Potentially Mobilizable Geogenic As and Sb in an Agricultural Wetland Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Characterization
2.2. Experimental Setup: Soil Incubation under Reducing Conditions
2.3. Soil Solution Analysis
2.4. Sequential Extractions Procedure
2.5. Mass Balance
2.6. Enrichment Factor
3. Results
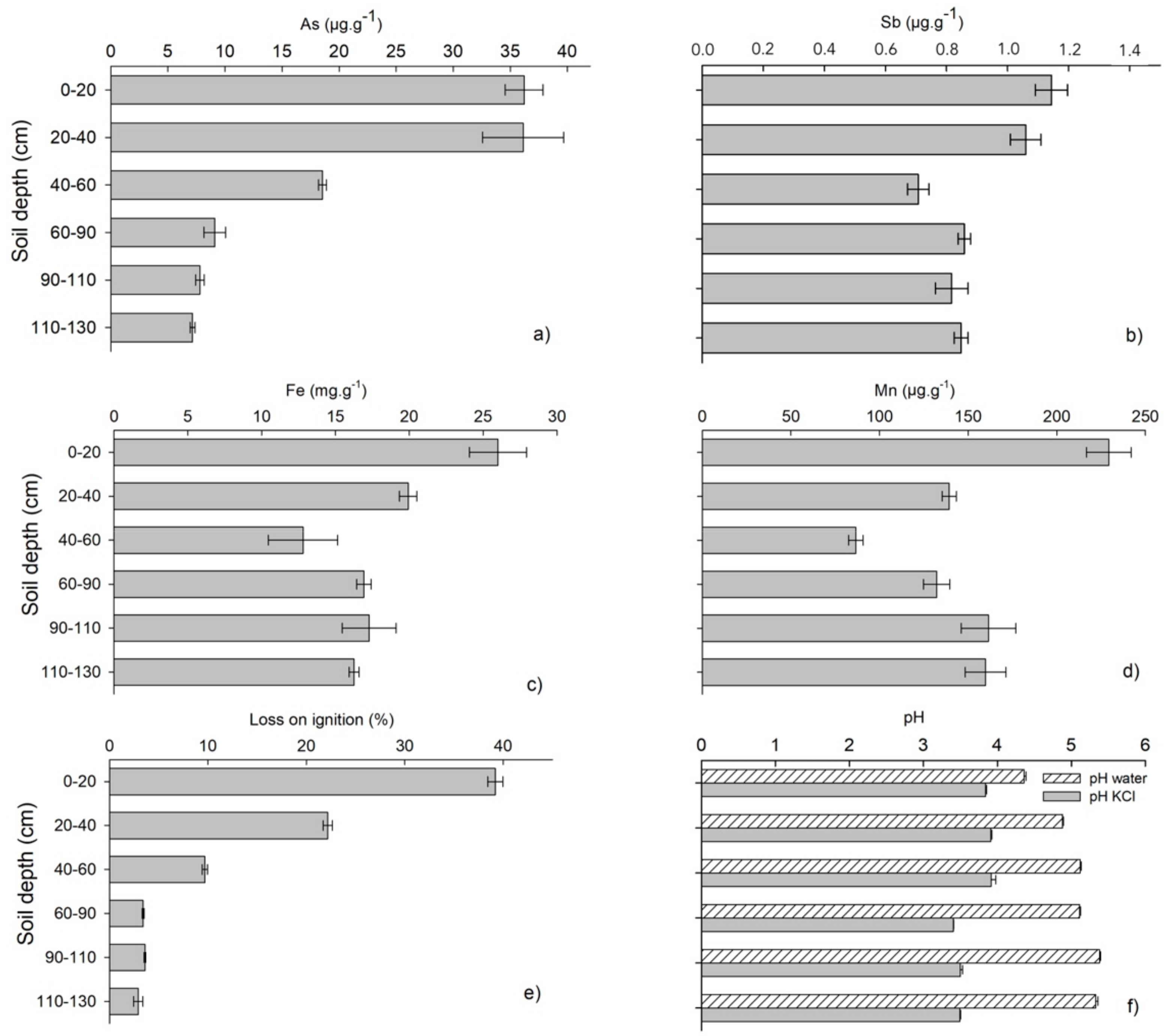
3.1. Depth Distribution of As, Sb, Fe, Mn, LOI and pH
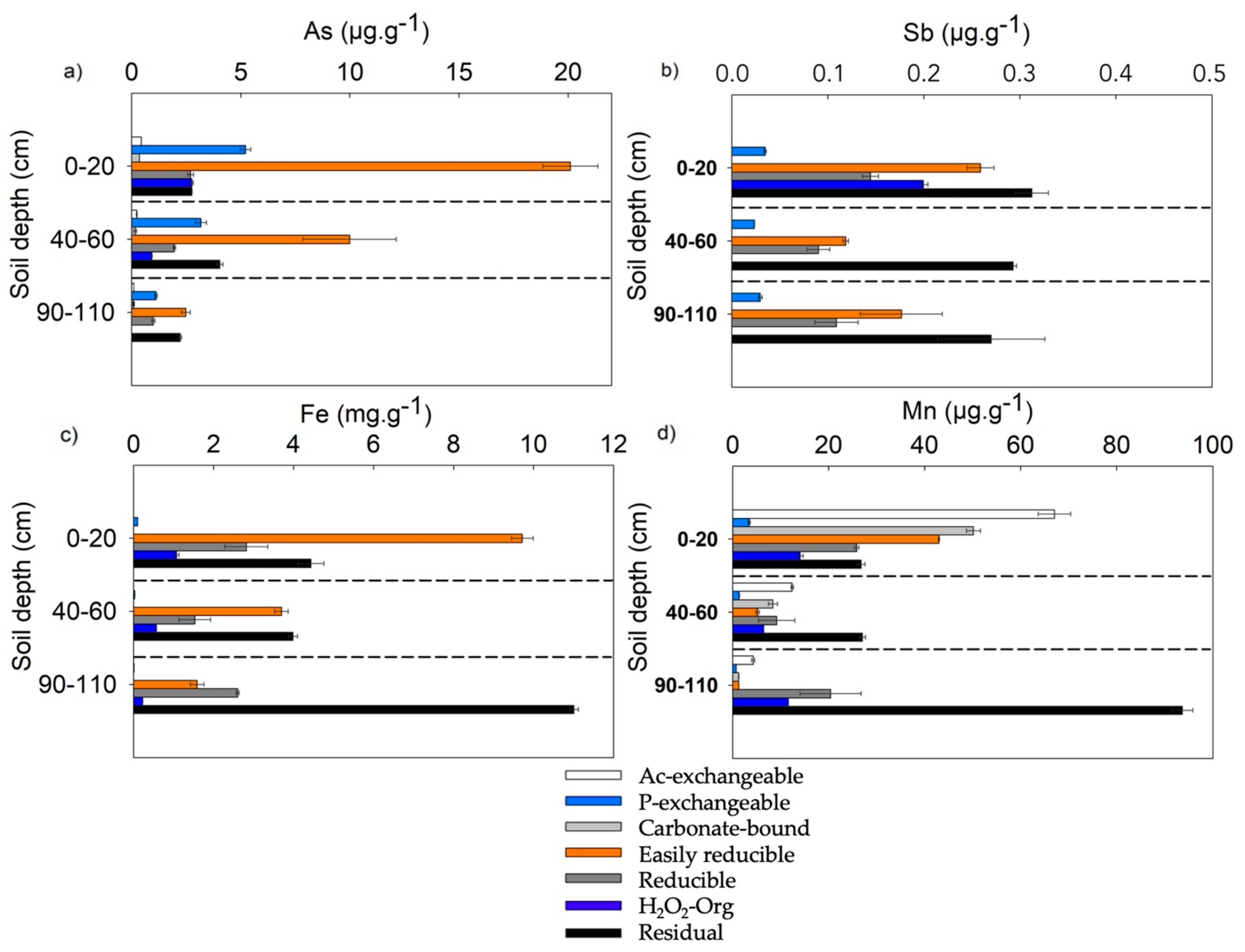
3.2. Solid-Phase Distribution of As, Sb, Fe and Mn along the Soil Depth
3.3. Laboratory Incubation: Mobility and Solid-phase Distribution of As in Wetland Soil (0–40 cm) during Development of the Reducing Conditions
4. Discussion
4.1. Fe and Organic Matter Impact As and Sb Dynamics in Wetland Soil
4.2. Dynamics of Strongly Exchangeable and Carbonate-bound As and Sb in Wetland Soil
4.3. Dynamics of Less Mobilizable As and Sb in Wetland Soil
4.4. Soil Hydrology Affect the Solid-Phase Distribution of As and Sb and, thus, the Content of Potentially Mobilizable As and Sb
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: New York, NY, USA, 2010; ISBN 978-0-429-19203-6. [Google Scholar]
- Martin, J.-M.; Whitfield, M. The Significance of the River Input of Chemical Elements to the Ocean. In Trace Metals in Sea Water; Wong, C.S., Boyle, E., Bruland, K.W., Burton, J.D., Goldberg, E.D., Eds.; Springer US: Boston, MA, USA, 1983; pp. 265–296. ISBN 978-1-4757-6866-4. [Google Scholar]
- Golfinopoulos, S.; Varnavas, S.; Alexakis, D. The Status of Arsenic Pollution in the Greek and Cyprus Environment: An Overview. Water 2021, 13, 224. [Google Scholar] [CrossRef]
- Alexakis, D.E.; Bathrellos, G.D.; Skilodimou, H.D.; Gamvroula, D.E. Spatial Distribution and Evaluation of Arsenic and Zinc Content in the Soil of a Karst Landscape. Sustainability 2021, 13, 6976. [Google Scholar] [CrossRef]
- Léonard, A.; Gerber, G.B. Mutagenicity, Carcinogenicity and Teratogenicity of Antimony Compounds. Mutat. Res. Genet. Toxicol. 1996, 366, 1–8. [Google Scholar] [CrossRef]
- Yoshida, T. Chronic Health Effects in People Exposed to Arsenic via the Drinking Water: Dose? Response Relationships in Review. Toxicol. Appl. Pharmacol. 2004, 198, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Smedley, P.L.; Kinniburgh, D.G. A Review of the Source, Behaviour and Distribution of Arsenic in Natural Waters. Appl. Geochem. 2002, 17, 517–568. [Google Scholar] [CrossRef] [Green Version]
- Filella, M.; Belzile, N.; Chen, Y.-W. Antimony in the Environment: A Review Focused on Natural Waters: I. Occurrence. Earth-Sci. Rev. 2002, 57, 125–176. [Google Scholar] [CrossRef]
- Filella, M.; Belzile, N.; Chen, Y.-W. Antimony in the Environment: A Review Focused on Natural Waters: II. Relevant Solution Chemistry. Earth-Sci. Rev. 2002, 59, 265–285. [Google Scholar] [CrossRef]
- Wilson, S.C.; Lockwood, P.V.; Ashley, P.M.; Tighe, M. The Chemistry and Behaviour of Antimony in the Soil Environment with Comparisons to Arsenic: A Critical Review. Environ. Pollut. 2010, 158, 1169–1181. [Google Scholar] [CrossRef]
- Herath, I.; Vithanage, M.; Bundschuh, J. Antimony as a Global Dilemma: Geochemistry, Mobility, Fate and Transport. Environ. Pollut. 2017, 223, 545–559. [Google Scholar] [CrossRef]
- Belzile, N.; Chen, Y.-W.; Wang, Z. Oxidation of Antimony (III) by Amorphous Iron and Manganese Oxyhydroxides. Chem. Geol. 2001, 174, 379–387. [Google Scholar] [CrossRef]
- McComb, K.A.; Craw, D.; McQuillan, A.J. ATR-IR Spectroscopic Study of Antimonate Adsorption to Iron Oxide. Langmuir 2007, 23, 12125–12130. [Google Scholar] [CrossRef]
- Waychunas, G.A.; Rea, B.A.; Fuller, C.C.; Davis, J.A. Surface Chemistry of Ferrihydrite: Part 1. EXAFS Studies of the Geometry of Coprecipitated and Adsorbed Arsenate. Geochim. Cosmochim. Acta 1993, 57, 2251–2269. [Google Scholar] [CrossRef]
- Sarkar, B.; Jacks, G.; Frisbie, S.; Smith, H.; Naidu, R.; Sarkar, B. Arsenic in the Environment: A Global Perspective. In Heavy Metals in The Environment; CRC Press: New York, NY, USA, 2002; pp. 147–215. ISBN 978-0-8247-0630-2. [Google Scholar]
- Zobrist, J.; Dowdle, P.R.; Davis, J.A.; Oremland, R.S. Mobilization of Arsenite by Dissimilatory Reduction of Adsorbed Arsenate. Environ. Sci. Technol. 2000, 34, 4747–4753. [Google Scholar] [CrossRef]
- Leuz, A.-K.; Mönch, H.; Johnson, C.A. Sorption of Sb(III) and Sb(V) to Goethite: Influence on Sb(III) Oxidation and Mobilization. Environ. Sci. Technol. 2006, 40, 7277–7282. [Google Scholar] [CrossRef]
- Griggs, C.S.; Martin, W.A.; Larson, S.L.; O’Connnor, G.; Fabian, G.; Zynda, G.; Mackie, D. The Effect of Phosphate Application on the Mobility of Antimony in Firing Range Soils. Sci. Total Environ. 2011, 409, 2397–2403. [Google Scholar] [CrossRef]
- Signes-Pastor, A.; Burló, F.; Mitra, K.; Carbonell-Barrachina, A.A. Arsenic Biogeochemistry as Affected by Phosphorus Fertilizer Addition, Redox Potential and PH in a West Bengal (India) Soil. Geoderma 2007, 137, 504–510. [Google Scholar] [CrossRef]
- Smith, E.; Naidu, R.; Alston, A.M. Chemistry of Inorganic Arsenic in Soils: II. Effect of Phosphorus, Sodium, and Calcium on Arsenic Sorption. J. Environ. Qual. 2002, 31, 557–563. [Google Scholar] [CrossRef]
- Spuller, C.; Weigand, H.; Marb, C. Trace Metal Stabilisation in a Shooting Range Soil: Mobility and Phytotoxicity. J. Hazard. Mater. 2007, 141, 378–387. [Google Scholar] [CrossRef]
- O’Day, P.A.; Vlassopoulos, D.; Root, R.; Rivera, N. The Influence of Sulfur and Iron on Dissolved Arsenic Concentrations in the Shallow Subsurface under Changing Redox Conditions. Proc. Natl. Acad. Sci. USA 2004, 101, 13703–13708. [Google Scholar] [CrossRef] [Green Version]
- Bostick, B.C.; Fendorf, S. Arsenite Sorption on Troilite (FeS) and Pyrite (FeS2). Geochim. Cosmochim. Acta 2003, 67, 909–921. [Google Scholar] [CrossRef]
- Mitsunobu, S.; Takahashi, Y.; Terada, Y.; Sakata, M. Antimony(V) Incorporation into Synthetic Ferrihydrite, Goethite, and Natural Iron Oxyhydroxides. Environ. Sci. Technol. 2010, 44, 3712–3718. [Google Scholar] [CrossRef]
- Hindersmann, I.; Mansfeldt, T. Trace Element Solubility in a Multimetal-Contaminated Soil as Affected by Redox Conditions. Water. Air. Soil Pollut. 2014, 225, 2158. [Google Scholar] [CrossRef]
- Pedersen, H.D.; Postma, D.; Jakobsen, R. Release of Arsenic Associated with the Reduction and Transformation of Iron Oxides. Geochim. Cosmochim. Acta 2006, 70, 4116–4129. [Google Scholar] [CrossRef]
- Schwertmann, U. Solubility and Dissolution of Iron Oxides. Plant Soil 1991, 130, 1–25. [Google Scholar] [CrossRef]
- Wan, X.; Tandy, S.; Hockmann, K.; Schulin, R. Effects of Waterlogging on the Solubility and Redox State of Sb in a Shooting Range Soil and Its Uptake by Grasses: A Tank Experiment. Plant Soil 2013, 371, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Dousova, B.; Buzek, F.; Herzogova, L.; Machovic, V.; Lhotka, M. Effect of Organic Matter on Arsenic(V) and Antimony(V) Adsorption in Soils: Comparison of Arsenic and Antimony Adsorption Properties. Eur. J. Soil Sci. 2015, 66, 74–82. [Google Scholar] [CrossRef]
- Buschmann, J.; Kappeler, A.; Lindauer, U.; Kistler, D.; Berg, M.; Sigg, L. Arsenite and Arsenate Binding to Dissolved Humic Acids: Influence of PH, Type of Humic Acid and Aluminum. Environ. Sci. Technol. 2006, 40, 6015–6020. [Google Scholar] [CrossRef] [Green Version]
- Redman, A.D.; Macalady, D.L.; Ahmann, D. Natural Organic Matter Affects Arsenic Speciation and Sorption onto Hematite. Environ. Sci. Technol. 2002, 36, 2889–2896. [Google Scholar] [CrossRef]
- Mikutta, C.; Kretzschmar, R. Spectroscopic Evidence for Ternary Complex Formation between Arsenate and Ferric Iron Complexes of Humic Substances. Environ. Sci. Technol. 2011, 45, 9550–9557. [Google Scholar] [CrossRef]
- Tella, M.; Pokrovski, G.S. Stability and Structure of Pentavalent Antimony Complexes with Aqueous Organic Ligands. Chem. Geol. 2012, 292–293, 57–68. [Google Scholar] [CrossRef]
- Tella, M.; Pokrovski, G.S. Antimony(III) Complexing with O-Bearing Organic Ligands in Aqueous Solution: An X-Ray Absorption Fine Structure Spectroscopy and Solubility Study. Geochim. Cosmochim. Acta 2009, 73, 268–290. [Google Scholar] [CrossRef]
- Hockmann, K.; Schulin, R. Leaching of Antimony from Contaminated Soils. In Competitive Sorption and Transport of Heavy Metals in Soils and Geological Media; Selim, H., Ed.; CRC Press: New York, NY, USA, 2012; pp. 119–145. ISBN 978-1-4398-8014-2. [Google Scholar]
- Verbeeck, M.; Thiry, Y.; Smolders, E. Soil Organic Matter Affects Arsenic and Antimony Sorption in Anaerobic Soils. Environ. Pollut. 2020, 257, 113566. [Google Scholar] [CrossRef] [PubMed]
- Mansfeldt, T.; Overesch, M. Arsenic Mobility and Speciation in a Gleysol with Petrogleyic Properties: A Field and Laboratory Approach. J. Environ. Qual. 2013, 42, 1130–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arsic, M.; Teasdale, P.R.; Welsh, D.T.; Johnston, S.G.; Burton, E.D.; Hockmann, K.; Bennett, W.W. Diffusive Gradients in Thin Films Reveals Differences in Antimony and Arsenic Mobility in a Contaminated Wetland Sediment during an Oxic-Anoxic Transition. Environ. Sci. Technol. 2018, 52, 1118–1127. [Google Scholar] [CrossRef]
- Rouwane, A.; Rabiet, M.; Grybos, M.; Bernard, G.; Guibaud, G. Effects of NO3 − and PO4 3− on the Release of Geogenic Arsenic and Antimony in Agricultural Wetland Soil: A Field and Laboratory Approach. Environ. Sci. Pollut. Res. 2016, 23, 4714–4728. [Google Scholar] [CrossRef]
- Rouwane, A.; Grybos, M.; Bourven, I.; Rabiet, M.; Guibaud, G. Waterlogging and Soil Reduction Affect the Amount and Apparent Molecular Weight Distribution of Dissolved Organic Matter in Wetland Soil: A Laboratory Study. Soil Res. 2018, 56, 28. [Google Scholar] [CrossRef]
- Grybos, M.; Davranche, M.; Gruau, G.; Petitjean, P.; Pédrot, M. Increasing PH Drives Organic Matter Solubilization from Wetland Soils under Reducing Conditions. Geoderma 2009, 154, 13–19. [Google Scholar] [CrossRef]
- Huang, G.; Chen, Z.; Zhang, Y.; Liu, F.; Wang, J.; Hou, Q. Changes of Arsenic Fractionation and Bioaccessibility in Wastewater-Irrigated Soils as a Function of Aging: Influence of Redox Condition and Arsenic Load. Geoderma 2016, 280, 1–7. [Google Scholar] [CrossRef]
- WRB. World Reference Base for Soil Resources. I: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Food and Agriculture Organization: Rome, Italy, 2014; p. 203. [Google Scholar]
- Bohn, H.L. Redox potentials. Soil Sci. 1971, 112, 39–45. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Wenzel, W.W.; Kirchbaumer, N.; Prohaska, T.; Stingeder, G.; Lombi, E.; Adriano, D.C. Arsenic Fractionation in Soils Using an Improved Sequential Extraction Procedure. Anal. Chim. Acta 2001, 436, 309–323. [Google Scholar] [CrossRef]
- Keon, N.E.; Swartz, C.H.; Brabander, D.J.; Harvey, C.; Hemond, H.F. Validation of an Arsenic Sequential Extraction Method for Evaluating Mobility in Sediments. Environ. Sci. Technol. 2001, 35, 2778–2784. [Google Scholar] [CrossRef]
- Chen, M.; Ma, L.Q. Comparison of Three Aqua Regia Digestion Methods for Twenty Florida Soils. Soil Sci. Soc. Am. J. 2001, 65, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Bauer, M.; Blodau, C. Arsenic Distribution in the Dissolved, Colloidal and Particulate Size Fraction of Experimental Solutions Rich in Dissolved Organic Matter and Ferric Iron. Geochim. Cosmochim. Acta 2009, 73, 529–542. [Google Scholar] [CrossRef]
- Tarvainen, T.; Salminen, R.; Vos, W.D. Geochemical Atlas of Europe. Background Information, Methodology and Maps Part 1; Geological Survey of Finland: Espoo, Finland, 2005; ISBN 978-951-690-913-7. [Google Scholar]
- Bossy, A.; Grosbois, C.; Beauchemin, S.; Courtin-Nomade, A.; Hendershot, W.; Bril, H. Alteration of As-Bearing Phases in a Small Watershed Located on a High Grade Arsenic-Geochemical Anomaly (French Massif Central). Appl. Geochem. 2010, 25, 1889–1901. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Ke, Z.; Yan, M.; Wang, W.; Nie, H.; Li, B.; Zhang, J.; Xu, X.; Wang, J. Concentrations, Distribution, and Ecological Risk Assessment of Heavy Metals in Daya Bay, China. Water 2018, 10, 780. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, C.L. Riverine Composition and Estuarine Geochemistry of Particulate Metals in China—Weathering Features, Anthropogenic Impact and Chemical Fluxes. Estuar. Coast. Shelf Sci. 2002, 54, 1051–1070. [Google Scholar] [CrossRef]
- Guénet, H.; Davranche, M.; Vantelon, D.; Pédrot, M.; Al-Sid-Cheikh, M.; Dia, A.; Jestin, J. Evidence of Organic Matter Control on As Oxidation by Iron Oxides in Riparian Wetlands. Chem. Geol. 2016, 439, 161–172. [Google Scholar] [CrossRef] [Green Version]
- Escudey, M.; Förster, J.E.; Galindo, G. Relevance of Organic Matter in Some Chemical and Physical Characteristics of Volcanic Ash-Derived Soils. Commun. Soil Sci. Plant Anal. 2004, 35, 781–797. [Google Scholar] [CrossRef]
- Catrouillet, C.; Davranche, M.; Dia, A.; Bouhnik-Le Coz, M.; Pédrot, M.; Marsac, R.; Gruau, G. Thiol Groups Controls on Arsenite Binding by Organic Matter: New Experimental and Modeling Evidence. J. Colloid Interface Sci. 2015, 460, 310–320. [Google Scholar] [CrossRef] [Green Version]
- Couture, R.-M.; Wallschläger, D.; Rose, J.; Van Cappellen, P. Arsenic Binding to Organic and Inorganic Sulfur Species during Microbial Sulfate Reduction: A Sediment Flow-through Reactor Experiment. Environ. Chem. 2013, 10, 285. [Google Scholar] [CrossRef] [Green Version]
- Al-Sid-Cheikh, M.; Pédrot, M.; Dia, A.; Guenet, H.; Vantelon, D.; Davranche, M.; Gruau, G.; Delhaye, T. Interactions between Natural Organic Matter, Sulfur, Arsenic and Iron Oxides in Re-Oxidation Compounds within Riparian Wetlands: NanoSIMS and X-Ray Adsorption Spectroscopy Evidences. Sci. Total Environ. 2015, 515–516, 118–128. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, S.; Jia, Y. Effect of Sulfide on As(III) and As(V) Sequestration by Ferrihydrite. Chemosphere 2017, 185, 321–328. [Google Scholar] [CrossRef]
- Hou, H.; Takamatsu, T.; Koshikawa, M.K.; Hosomi, M. Concentrations of Ag, In, Sn, Sb and Bi, and Their Chemical Fractionation in Typical Soils in Japan: Fractionation of Ag, In, Sn, Sb and Bi in Soils. Eur. J. Soil Sci. 2006, 57, 214–227. [Google Scholar] [CrossRef]
- Ceriotti, G.; Amarasiriwardena, D. A Study of Antimony Complexed to Soil-Derived Humic Acids and Inorganic Antimony Species along a Massachusetts Highway. Microchem. J. 2009, 91, 85–93. [Google Scholar] [CrossRef]
- Hamon, R.E.; Lombi, E.; Fortunati, P.; Nolan, A.L.; McLaughlin, M.J. Coupling Speciation and Isotope Dilution Techniques to Study Arsenic Mobilization in the Environment. Environ. Sci. Technol. 2004, 38, 1794–1798. [Google Scholar] [CrossRef]
- Hou, H.; Takamatsu, T.; Koshikawa, M.K.; Hosomi, M. Migration of Silver, Indium, Tin, Antimony, and Bismuth and Variations in Their Chemical Fractions on Addition to Uncontaminated Soils. Soil Sci. 2005, 170, 624–639. [Google Scholar] [CrossRef]
- Krysiak, A.; Karczewska, A. Arsenic Extractability in Soils in the Areas of Former Arsenic Mining and Smelting, SW Poland. Sci. Total Environ. 2007, 379, 190–200. [Google Scholar] [CrossRef]
- Pfeifer, H.-R.; Gueye-Girardet, A.; Reymond, D.; Schlegel, C.; Temgoua, E.; Hesterberg, D.L.; Chou, J.W. Dispersion of Natural Arsenic in the Malcantone Watershed, Southern Switzerland: Field Evidence for Repeated Sorption–Desorption and Oxidation–Reduction Processes. Geoderma 2004, 122, 205–234. [Google Scholar] [CrossRef]
- Blodau, C.; Fulda, B.; Bauer, M.; Knorr, K.-H. Arsenic Speciation and Turnover in Intact Organic Soil Mesocosms during Experimental Drought and Rewetting. Geochim. Cosmochim. Acta 2008, 72, 3991–4007. [Google Scholar] [CrossRef]
- Xue, Q.; Ran, Y.; Tan, Y.; Peacock, C.L.; Du, H. Arsenite and Arsenate Binding to Ferrihydrite Organo-Mineral Coprecipitate: Implications for Arsenic Mobility and Fate in Natural Environments. Chemosphere 2019, 224, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Johnston, S.G.; Bennett, W.W.; Doriean, N.; Hockmann, K.; Karimian, N.; Burton, E.D. Antimony and Arsenic Speciation, Redox-Cycling and Contrasting Mobility in a Mining-Impacted River System. Sci. Total Environ. 2020, 710, 136354. [Google Scholar] [CrossRef] [PubMed]


| Step | Target Phase/Mechanisms | Labels/Abbreviation in Figures and Text | Extracting Agent | Reference |
|---|---|---|---|---|
| F1 | Loosely adsorbed | Ac-exchangeable | 1-M sodium acetate pH 8.2 2 h of shaking at 20 ± 2 °C | [45] |
| F2 | Strongly adsorbed | P-exchangeable | 0.05-M sodium phosphates pH 5 16 h of shaking at 20 ± 2 °C | [46,47] |
| F3 | Carbonates bound | Carbonate-bound | 1-M sodium acetate pH 5 5 h of shaking at 20 ± 2 °C | [45] |
| F4 | Expected to be bound to easily reducible fraction | Easily reducible | 0.2-M ammonium oxalate/oxalic acid pH 3 2 h of shaking at 20 ± 2 °C in the dark * | [47] |
| F5 | Expected to be reducible fraction | Reducible | 0.2-M ammonium oxalate/oxalic acid + ascorbic acid (0.1 M) pH 3.25 30 min at 96 °C * | [46] |
| F6 | Expected to be associated with persistent OM and sulfides | H2O2–Org | (1) 6 mL of 0.02-M HNO3 + 10 mL of 30% H2O2 (pH 2) 2h at 85 °C (2) 6 mL of 30% H2O2 (pH 2) 3 h at 85 °C (3) 10 mL of ammonium acetate in HNO3 20% (v/v) + ultrapure water up to 40 mL 30 min at 20 ± 2 °C ** | [45] |
| F7 | Residual | Residual | 4-mL HCl (37%) + 4-mL HNO3 (65%) by microwave heating (Multiwave Go) 10 min at 180 °C | – |
| Soil Layers | ||||||
|---|---|---|---|---|---|---|
| 0–20 cm | 40–60 cm | 90–110 cm | 0–20 cm | 40–60 cm | 90–110 cm | |
| Fraction | Extracted As (%) | Extracted Sb (%) | ||||
| Ac-exchangeable | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.4 ± 0.1 | n.q | n.q | n.q |
| P-exchangeable | 15.2 ± 0.1 | 15.5 ± 0.5 | 15.9 ± 0.1 | 3.6 ± 0.2 | 4.5 ± 0.2 | 5.1 ± 0.8 |
| Carbonate-bound | 1.0 ± 0.0 | 0.7 ± 0.2 | 1.2 ± 0.6 | n.q | n.q | n.q |
| Easily reducible | 58.6 ± 0.7 | 48 ± 5 | 35 ± 2 | 27 ± 1 | 22.6 ± 0.2 | 30 ± 1 |
| Reducible | 7.9 ± 0.0 | 10 ± 2 | 14.2 ± 0.3 | 15 ± 1 | 17 ± 2 | 18.6 ± 0.0 |
| H2O2–Org | 8.0 ± 0.2 | 4.5 ± 0.4 | n.q | 21.0 ± 0.9 | n.q | n.q |
| Residual | 8.0 ± 0.4 | 19.9 ± 0.3 | 31.9 ± 0.8 | 33 ± 1 | 55.8 ± 0.1 | 46.2 ± 0.1 |
| Fraction | Extracted Fe (%) | Extracted Mn (%) | ||||
| Ac-exchangeable | n.q | n.q | n.q | 29 ± 2 | 17.7 ± 0.9 | 3.2 ± 0.0 |
| P-exchangeable | <1 | <1 | <1 | 1.5 ± 0.1 | 1.9 ± 0.2 | <1 |
| Carbonate-bound | n.q | n.q | n.q | 21.8 ± 0.6 | 12.0 ± 0.4 | 0.9 ± 0.1 |
| Easily reducible | 54 ± 2 | 38 ± 3 | 10.3 ± 0.5 | 18.7 ± 0.1 | 8 ± 1 | 1.0 ± 0.0 |
| Reducible | 16 ± 4 | 16 ± 4 | 17 ± 4 | 11.2 ± 0.2 | 13 ± 5 | 15 ± 4 |
| H2O2–Org | 5.9 ± 0.3 | 5.8 ± 0.1 | 1.5 ± 0.1 | 6.1 ± 0.3 | 9.3 ± 0.7 | 8.7 ± 0.6 |
| Residual | 24 ± 2 | 40.7 ± 0.2 | 72 ± 4 | 11.6 ± 0.3 | 38.7 ± 0.2 | 70 ± 3 |
| Incubation Time | |||
|---|---|---|---|
| Day 0 | Day 22 | Day 36 | |
| As (µg·L−1) | 3.4 ± 0.1 | 138 ± 4 | 370 ± 50 |
| Sb (µg·L−1) | 0.22 ± 0.01 | 0.89 ± 0.02 | 0.51 ± 0.12 |
| pH | 5.7 ± 0.1 | 6.7 ± 0.1 | 6.7 ± 0.1 |
| Eh (mV) | 290 ± 20 | 3 ± 5 | −11 ± 10 |
| Fe(II) (mg·L−1) | 0.2 ± 0.1 | 47 ± 4 | 39 ± 4 |
| Mn (µg·L−1) | 65 ± 2 | 440 ± 20 | 430 ± 20 |
| DOC (mgC·L−1) | 51 ± 1 | 267 ± 3 | 250 ± 20 |
| HCO3− (mg·L−1) | 27 ± 1 | 370 ± 20 | 414 ± 5 |
| SO42− (mg·L−1) | 17.7 ± 1.6 | Not mesured | 0.2 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rouwane, A.; Grybos, M.; Rabiet, M.; Guibaud, G. Potentially Mobilizable Geogenic As and Sb in an Agricultural Wetland Soil. Geosciences 2021, 11, 444. https://doi.org/10.3390/geosciences11110444
Rouwane A, Grybos M, Rabiet M, Guibaud G. Potentially Mobilizable Geogenic As and Sb in an Agricultural Wetland Soil. Geosciences. 2021; 11(11):444. https://doi.org/10.3390/geosciences11110444
Chicago/Turabian StyleRouwane, Asmaa, Malgorzata Grybos, Marion Rabiet, and Gilles Guibaud. 2021. "Potentially Mobilizable Geogenic As and Sb in an Agricultural Wetland Soil" Geosciences 11, no. 11: 444. https://doi.org/10.3390/geosciences11110444





