, S.; Kuhnt, W. Deep-sea benthic foraminiferal recolonization of the 1991 Mt. Pinatubo ash layer in the South China Sea. Mar. Micropaleontol. 1996, 28, 171–197. [Google Scholar]
- Mc Gann, M. Historical and modern distributions of benthic foraminifers on the continental shelf of Monterey Bay, California. Mar. Geol. 2002, 181, 115–156. [Google Scholar]
- Dessandier, P.A.; Bonnin, J.; Kim, J.H.; Bichon, S.; Grémare, A.; Deflandre, B.; de Stigter, H.; Malaizé, B. Lateral and vertical distributions of living benthic foraminifera off the Douro River (western Iberian margin): Impact of the organic matter quality. Mar. Micropaleontol. 2015, 120, 31–45. [Google Scholar]
- Di Bella, L.; Ingrassia, M.; Frezza, V.; Chiocci, F.L.; Martorelli, E. The response of benthic meiofauna to hydrothermal emissions in the Pontine Archipelago, Tyrrhenian Sea (central Mediterranean Basin). J. Mar. Syst. 2016, 164, 53–66. [Google Scholar]
- Di Bella, L.; Ingrassia, M.; Frezza, V.; Chiocci, F.L.; Pecci, R.; Bedini, R.; Martorelli, E. Spiculosiphon oceana (Foraminifera) a new bio-indicator of acidic environments related to fluid emissions of the Zannone Hydrothermal Field (central Tyrrhenian Sea). Mar. Environ. Res. 2018, 136, 89–98. [Google Scholar] [PubMed]
- Romano, E.; Bergamin, L.; Pierfranceschi, G.; Provenzani, C.; Marassich, A. The distribution of benthic foraminifera in Bel Torrente submarine cave (Sardinia, Italy) and their environmental significance. Mar. Environ. Res. 2018, 133, 114–127. [Google Scholar] [PubMed]
- Romano, E.; Bergamin, L.; Di Bella, L.; Frezza, V.; Marassich, A.; Pierfranceschi, G.; Provenzani, C. Benthic foraminifera as proxies of marine influence in the Orosei marine caves (Sardinia, Italy). Aquat. Conserv: Mar. Freshw Ecosyst. 2020, 30, 701–716. [Google Scholar]
- Dias, B.B.; Hart, M.B.; Smart, C.W.; Hall-Spencer, J.M. Modern seawater acidification: The response of foraminifera to high-CO2 conditions in the Mediterranean Sea. J. Geol. Soc. 2010, 167, 843–846. [Google Scholar]
- Panieri, G.; Gamberi, F.; Marani, M.; Barbieri, R. Benthic foraminifera from a recent, shallow-water hydrothermal environment in the Aeolian Arc (Tyrrhenian Sea). Mar. Geol. 2005, 218, 207–229. [Google Scholar]
- Lei, Y.L.; Li, T.G.; Bi, H.; Cui, W.L.; Song, W.P.; Li, J.Y.; Li, C.C. Responses of benthic foraminifera to the 2011 oil spill in the Bohai Sea, PR China. Mar. Pollut. Bull. 2015, 96, 245–260. [Google Scholar]
- Diz, P.; Francés, G. Distribution of live benthic foraminifera in the Ria de Vigo (NW Spain). Mar. Micropaleontol. 2008, 66, 165–191. [Google Scholar]
- Alve, E.; Goldstein, S.T. Dispersal, survival and delayed growth of benthic foraminiferal propagules. J. Sea Res. 2010, 63, 36–51. [Google Scholar]
- Nardelli, M.P.; Jorissen, F.J.; Pusceddu, A.; Morigi, C.; Dell’Anno, A.; Danovaro, R.; De Stigter, H.C.; Negri, A. Living benthic foraminiferal assemblages along a latitudinal transect at 1000 m depth off the Portuguese margin. Micropaleontology 2010, 56, 323–344. [Google Scholar]
- Mendes, I.; Gonzalez, R.; Dias, J.M.A.; Lobo, P.; Martins, V. Factors influencing recent benthic foraminifera distribution on the Guadiana shelf (southwestern Iberia). Mar. Micropaleontol. 2004, 51, 171–192. [Google Scholar]
- De Rijk, S.; Jorissen, F.J.; Rohling, E.J.; Troelstra, S.R. Organic flux on bathymetric zonation of Mediterranean benthic foraminifera. Mar. Micropaleontol. 2000, 40, 151–166. [Google Scholar]
- Frezza, V.; Pignatti, J.; Matteucci, R. Benthic foraminiferal biofacies in temperate carbonate sediment in the western pontine archipelago (Tyrrhenian sea, Italy). J. Foraminifer. Res. 2010, 40, 313–326. [Google Scholar]
- Jonasson, K.E.; Schrijder-Adams, C.J.; Patterson, R.T. Benthic foraminiferal distribution at Middle Valley, Juan de Fuca Ridge, a northeast Pacific hydrothermal venting site. Mar. Micropaleontol. 1995, 25, 151–167. [Google Scholar]
- Contreras-Rosales, L.A.; Koho, K.A.; Duijnstee, I.A.P.; De Stigter, H.C.; García, R.; Koning, E.; Epping, E. Living deep-sea benthic foraminifera from the Cap de Creus Canyon (western Mediterranean): Faunal-geochemical interactions. Deep Sea Res. Part I Oceanogr. Res. 2012, 64, 22–42. [Google Scholar]
- Fontanier, C.; Jorissen, F.J.; Lansard, B.; Mouret, A.; Buscail, R.; Schmidt, S.; Kerhervé, P.; Buron, F.; Zaragosi, S.; Hunault, G.; et al. Live foraminifera from the open slope between Grand Rhone and Petit Rhone Canyons (Gulf of Lions, NW Mediterranean). Deep Sea Res. Part I Oceanogr. Res. 2008, 55, 1532–1553. [Google Scholar]
- Alavi, S.N. Late Holocene deep-sea benthic foraminifera from the Sea of Marmara. Mar. Micropaleontol. 1988, 13, 213–237. [Google Scholar]
- Sen Gupta, B.K.; Machain-Castillo, M.L. Benthic foraminifera in oxygen-poor habitats. Mar. Micropaleontol. 1993, 20, 183–201. [Google Scholar]
- Bernhard, J.M.; Sen Gupta, B.K. Foraminifera of Oxygen-Depleted Environments. In Modern Foraminifera; Sen Gupta, B., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1999; pp. 201–216. [Google Scholar]
- Jorissen, F.J. The distribution of benthic foraminifera in the Adriatic Sea. Mar. Micropaleontol. 1987, 12, 21–48. [Google Scholar]
- Schmiedl, G.; Mackensen, A.; Müller, P.J. Recent benthic foraminifera from the eastern South Atlantic Ocean: Dependence on food supply and water masses. Mar. Micropaleontol. 1997, 32, 249–287. [Google Scholar]
- Martins, M.V.; Dubert, J.; Jouanneau, J.-M.; Weber, O.; Da Silva, E.F.; Patinha, C.; Alveirinho Dias, J.M.; Rocha, F. A multiproxy approach of the Holocene evolution of shelf-slope circulation on the NW Iberian Continental Shelf. Mar. Geol. 2007, 239, 1–18. [Google Scholar]
- Sorokin, P.Y. Oxidation of reduced compounds of sulfur in volcanically active areas of the Bay of Plenty (New Zealand) and Matupi Harbor (The New Britain Island, Papua New Guinea). Izv. AN USSR Ser. Biol. 1991, 3, 376–387. [Google Scholar]
- Tarasov, V.G.; Gebruk, A.V.; Mironov, A.N.; Moskalev, L.I. Deep-sea and shallowwater hydrothermal vent communities: Two different phenomena? Chem. Geol. 2005, 224, 5–39. [Google Scholar]
- Bárcenas, P.; Lobo, F.; Macías, J.; Fernández-Salas, L.; del Río, V.D. Spatial variability of surficial sediments on the northern shelf of the Alboran Sea: The effects of hydrodynamic forcing and supply of sediment by rivers. J. Iber. Geol. 2011, 37, 195–214. [Google Scholar]
- Tortora, P. La superficie deposizionale del delta sottomarino del Tevere: Zonazione del sedimento e processi associati. Boll. Soc. Geol. Ital. 1995, 114, 89–105. [Google Scholar]
- Díaz, J.; Palanques, A.; Nelson, C.H.; Guillén, J. Morpho-structure and sedimentology of the Holocene Ebro prodelta mud belt (northwestern Mediterranean Sea). Cont. Shelf Res. 1996, 16, 435–445. [Google Scholar]
- Mulder, T.; Syvitski, J.P.M. Turbidity currents generated at river mouths during exceptional discharges to the world oceans. J. Geol. 1995, 103, 285–299. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).