Sabellaria alveolata versus Sabellaria spinulosa Reefs along the Italian Coasts: A New Methodological Proposal to Compare Different Growth Models
Abstract
:1. Introduction
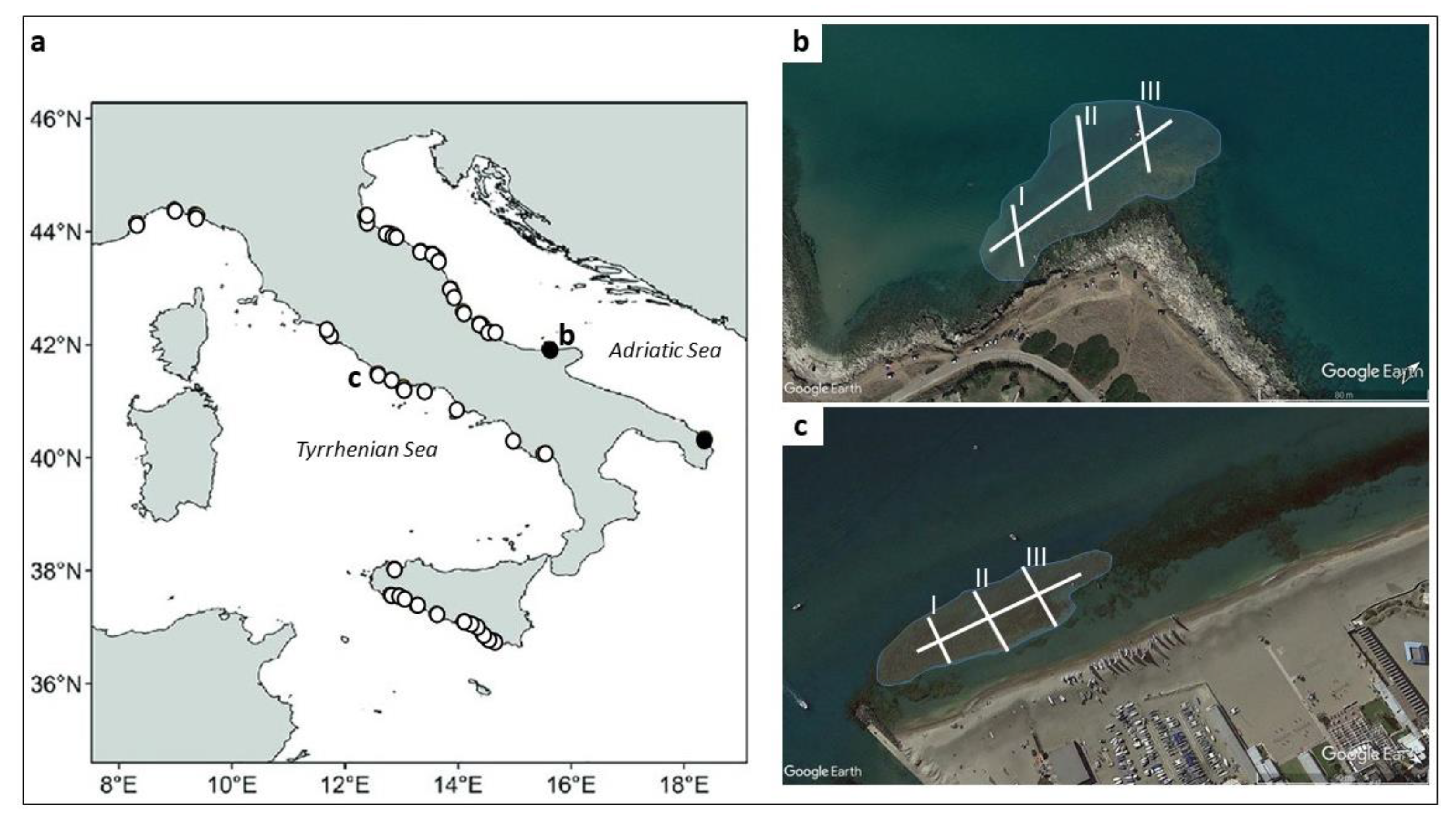
2. Study Area
2.1. Torre Mileto Site
2.2. Ostia Site
3. Materials and Methods
- -
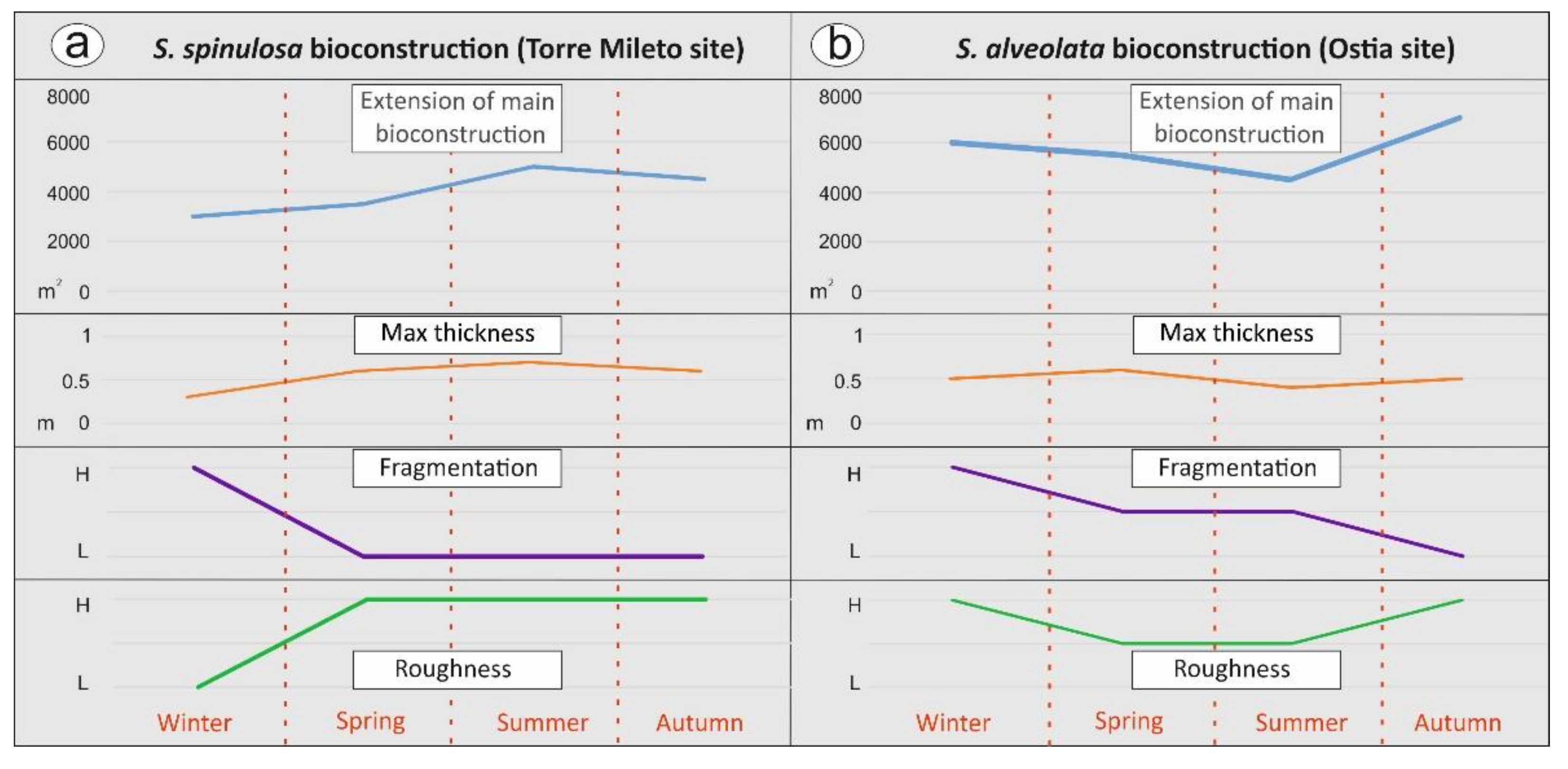
- distributional area of the main bioconstruction (calculated in m2). Five georeferenced points were used to establish the approximate reef extension and its eventual lateral displacement;
- -
- fragmentation degree. Across five test square areas (3m × 3 m), located in the same zone as the five georeferenced points, we used a qualitative scale assigning: (i) a low fragmentation value (L) in the absence of interruptions, (ii) a moderate fragmentation value (M) if the discontinuities in the presence of recurring structure interruptions are equal to 30% of the entire test area and (iii) a high fragmentation value (H) in the presence of continuous structure interruptions higher than 50%;
- -
- the roughness of the bioconstruction surface, evaluated in the central portions of the bioconstruction and in the same five test square areas (3 × 3 m). A high roughness value (H) was assigned in the presence of highly articulated surfaces, an average roughness value (M) for poorly articulated surfaces and a low roughness value (L) when smooth surfaces covered more than 50%;
- -
- thickness, measured at five georeferenced points; the maximum thickness was measured in centimeters vertically by inserting a thin graduated iron rod into the bioconstruction.
4. Results
4.1. Large-Scale Monitoring Results
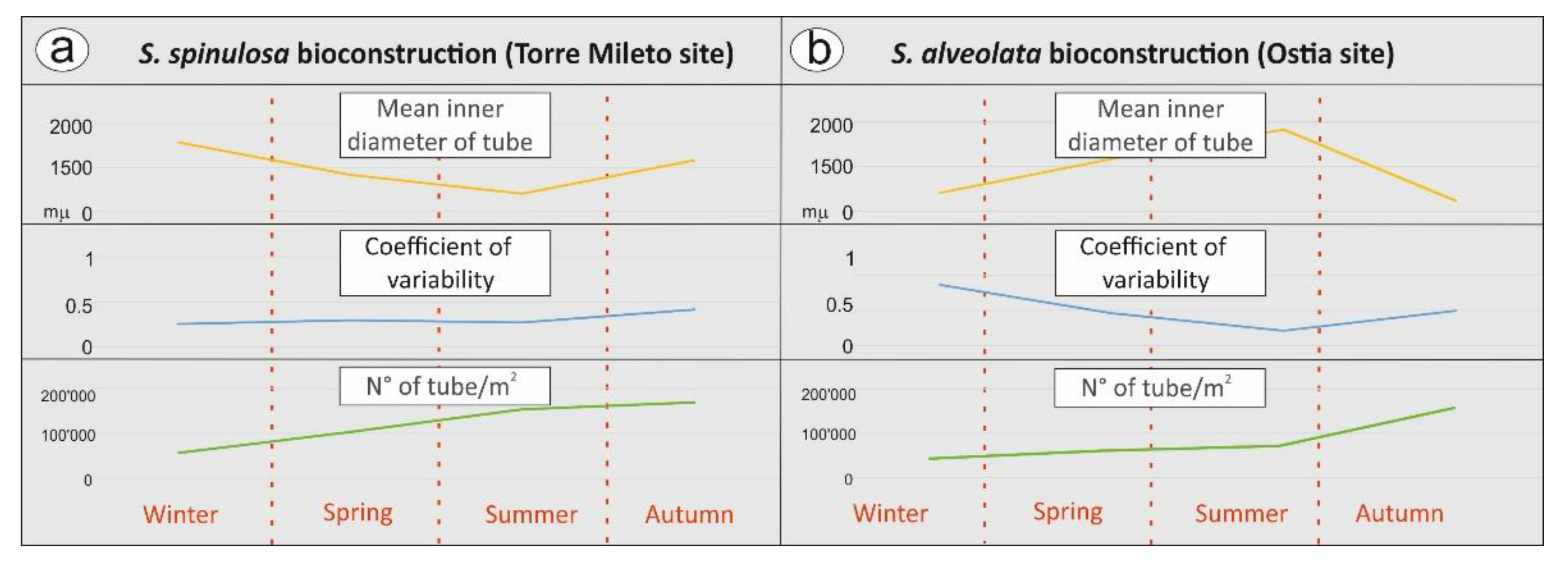
4.2. Meso- and Microscopic Monitoring Results
5. Discussion
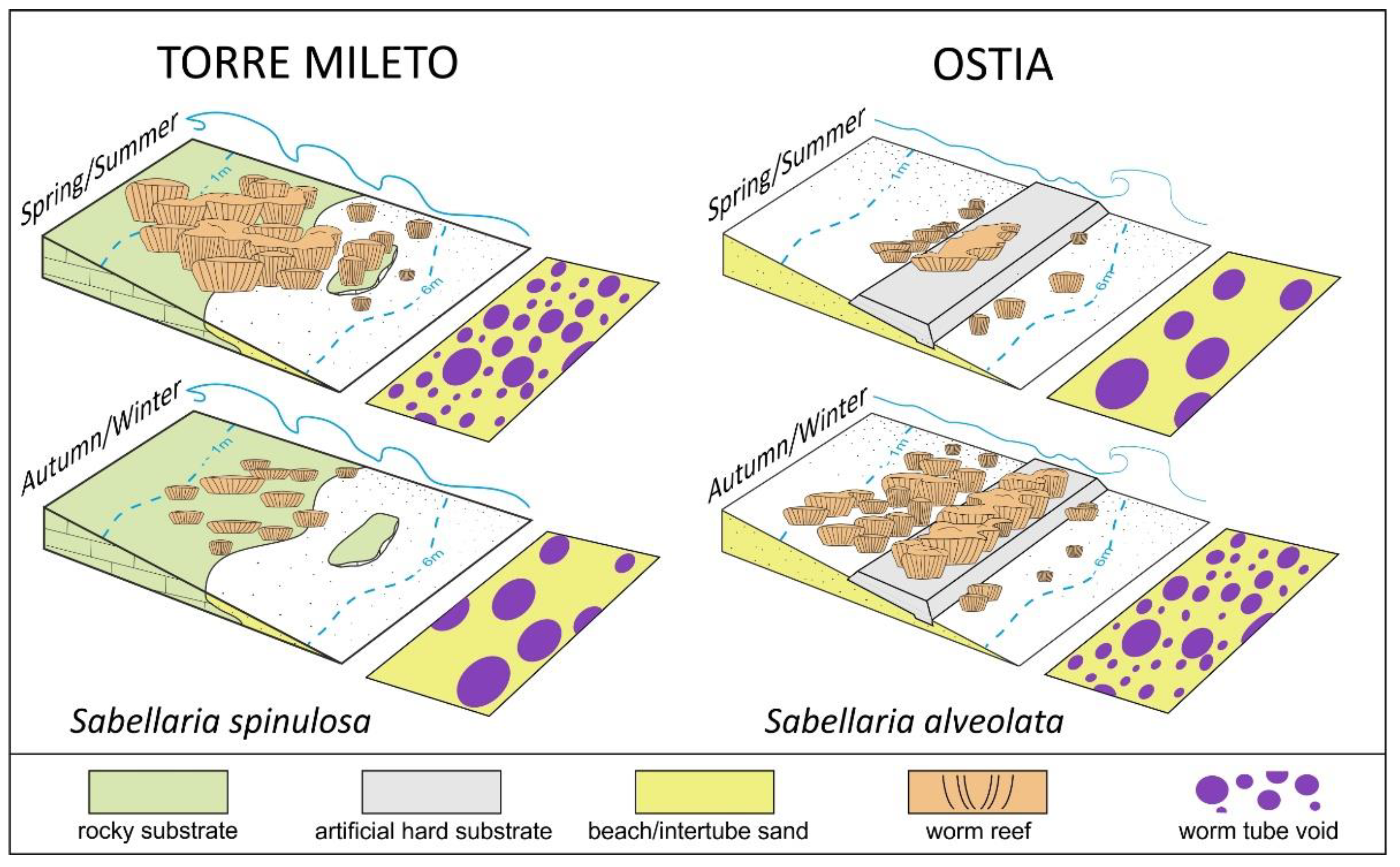
5.1. A Stage-Based Model for the Temporal/Spatial Evolution of the Worm Reefs
5.2. Towards an Integrated Approach for the Description of Growth/Degenerative Phases of Worm Reefs
- microscale textural features (Table 3).
6. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Ten Hove, H.A.; Van Den Hurk, P. A review of recent and fossil serpulid ‘reefs’; actuopalaeontology and the ‘Upper malm’ serpulid limestones in NW Germany. Geologie en Mijnbouw 1993, 72, 23–67. [Google Scholar]
- Sanfilippo, R.; Vertino, A.; Rosso, A.; Beuck, L.; Freiwald, A.; Taviani, M. Serpula aggregates and their role in deep-sea coral communities in the southern Adriatic Sea. Facies 2013, 59, 663–677. [Google Scholar] [CrossRef]
- Gruet, Y.; Bodeur, Y. Sélection des grains de sable selon leur nature et leur forme par Sabellaria alveolata (Linné) (Polychète Sabellariide) lors de la reconstruction expérimentale de son tube. Mémoires du Muséum National d’Histoire Naturelle 1994, 162, 425–432. [Google Scholar]
- Vovelle, J. Le tube de Sabellaria alveolata (L.) Annélide Polychète Hermellidae et son ciment. Étude écologique, expérimentale, histologique et histochimique. Archives de Zoologie Expérimentale et Générale 1965, 106, 1–180. [Google Scholar]
- Dubois, S.; Laurent, B.; Cognie, B.; Beninger, P.G. Particle capture and processing mechanism in Sabellaria alveolata (Polychaeta: Sabellariidae). Mar. Ecol. Prog. Ser. 2005, 301, 159–171. [Google Scholar] [CrossRef]
- Naylor, L.A.; Viles, H.A. A temperate reef builder: An evaluation of the growth, morphology and composition of Sabellaria alveolata (L.) colonies on carbonate platforms in South Wales. In Carbonate Platform Systems: Components and Interactions; Insalaco, E., Skelton, P.W., Palmer, T.J., Eds.; Geological Society, Special Publications: London, UK, 2000; Volume 178, pp. 9–19. [Google Scholar]
- Bonifazi, A.; Lezzi, M.; Ventura, D.; Lisco, S.N.; Cardone, F.; Gravina, M.F. Macrofaunal biodiversity associated with different developmental phases of a threatened Mediterranean Sabellaria alveolata (Linnaeus, 1767) reef. Mar. Environ. Res. 2019, 145, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Gruet, Y. Spatio-temporal changes of Sabellarian reefs built by the sedentary Polychaete Sabellaria alveolata (LINNE). Marine Ecology. Pubblicazioni della Stazione Zoologica di Napoli 1986, 73, 303–319. [Google Scholar] [CrossRef]
- Holt, T.J.; Rees, E.I.; Hawkins, S.J.; Seed, R. Biogenic reefs (volume IX). In An Overview of Dynamic and Sensitivity Characteristics for Conservation Management of Marine SACs; Scottish Association for Marine Science (UK Marine SACs Project): Oban, UK, 1998; 170p. [Google Scholar]
- Nicoletti, L.; Lattanzi, L.; La Porta, B.; La Valle, P.; Gambi, M.C.; Tomassetti, P.; Tucci, P.; Chimenz Gusso, C. Sabellaria reefs from the Latium coast (Central Tyrrhenian Sea). Biologia Marina Mediterranea 2001, 8, 252–258. [Google Scholar]
- Dubois, S.; Comtet, T.; Retière, C.; Thiébaut, E. Distribution and retention of Sabellaria alveolata larvae (Polychaeta: Sabellariidae) in the bay of Mont Saint Michel, France. Mar. Ecol. Prog. Ser. 2007, 346, 243–254. [Google Scholar] [CrossRef]
- Delbono, I.; Bianchi, C.N.; Morri, C. Le biocostruzioni di Sabellaria alveolata come indicatori ambientali: Area costiera fra Chiavari e Sestri Levante. In Studi per la Creazione di Strumenti di Gestione Costiera; Ferretti, O., Ed.; Golfo del Tigullio: Genoa, Italy, 2003; pp. 130–140. [Google Scholar]
- Braithwaite, C.J.R.; Robinson, R.J.; Jones, G. Sabellarids: A hidden danger or an aid to subsea pipelines? Q. J. Eng. Geol. Hydrogeol. 2006, 39, 259–265. [Google Scholar] [CrossRef]
- La Porta, B.; Nicoletti, L. Sabellaria alveolata (Linnaeus) reefs in the Central Tyrrhenian Sea (Italy) and associated polychaete fauna. Zoosymposia 2009, 2, 527–536. [Google Scholar] [CrossRef] [Green Version]
- Lisco, S.; Moretti, M.; Moretti, V.; Cardone, F.; Longo, C.; Corriero, G. Sedimentological features of Sabellaria spinulosa bioconstructions. Mar. Pet. Geol. 2017, 87, 203–212. [Google Scholar] [CrossRef]
- Desroy, N.; Dubois, S.; Fournier, J.; Ricquiers, L.; Le Mao, P.; Guérin, L.; Gerla, D.; Rougerie, M.; Legendre, A. Conservation status of Sabellaria alveolata (L.) (Polychaeta: Sabellariidae) reefs in the Mont-saint-Michel bay. Aquat. Conserv. Mar. Freshw. Ecosyst. 2011, 21, 462–471. [Google Scholar] [CrossRef]
- Schlund, E.; Basuyaux, O.; Lecornu, B.; Pezy, J.P.; Baffreau, A.; Dauvin, J.C. Macrofauna associated with temporary Sabellaria alveolata reefs on the west coast of Cotentin (France). SpringerPlus 2016, 5, 1260–1281. [Google Scholar] [CrossRef]
- Ayata, S.D.; Ellien, C.; Dumas, F.; Dubois, S.; Thiébaut, É. Modelling larval dispersal and settlement of the reef-building polychaete Sabellaria alveolata: Role of hydroclimatic processes on the sustainability of biogenic reefs. Cont. Shelf Res. 2009, 29, 1605–1623. [Google Scholar] [CrossRef] [Green Version]
- Gruet, Y. Aspects morphologiques et dynamiques de constructions de l’annelide polychete Sabellaria alveolata (Linne). Revue des Travaux de l’Institut des Pêches Maritimes 1972, 36, 131–161. [Google Scholar]
- Allen, J.H.; Billings, I.; Cutts, N.; Elliott, M. Mapping, Condition & Conservation Assessment of Honeycomb Worm, Sabellaria alveolata Reefs on the Eastern Irish Sea Coast; Report to English Nature, Reference No: Z122-F-2002; Institute of Estuarine and Coastal Studies University of Hull: Hull, UK, 2002. [Google Scholar]
- Lezzi, M.; Cardone, F.; Mikac, B.; Giangrande, A. Variation and ontogenetic changes of opercularpaleae in a population of Sabellaria spinulosa (Polychaeta: Sabellariidae) from the South Adriatic Sea, with remarks on larval development. Sci. Mar. 2015, 79, 1–14. [Google Scholar]
- Pearce, B.; Hill, J.M.; Wilson, C.; Griffin, R.; Earnshaw, S.; Pitts, J. Sabellaria spinulosa Reef Ecology and Ecosystem Services; The Crown Estate: London, UK, 2011; p. 120. ISBN 978-1-906410-27-8. [Google Scholar]
- Gruet, Y.; Bodeur, Y. Ecological conditions of modern sabellarian reefs development: Geological implications. Pubbl. Serv. Géol. Lux 1995, 29, 73–80. [Google Scholar]
- Griffin, R.A.; Jones, R.E.; Lough, N.E.L.; Lindenbaum, C.P.; Alvarez, M.C.; Clark, K.A.J.; Griffiths, J.D.; Clabburn, P.A.T. Effectiveness of acoustic cameras as tools for assessing biogenic structures formed by Sabellaria in highly turbid environments. Aquat. Conserv. Mar. Freshw. Ecosyst. 2020, 30, 1121–1136. [Google Scholar] [CrossRef]
- Le Cam, J.B.; Fournier, J.; Etienne, S.; Couden, J. The strength of biogenic sand reefs: Visco-elastic behaviour of cement secreted by the tube building polychaete Sabellaria alveolata, Linnaeus, 1767. Estuar. Coast. Shelf Sci. 2011, 91, 333–339. [Google Scholar] [CrossRef]
- Lisco, S.; Acquafredda, P.; Gallicchio, S.; Sabato, L.; Bonifazi, A.; Cardone, F.; Corriero, G.; Gravina, M.F.; Pierri, C.; Moretti, M. The sedimentary dynamics of Sabellaria alveolata bioconstructions (Ostia, Tyrrhenian Sea, central Italy). J. Palaeogeogr. 2020, 9, 2. [Google Scholar] [CrossRef] [Green Version]
- Sanfilippo, R.; Rosso, A.; Mastandrea, A.; Viola, A.; Deias, C.; Guido, A. Sabellaria alveolata sandcastle worm from the Mediterranean Sea: New insights on tube architecture and biocement. J. Morphol. 2019, 280, 1839–1849. [Google Scholar] [CrossRef] [PubMed]
- Scholl, D.W. Effects of an arenaceous tube- building polychaete upon the sorting of a beach sand at Abalone Cove, California. Canpass Mag. 1958, 35, 276–283. [Google Scholar]
- Bodeur, Y.; Garcin, M.; Gruet, Y.; Vachard, D. Biosédimentologiedes Constructions à Hermelles de l’île de Noirmoutier (France). Relations Avec des Équivalents Fossiles Découverts Dans le Messinien des Cordillères Bétiques (Espagne), 8th ed.; IAS: Tunis, Tunisia, 1987; 2p. [Google Scholar]
- Gravina, M.F.; Chimienti, G.; Longo, C.; Corriero, G.; Lisco, S.; Moretti, M.; Cardone, F.; Nonnis Marzano, C.; Bertrandino, M.; Giangrande, A.; et al. Sabellaria spinulosa (Annelida, Polychaeta) reefs in the Mediterranean Sea: Habitat mapping, dynamics and associated fauna for conservation management. Estuar. Coast. Shelf Sci. 2018, 200, 248–257. [Google Scholar] [CrossRef]
- Ingrosso, G.; Abbiati, M.; Badalamenti, F.; Bavestrello, G.; Belmonte, G.; Cannas, R.; Benedetti-Cecchi, L.; Bertolino, M.; Bevilacqua, S.; Bianchi, C.N.; et al. Mediterranean Bioconstructions Along the Italian Coast. Adv. Mar. Biol. 2018, 79, 61–136. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, R.; Guido, A.; Insacco, G.; Deias, C.; Catania, G.; Reitano, A.; Leonardi, R.; Rosso, A. Distribution of Sabellaria alveolata (Polychaeta Sabellariidae) in the Mediterranean Sea: Update and new findings. Zoosymposia. In Proceedings of the 13° IPC Conference, Long Beach, CA, USA, 2–8 August 2019; Volume 19, pp. 198–208. [Google Scholar]
- BIOMAP Project. Biocostruzioni Marine in Puglia. 2014. Available online: http://www.sit.puglia.it/portal/portale_rete_ecologica/biomap (accessed on 20 July 2021).
- Capelli, G.; Mazza, R.; Papiccio, C. Intrusione salina nel Delta del Fiume Tevere. Geologia, idrologia e idrogeologia del settore romano della piana costiera. Giornale di Geologia Applicata 2007, 5, 13–28. [Google Scholar]
- Franco, L.; Di Risio, M.; Riccardi, C.; Scaloni, P.; Conti, M. Monitoraggio del ripascimento protetto con barriera sommersa nella spiaggia di Ostia Centro. Studi Costieri 2004, 8, 3–16. [Google Scholar]
- Wilson, D.P. Sabellaria colonies at Duckpool, North Cornwall, 1961–1970. J. Mar. Biol. Assoc. UK 1971, 51, 509–580. [Google Scholar] [CrossRef]
- Cunningham, P.N.; Hawkins, S.J.; Jones, H.D.; Burrows, M.T. The Geographical Distribution of Sabellaria alveolata (L.) in England, Wales and Scotland, with Investigations into the Community Structure of, and the Effects of Trampling on Sabellaria alveolata Colonies; Nature Conservancy Council, CSD Report, No. 535; Department of Zoology, University of Manchester: Manchester, UK, 1984. [Google Scholar]


| Season | Year | Site | Survey | Mapping | Sampling | Macrofeat. Measuring |
|---|---|---|---|---|---|---|
| Autumn | 2012 | Torre Mileto | x | x | x | x |
| Spring | 2013 | Torre Mileto | x | x | x | x |
| Winter | 2013 | Torre Mileto | x | x | x | x |
| Summer | 2015 | Torre Mileto | x | x | x | x |
| Autumn | 2016 | Torre Mileto | x | x | x | x |
| Spring | 2017 | Torre Mileto | x | x | x | x |
| Summer | 2017 | Torre Mileto | x | x | x | x |
| Autumn | 2017 | Torre Mileto | x | x | x | x |
| Spring | 2018 | Torre Mileto | x | x | x | x |
| Summer | 2018 | Torre Mileto | x | x | x | x |
| Autumn | 2018 | Torre Mileto | x | x | x | |
| Winter | 2018 | Torre Mileto | x | x | ||
| Spring | 2019 | Torre Mileto | x | x | x | x |
| Summer | 2019 | Torre Mileto | x | x | x | x |
| Autumn | 2019 | Torre Mileto | x | x | x | x |
| Winter | 2019 | Torre Mileto | x | x | x | x |
| Summer | 2020 | Torre Mileto | x | x | x | x |
| Winter | 2020 | Torre Mileto | x | x | x | x |
| Autumn | 2012 | Ostia | x | x | x | x |
| Autumn | 2013 | Ostia | x | x | x | x |
| Spring | 2014 | Ostia | x | x | x | x |
| Summer | 2014 | Ostia | x | x | x | x |
| Winter | 2014 | Ostia | x | x | ||
| Spring | 2017 | Ostia | x | x | x | x |
| Spring | 2018 | Ostia | x | x | x | x |
| Summer | 2018 | Ostia | x | x | x | x |
| Autumn | 2018 | Ostia | x | x | x | x |
| Winter | 2018 | Ostia | x | x | x | x |
| Spring | 2019 | Ostia | x | x | x | x |
| Summer | 2019 | Ostia | x | x | x | x |
| Autumn | 2019 | Ostia | x | x | x | x |
| Winter | 2019 | Ostia | x | x | x | x |
| Summer | 2020 | Ostia | x | x | x | x |
| Winter | 2020 | Ostia | x | x | x |
| Macroscale Features | Description |
|---|---|
| A | Variation in the overall distributional area of the main bioconstruction. |
| B | Fragmentation degree of the main bioconstruction. It is a qualitative measure carried out in the central portion of the bioconstruction based on three classes of relative lateral reef continuity: low fragmentation (L), moderate fragmentation (M) and high fragmentation (H) values. |
| C | Roughness of the bioconstruction surface. It is a qualitative measure carried out in the central portion of the bioconstruction and is based on three classes: high roughness (H), moderate roughness (M) and low roughness (L) values. |
| D | Thickness. |
| Microscale Features | Description |
|---|---|
| E | Inner mean diameter of tubes composing bioconstruction. It is defined as the arithmetic mean of the average tube diameters in different seasons. |
| F | Coefficient of variation as a measure of the relative variability. It is defined as the ratio of the standard deviation of mean (average) tube values for one square meter of surface. |
| G | Number of tubes/square meter. |
| H | Presence/absence of tubes filled with external random sediment. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisco, S.N.; Pierri, C.; Lazic, T.; Bonifazi, A.; Gravina, M.F.; Giangrande, A.; Acquafredda, P.; Moretti, M. Sabellaria alveolata versus Sabellaria spinulosa Reefs along the Italian Coasts: A New Methodological Proposal to Compare Different Growth Models. Geosciences 2021, 11, 426. https://doi.org/10.3390/geosciences11100426
Lisco SN, Pierri C, Lazic T, Bonifazi A, Gravina MF, Giangrande A, Acquafredda P, Moretti M. Sabellaria alveolata versus Sabellaria spinulosa Reefs along the Italian Coasts: A New Methodological Proposal to Compare Different Growth Models. Geosciences. 2021; 11(10):426. https://doi.org/10.3390/geosciences11100426
Chicago/Turabian StyleLisco, Stefania Nunzia, Cataldo Pierri, Tamara Lazic, Andrea Bonifazi, Maria Flavia Gravina, Adriana Giangrande, Pasquale Acquafredda, and Massimo Moretti. 2021. "Sabellaria alveolata versus Sabellaria spinulosa Reefs along the Italian Coasts: A New Methodological Proposal to Compare Different Growth Models" Geosciences 11, no. 10: 426. https://doi.org/10.3390/geosciences11100426
APA StyleLisco, S. N., Pierri, C., Lazic, T., Bonifazi, A., Gravina, M. F., Giangrande, A., Acquafredda, P., & Moretti, M. (2021). Sabellaria alveolata versus Sabellaria spinulosa Reefs along the Italian Coasts: A New Methodological Proposal to Compare Different Growth Models. Geosciences, 11(10), 426. https://doi.org/10.3390/geosciences11100426









