Long-Term Evolution of Fracture Permeability in Slate: An Experimental Study with Implications for Enhanced Geothermal Systems (EGS)
Abstract
:1. Introduction
2. Materials and Methods
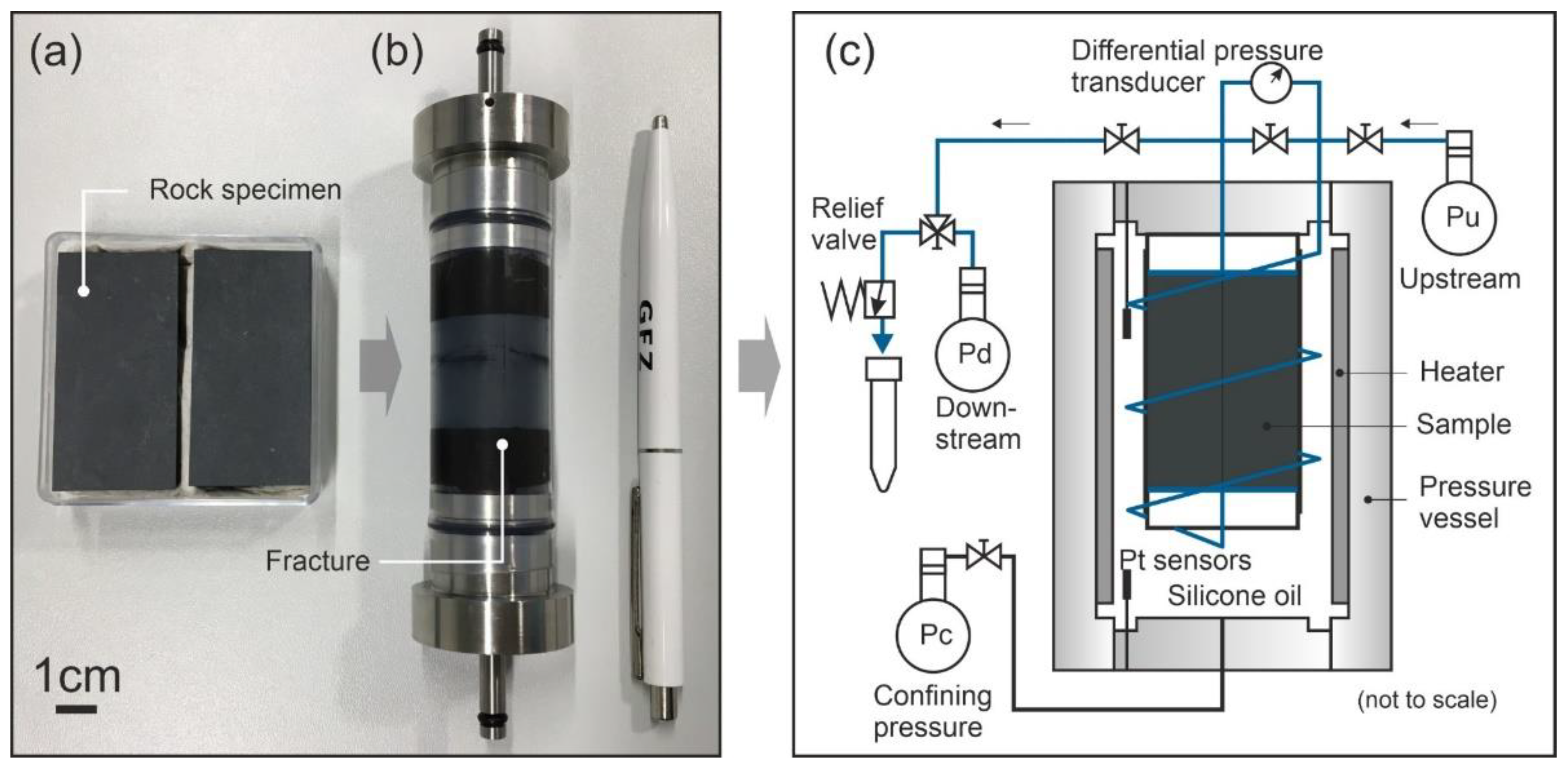
2.1. Rock Samples
2.2. Experimental Procedures
- (1)
- The purpose of stage one was to investigate the potential transient fracture permeability degradation after pressure build-up, which exerts a force on the fracture surfaces. Such initial fracture permeability decline was widely observed at the first dozens to hundreds of hours of continuous fluid flow through fractured granitic rocks [4,21], shale [22], novaculite [15,17], limestone [16], and dolomitic anhydrite [18]. However, this time-dependent fracture permeability decay did not occur in some fractured sandstones and mudstones [23,24] with the intermittent flow (flow-stop-flow with a certain time interval). To monitor the influence of pressure on fracture permeability evolution in slates, we continuously measured permeability for several to dozens of hours, followed by stopping the flow for dozens of hours and measured fracture permeability again. This initial stage was performed at room temperature immediately after pressurization.
- (2)
- The second stage was to reveal thermal effects on fracture permeability evolution and to eliminate any irreversible fracture permeability changes upon thermal expansion of the rock matrix. The temperature was increased and decreased stepwise between room temperature and 70 °C for sample SM2 and between 25 °C and 90 °C for sample SM1 and SM3 (Table 2). Fracture permeability was measured after stabilization of temperature in each step.
- (3)
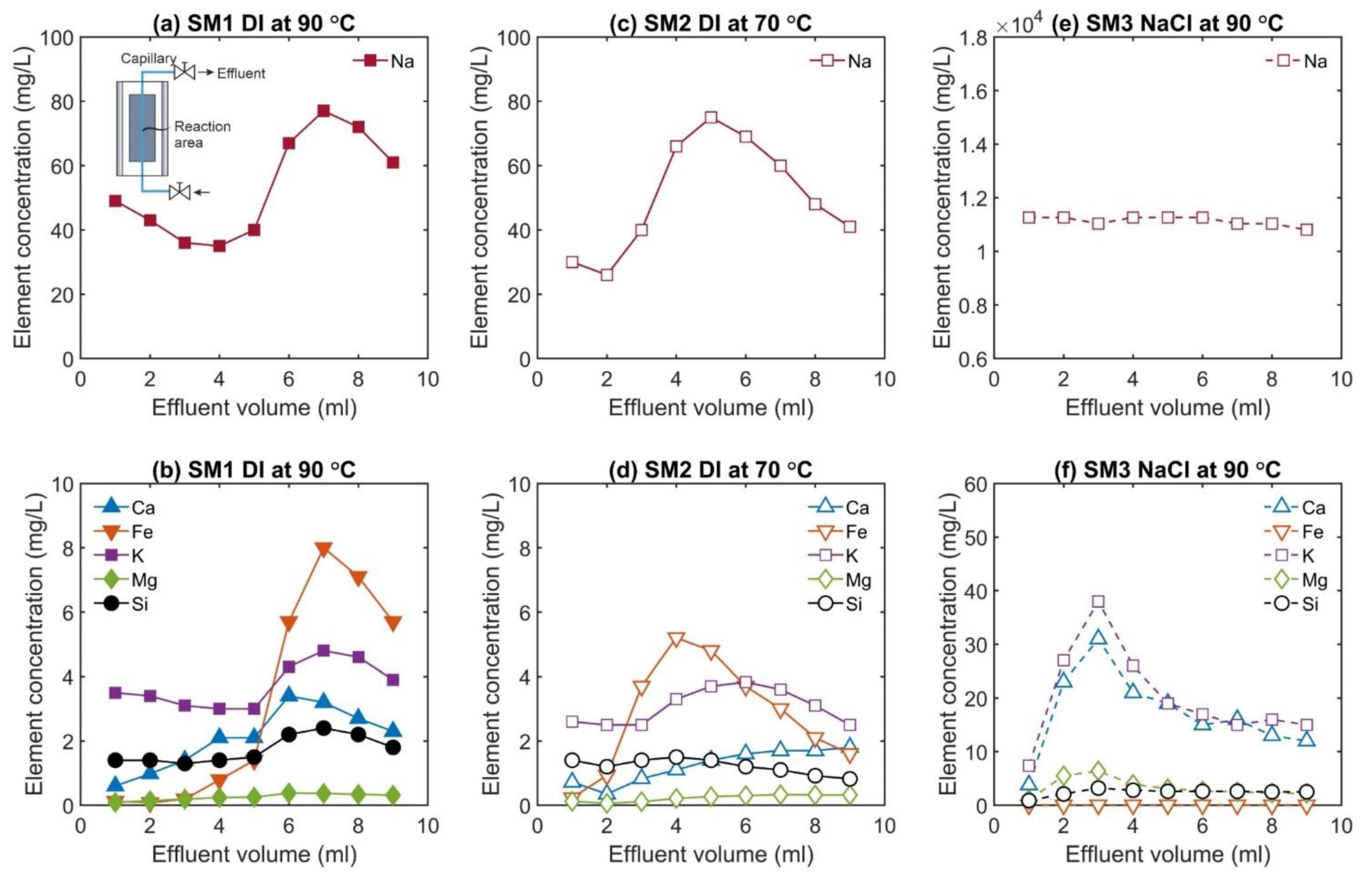
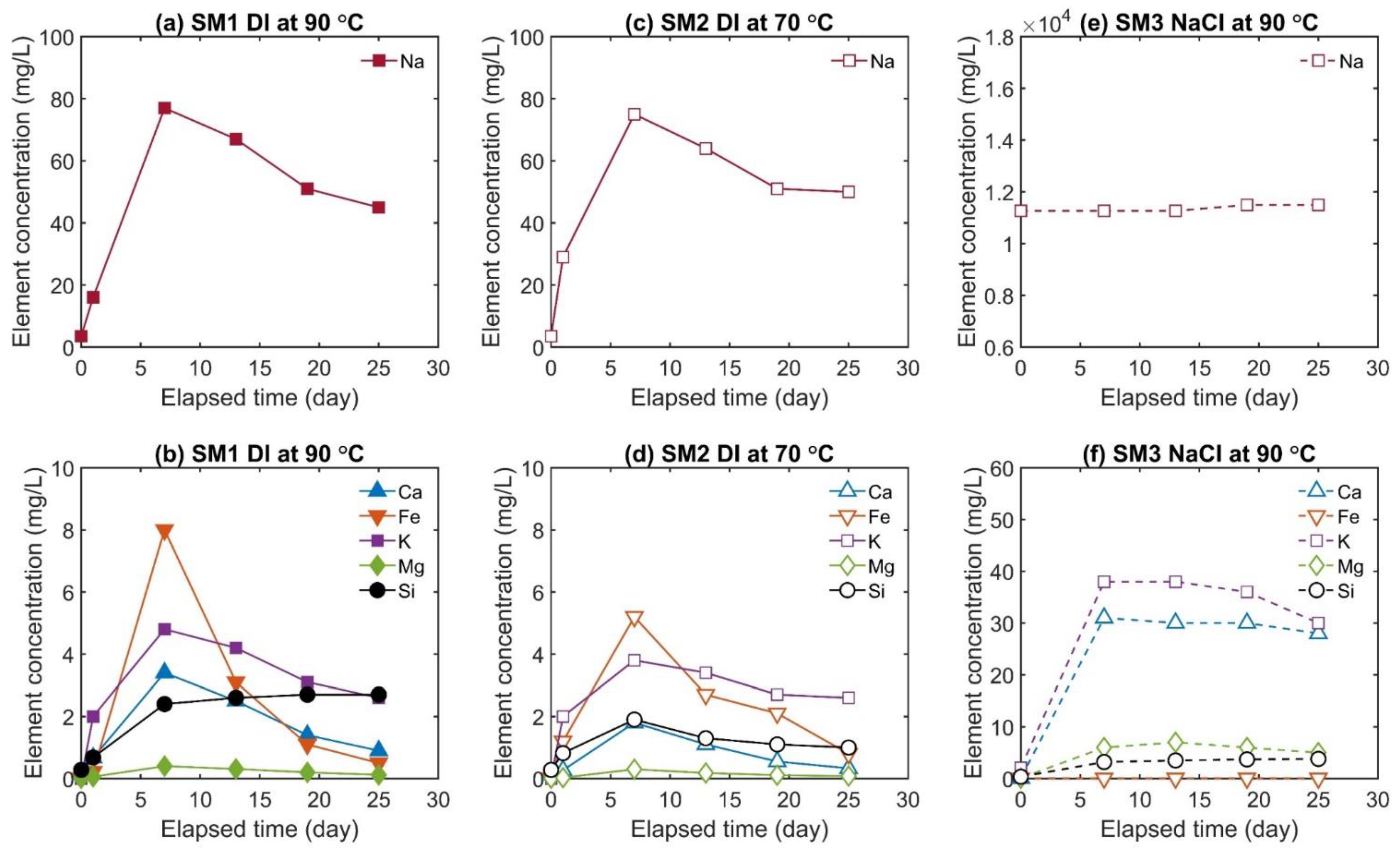
- In the last stage, the temperature was kept at the highest value (70 or 90 °C), and permeability was measured regularly after a time interval of 6 days. In between the time intervals, the valve of the upstream pump was closed, and the downstream pump maintained constant pressure so that the pore fluid could be considered as a semi-closed system. Before each permeability measurement, the effluent was sampled through the relief valve at a constant flow rate of Q = 0.1 mL/min. Each time, seven to nine subsamples with a volume of V = 1.0 mL at constant pore fluid pressure of Pp = 1 MPa were collected (downstream side). Each sample was acidified by addition of 0.01 ml super-pure HNO3 to minimize any potential precipitation or alteration of the fluid. The purpose of the chosen sampling strategy with collecting small-volume subsamples (V = 1.0 mL) was to better specify the fluid composition within the fracture. Otherwise, a large volume of effluent would have mixed the fluid within the fracture with the fluid in the capillaries connected to the sample.
2.3. Analytical Methods
2.3.1. Effluent Element Concentrations
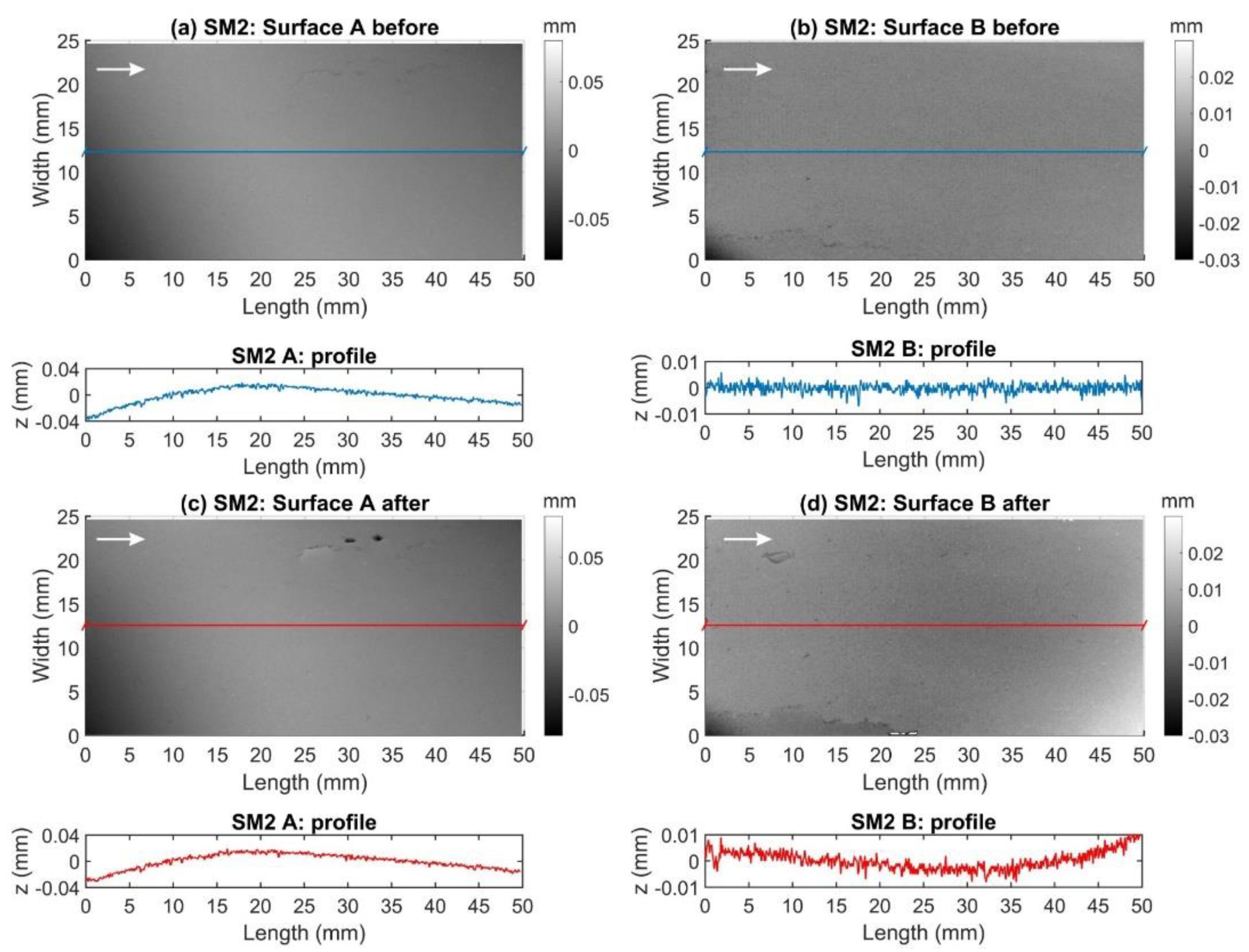
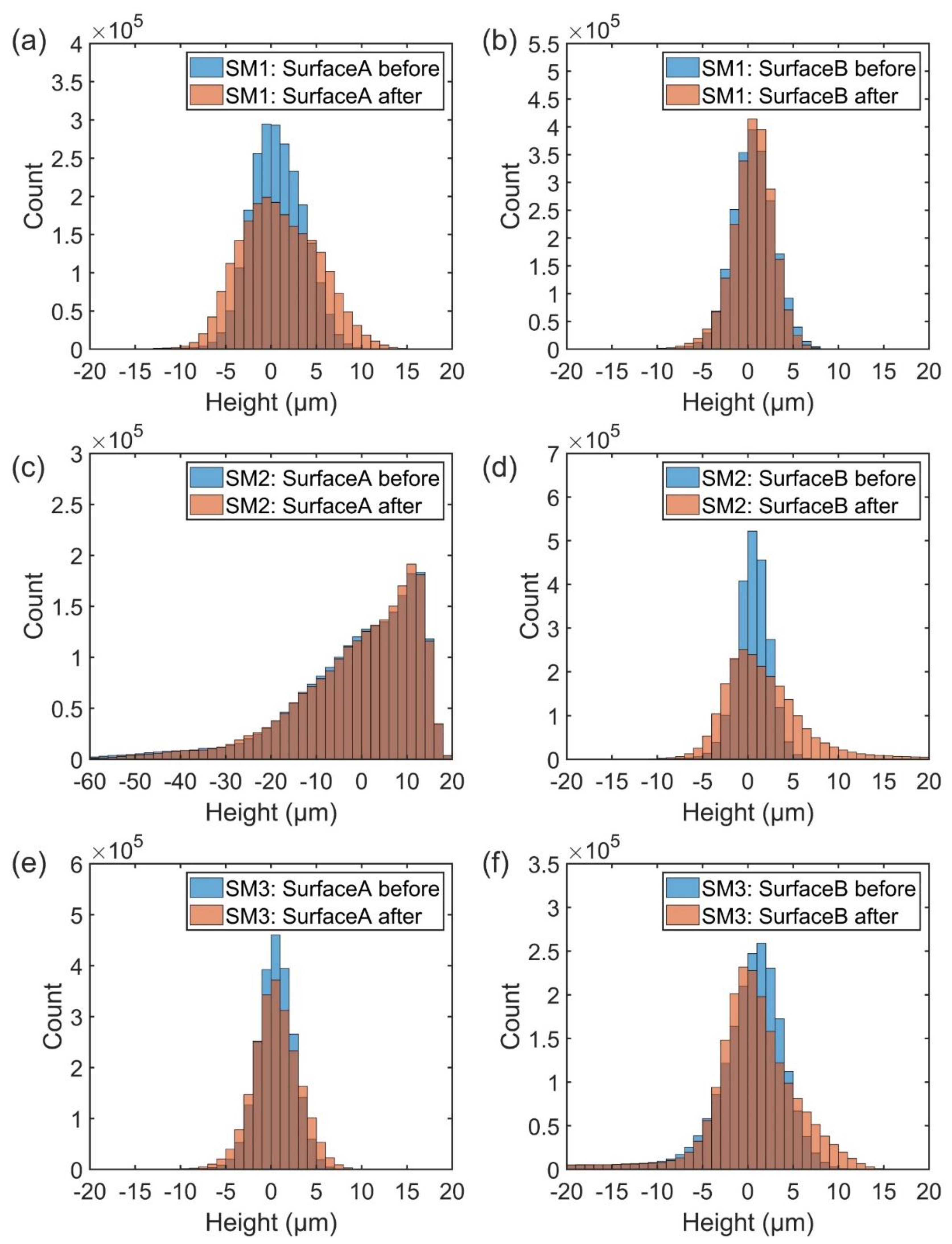
2.3.2. Fracture Surface Topographies
2.3.3. SEM-EDX
3. Results
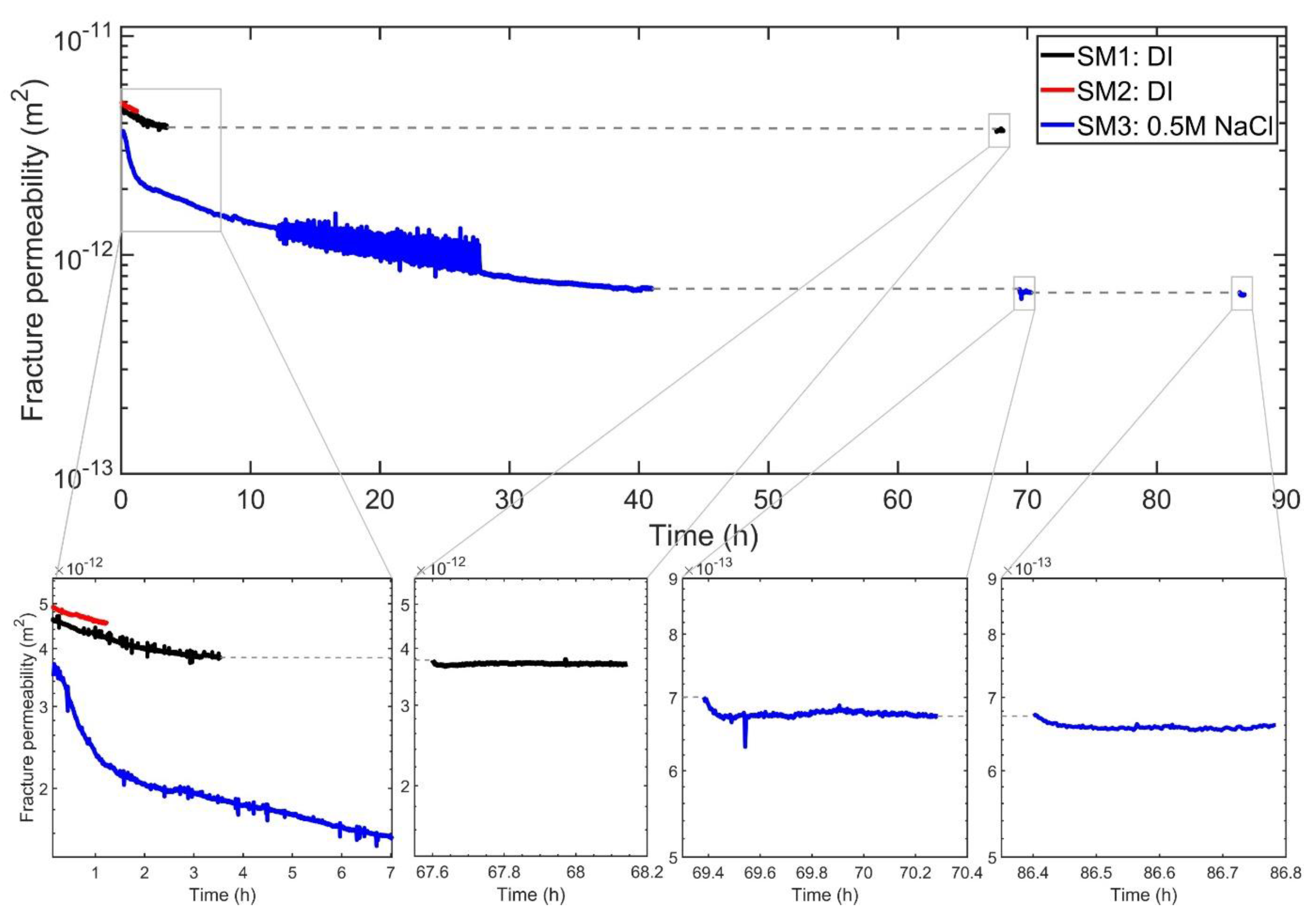
3.1. Variations of Fracture Permeability
3.1.1. Initial Fracture Permeability Decline with Continuous Flow (Stage 1)
3.1.2. Temperature Effects (Stage 2)
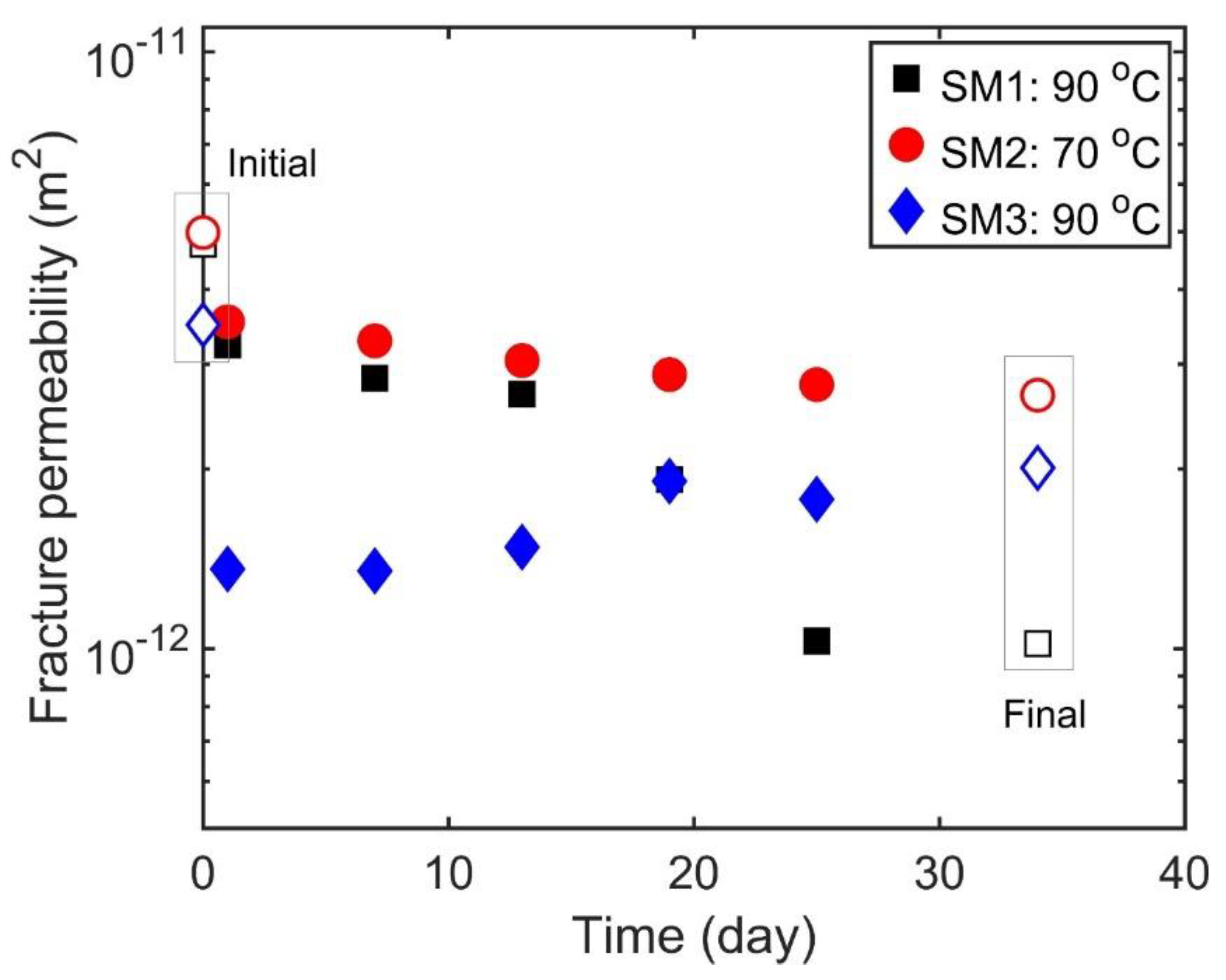
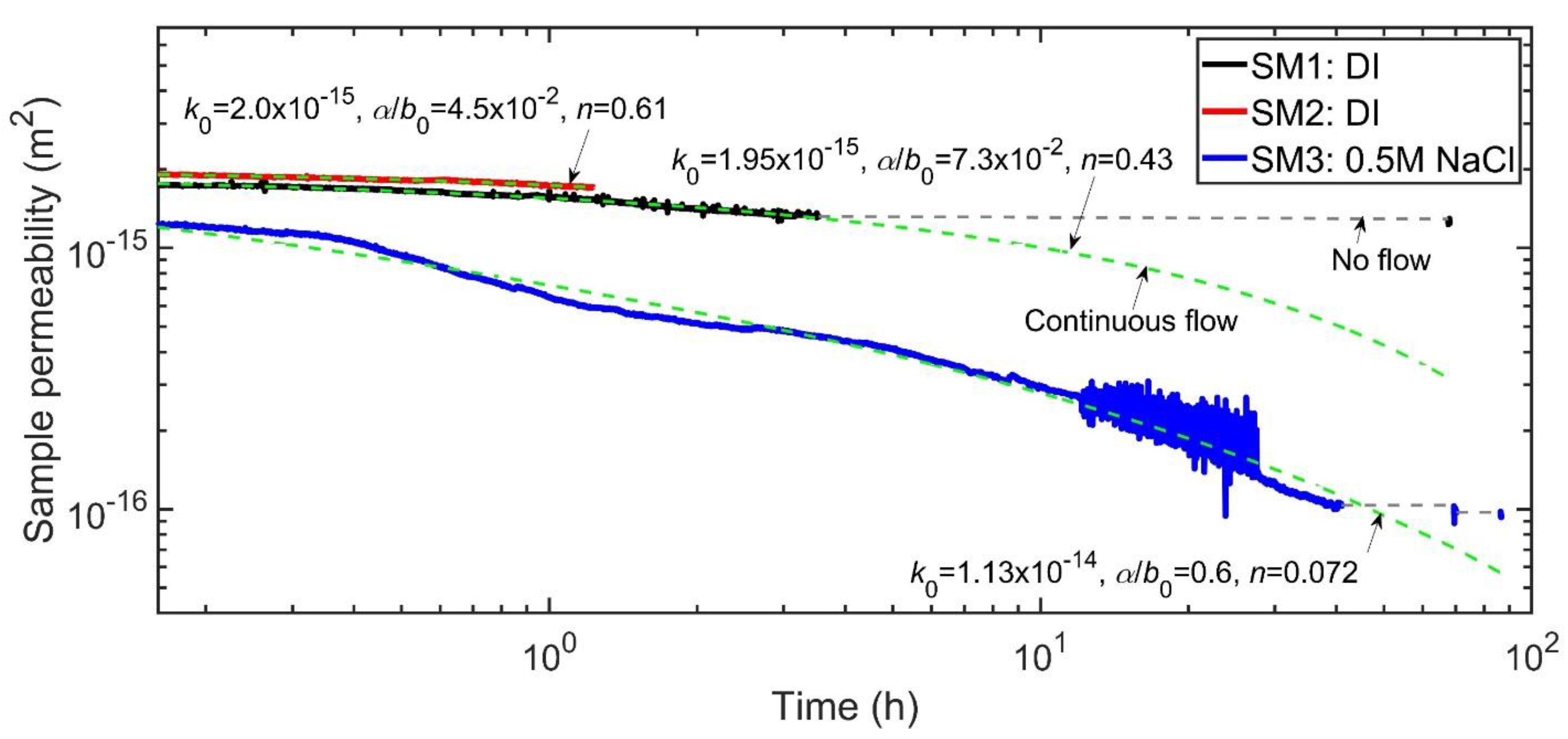
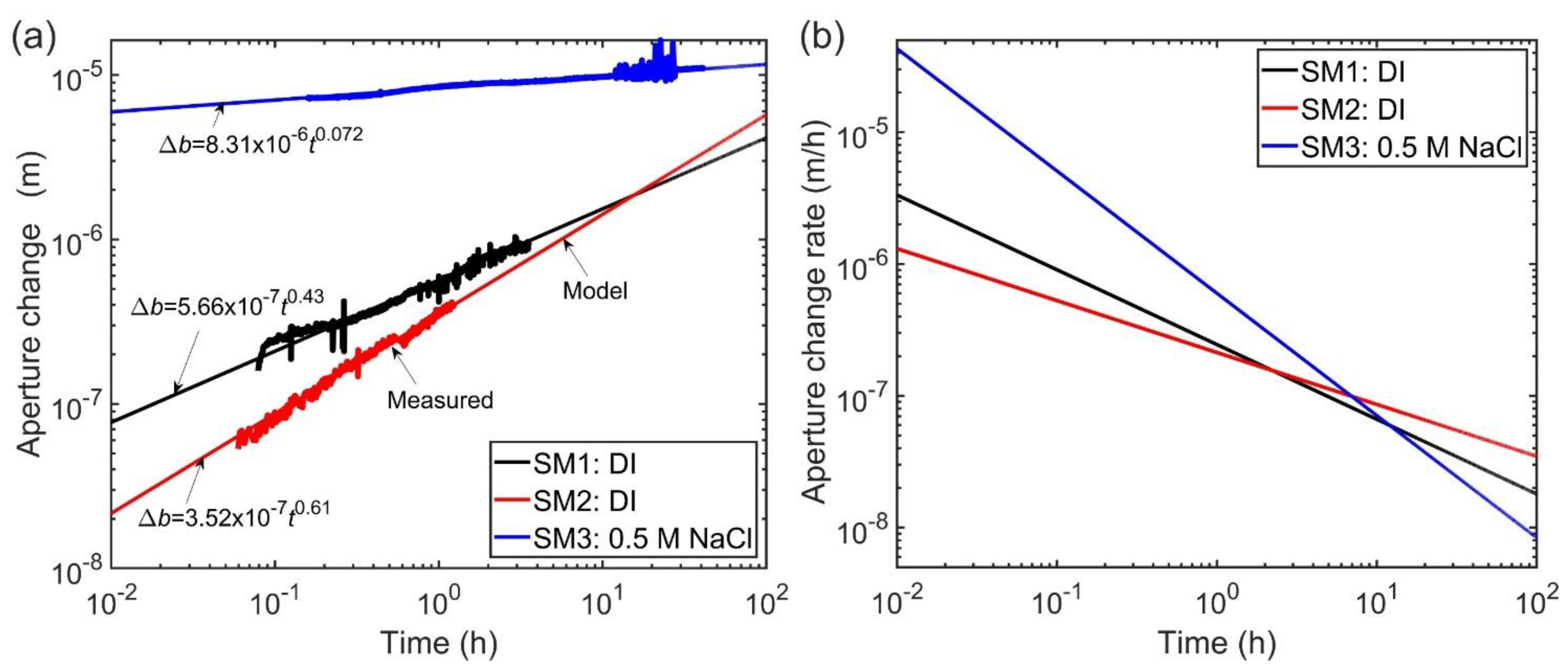
3.1.3. Time Dependence of Permeability with Intermittent Flow (Stage 3)
3.2. Fluid Chemistry Evolution
3.3. Fracture Surface Topography
3.4. Microstructures
4. Discussion
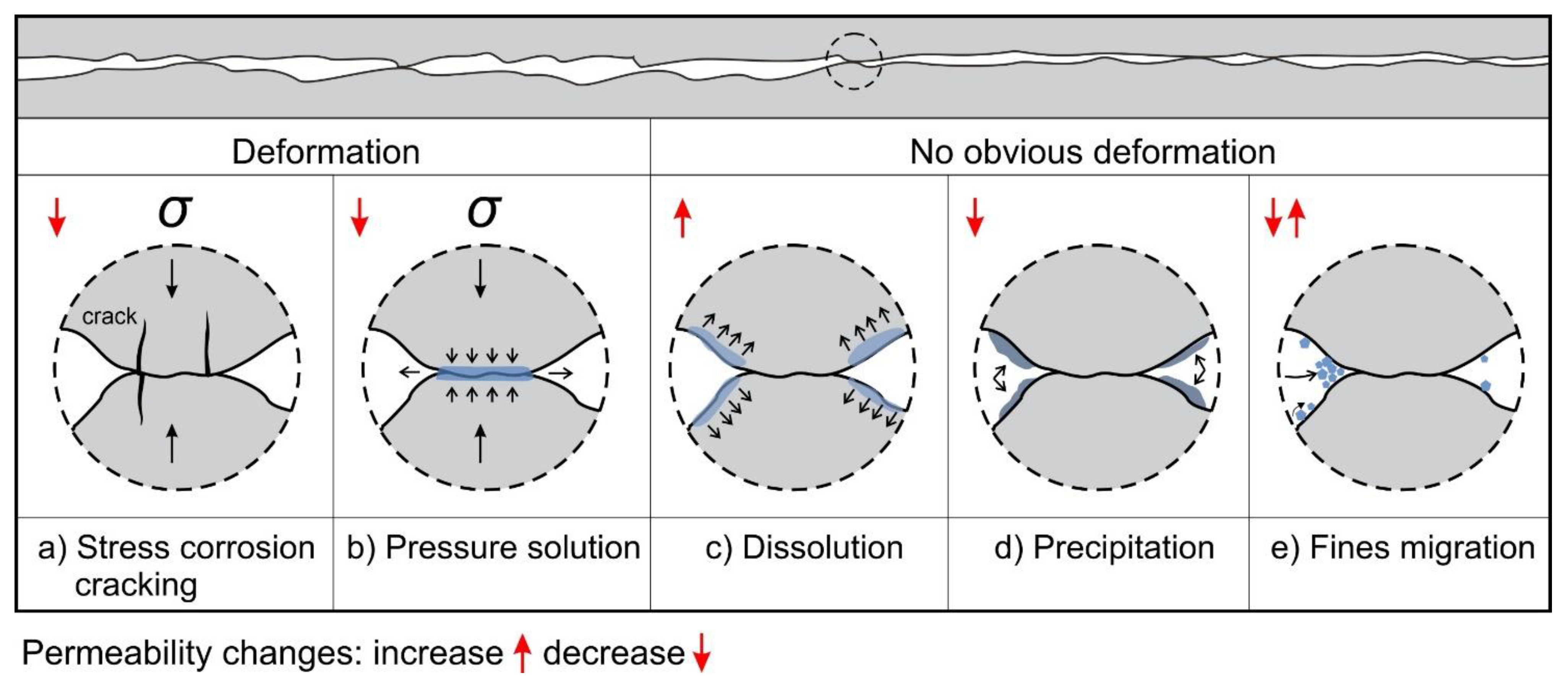
4.1. Mechanism of Initial Permeability Decline under Constant Conditions
4.2. Thermal Effects on Permeability
4.3. Potential Fluid–Rock Interactions on Time-Dependent Permeability Changes
4.4. Implications for EGS
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Jolie, E.; Scott, S.; Faulds, J.; Chambefort, I.; Axelsson, G.; Gutiérrez-Negrín, L.C.; Regenspurg, S.; Ziegler, M.; Ayling, B.; Richter, A. Geological controls on geothermal resources for power generation. Nat. Rev. Earth Environ. 2021, 2, 5. [Google Scholar] [CrossRef]
- Huenges, E. Geothermal Energy Systems: Exploration, Development, and Utilization; Wiley-VCH: Weinheim, Germany, 2010; Volume xxii, 463p. [Google Scholar]
- Cheng, C.; Milsch, H. Hydromechanical investigations on the self-propping potential of fractures in tight sandstones. Rock Mech. Rock Eng. 2021, 54, 5407–5432. [Google Scholar] [CrossRef]
- Hofmann, H.; Blocher, G.; Milsch, H.; Babadagli, T.; Zimmermann, G. Transmissivity of aligned and displaced tensile fractures in granitic rocks during cyclic loading. Int. J. Rock Mech. Min. Sci. 2016, 87, 69–84. [Google Scholar] [CrossRef]
- Vogler, D.; Amann, F.; Bayer, P.; Elsworth, D. Permeability evolution in natural fractures subject to cyclic loading and gouge formation. Rock Mech. Rock Eng. 2016, 49, 3463–3479. [Google Scholar] [CrossRef]
- Kluge, C.; Blöcher, G.; Barnhoorn, A.; Schmittbuhl, J.; Bruhn, D. Permeability evolution during shear zone initiation in low-porosity rocks. Rock Mech. Rock Eng. 2021, 54, 5221–5244. [Google Scholar] [CrossRef]
- Milsch, H.; Hofmann, H.; Blocher, G. An experimental and numerical evaluation of continuous fracture permeability measurements during effective pressure cycles. Int. J. Rock Mech. Min. Sci. 2016, 89, 109–115. [Google Scholar] [CrossRef]
- Dobson, P.F.; Kneafsey, T.J.; Nakagawa, S.; Sonnenthal, E.L.; Voltolini, M.; Smith, J.T.; Borglin, S.E. Fracture sustainability in Enhanced Geothermal Systems: Experimental and modeling constraints. J. Energy Res. Technol. 2020, 143, 1–37. [Google Scholar] [CrossRef]
- Phillips, T.; Bultreys, T.; Bisdom, K.; Kampman, N.; Van Offenwert, S.; Mascini, A.; Cnudde, V.; Busch, A. A systematic investigation into the control of roughness on the flow properties of 3D-printed fractures. Water Resour. Res. 2021, 57, 4. [Google Scholar] [CrossRef]
- Rutter, E.H.; Mecklenburgh, J. Influence of normal and shear stress on the hydraulic transmissivity of thin cracks in a tight quartz sandstone, a granite, and a shale. J. Geophys. Res. Solid Earth 2018, 123, 1262–1285. [Google Scholar] [CrossRef] [Green Version]
- Gutierrez, M.; Oino, L.E.; Nygard, R. Stress-dependent permeability of a de-mineralised fracture in shale. Mar. Petrol. Geol. 2000, 17, 895–907. [Google Scholar] [CrossRef]
- Durham, W.B.; Bonner, B.P. Self-propping and fluid flow in slightly offset joints at high effective pressures. J. Geophys. Res. 1994, 99, 9391–9399. [Google Scholar] [CrossRef]
- Blöcher, G.; Reinsch, T.; Henninges, J.; Milsch, H.; Regenspurg, S.; Kummerow, J.; Francke, H.; Kranz, S.; Saadat, A.; Zimmermann, G.; et al. Hydraulic history and current state of the deep geothermal reservoir Groß Schönebeck. Geothermics 2016, 63, 27–43. [Google Scholar] [CrossRef]
- Regenspurg, S.; Feldbusch, E.; Norden, B.; Tichomirowa, M. Fluid-rock interactions in a geothermal Rotliegend/Permo-Carboniferous reservoir (North German Basin). Appl. Geochem. 2016, 69, 12–27. [Google Scholar] [CrossRef]
- Polak, A.; Elsworth, D.; Yasuhara, H.; Grader, A.S.; Halleck, P.M. Permeability reduction of a natural fracture under net dissolution by hydrothermal fluids. Geophys. Res. Lett. 2003, 30, 20. [Google Scholar] [CrossRef]
- Polak, A.; Elsworth, D.; Liu, J.; Grader, A.S. Spontaneous switching of permeability changes in a limestone fracture with net dissolution. Water Resour. Res. 2004, 40, 40. [Google Scholar] [CrossRef]
- Yasuhara, H.; Polak, A.; Mitani, Y.; Grader, A.; Halleck, P.; Elsworth, D. Evolution of fracture permeability through fluid–rock reaction under hydrothermal conditions. Earth Planet. Sci. Lett. 2006, 244, 186–200. [Google Scholar] [CrossRef]
- Elkhoury, J.E.; Detwiler, R.L.; Ameli, P. Can a fractured caprock self-heal? Earth Planet. Sci. Lett. 2015, 417, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Yasuhara, H.; Kinoshita, N.; Ohfuji, H.; Lee, D.S.; Nakashima, S.; Kishida, K. Temporal alteration of fracture permeability in granite under hydrothermal conditions and its interpretation by coupled chemo-mechanical model. Appl. Geochem. 2011, 26, 2074–2088. [Google Scholar] [CrossRef]
- Kamali-Asl, A.; Ghazanfari, E.; Perdrial, N.; Bredice, N. Experimental study of fracture response in granite specimens subjected to hydrothermal conditions relevant for enhanced geothermal systems. Geothermics 2018, 72, 205–224. [Google Scholar] [CrossRef]
- Im, K.; Elsworth, D.; Fang, Y. The influence of preslip sealing on the permeability evolution of fractures and faults. Geophys. Res. Lett. 2018, 45, 166–175. [Google Scholar] [CrossRef] [Green Version]
- Im, K.; Elsworth, D.; Wang, C. Cyclic permeability evolution during repose then reactivation of fractures and faults. J. Geophys. Res. Solid Earth 2019, 124, 4492–4506. [Google Scholar] [CrossRef]
- Yasuhara, H.; Kinoshita, N.; Ohfuji, H.; Takahashi, M.; Ito, K.; Kishida, K. Long-term observation of permeability in sedimentary rocks under high-temperature and stress conditions and its interpretation mediated by microstructural investigations. Water Resour. Res. 2015, 51, 5425–5449. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.; Milsch, H. Evolution of fracture aperture in quartz sandstone under hydrothermal conditions: Mechanical and chemical effects. Minerals 2020, 10, 657. [Google Scholar] [CrossRef]
- Moore, D.E.; Lockner, D.A.; Byerlee, J.D. Reduction of permeability in granite at elevated temperatures. Science 1994, 265, 1558–1561. [Google Scholar] [CrossRef] [Green Version]
- Morrow, C.A.; Moore, D.E.; Lockner, D.A. Permeability reduction in granite under hydrothermal conditions. J. Geophys. Res. 2001, 106, 30551–30560. [Google Scholar] [CrossRef]
- Elsworth, D.; Yasuhara, H. Short-timescale chemo-mechanical effects and their influence on the transport properties of fractured rock. Pure Appl. Geophys. 2006, 163, 2051–2070. [Google Scholar] [CrossRef] [Green Version]
- Yasuhara, H.; Elsworth, D. Compaction of a rock fracture moderated by competing roles of stress corrosion and pressure solution. Pure Appl. Geophys. 2008, 165, 1289–1306. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, L.; Heap, M.J.; Wang, F.; Daval, D.; Gilg, H.A.; Baud, P.; Schmittbuhl, J.; Genter, A. Geothermal implications for fracture-filling hydrothermal precipitation. Geothermics 2016, 64, 235–245. [Google Scholar] [CrossRef]
- Aben, F.; Doan, M.L.; Gratier, J.P.; Renard, F. Experimental postseismic recovery of fractured rocks assisted by calcite sealing. Geophys. Res. Lett. 2017, 44, 7228–7238. [Google Scholar] [CrossRef] [Green Version]
- Rutter, E. Pressure solution in nature, theory and experiment. J. Geol. Soc. 1983, 140, 725–740. [Google Scholar] [CrossRef]
- Anderson, O.L.; Grew, P.C. Stress corrosion theory of crack propagation with applications to geophysics. Rev. Geophys. 1977, 15, 77–104. [Google Scholar] [CrossRef]
- Atkinson, B.K. Subcritical crack growth in geological materials. J. Geophys. Res. 1984, 89, 4077–4114. [Google Scholar] [CrossRef]
- Brantut, N.; Heap, M.; Meredith, P.; Baud, P. Time-dependent cracking and brittle creep in crustal rocks: A review. J. Struct. Geol. 2013, 52, 17–43. [Google Scholar] [CrossRef]
- Cheng, C.; Milsch, H. Permeability variations in illite-bearing sandstone: Effects of temperature and NaCl fluid salinity. J. Geophys. Res. Solid Earth 2020, 125, 9. [Google Scholar] [CrossRef]
- Farough, A.; Moore, D.E.; Lockner, D.A.; Lowell, R. Evolution of fracture permeability of ultramafic rocks undergoing serpentinization at hydrothermal conditions: An experimental study. Geochem. Geophy. Geosyst. 2016, 17, 44–55. [Google Scholar] [CrossRef]
- Hilgers, C.; Tenthorey, E. Fracture sealing of quartzite under a temperature gradient: Experimental results. Terra Nova 2004, 16, 173–178. [Google Scholar] [CrossRef]
- Sanchez-Roa, C.; Saldi, G.D.; Mitchell, T.M.; Iacoviello, F.; Bailey, J.; Shearing, P.R.; Oelkers, E.H.; Meredith, P.G.; Jones, A.P.; Striolo, A. The role of fluid chemistry on permeability evolution in granite: Applications to natural and anthropogenic systems. Earth Planet. Sci. Lett. 2021, 533, 116641. [Google Scholar] [CrossRef]
- Kamali-Asl, A.; Ghazanfari, E.; Perdrial, N.; Cladouhos, T. Effects of injection fluid type on pressure-dependent permeability evolution of fractured rocks in geothermal reservoirs: An experimental chemo-mechanical study. Geothermics 2020, 87, 101832. [Google Scholar] [CrossRef]
- Fazeli, H.; Vandeginste, V.; Rabbani, A.; Babaei, M.; Muljadi, B. Pore-scale modeling of fluid–rock chemical interactions in shale during hydraulic fracturing. Energy Fuels 2021, 35, 13. [Google Scholar] [CrossRef]
- Farquharson, J.I.; Kushnir, A.R.; Wild, B.; Baud, P. Physical property evolution of granite during experimental chemical stimulation. Geotherm. Energy 2020, 8, 1–24. [Google Scholar] [CrossRef]
- Durham, W.B.; Bourcier, W.L.; Burton, E.A. Direct observation of reactive flow in a single fracture. Water Resour. Res. 2001, 37, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.G.; Vogler, D.; Querci, L.; Madonna, C.; Hattendorf, B.; Saar, M.O.; Kong, X.Z. Thermally driven fracture aperture variation in naturally fractured granites. Geotherm. Energy 2019, 7, 1–28. [Google Scholar] [CrossRef]
- Lu, R.; Watanabe, N.; He, W.; Jang, E.; Shao, H.; Kolditz, O.; Shao, H. Calibration of water–granite interaction with pressure solution in a flow-through fracture under confining pressure. Environ. Earth Sci. 2017, 76, 12. [Google Scholar] [CrossRef]
- Lu, R.; Nagel, T.; Shao, H.; Kolditz, O.; Shao, H. Modeling of dissolution-induced permeability evolution of a granite fracture under crustal conditions. J. Geophys. Res.-Sol. Earth 2018, 123, 5609–5627. [Google Scholar] [CrossRef]
- Dewers, T.; Hajash, A. Rate laws for water-assisted compaction and stress-induced water-rock interaction in sandstones. J. Geophys. Res. 1995, 100, 13093–13112. [Google Scholar] [CrossRef]
- Bijay, K.; Ghazanfari, E. Geothermal reservoir stimulation through hydro-shearing: An experimental study under conditions close to enhanced geothermal systems. Geothermics 2021, 96, 102200. [Google Scholar] [CrossRef]
- Ishibashi, T.; McGuire, T.P.; Watanabe, N.; Tsuchiya, N.; Elsworth, D. Permeability evolution in carbonate fractures: Competing roles of confining stress and fluid pH. Water Resour. Res. 2013, 49, 2828–2842. [Google Scholar] [CrossRef]
- Milsch, H.; Spangenberg, E.; Kulenkampff, J.; Meyhöfer, S. A new apparatus for long-term petrophysical investigations on geothermal reservoir rocks at simulated in-situ conditions. Transp. Porous Med. 2008, 74, 73–85. [Google Scholar] [CrossRef] [Green Version]
- Kestin, J.; Sokolov, M.; Wakeham, W.A. Viscosity of liquid water in the range −8 °C to 150 °C. J. Phys. Chem. Ref. Data 1978, 7, 941–948. [Google Scholar] [CrossRef] [Green Version]
- Kestin, J.; Khalifa, H.E.; Correia, R.J. Tables of the dynamic and kinematic viscosity of aqueous NaCl solutions in the temperature range 20–150 °C and the pressure range 0.1–35 MPa. J. Phys. Chem. Ref. Data 1981, 10, 71–88. [Google Scholar] [CrossRef]
- Witherspoon, P.A.; Wang, J.S.; Iwai, K.; Gale, J.E. Validity of cubic law for fluid flow in a deformable rock fracture. Water Resour. Res. 1980, 16, 1016–1024. [Google Scholar] [CrossRef] [Green Version]
- Zimmerman, R.W.; Bodvarsson, G.S. Hydraulic conductivity of rock fractures. Transp. Porous Med. 1996, 23, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Gratier, J.P.; Renard, F.; Vial, B. Postseismic pressure solution creep: Evidence and time-dependent change from dynamic indenting experiments. J. Geophys. Res.-Sol. Earth 2014, 119, 2764–2779. [Google Scholar] [CrossRef]
- Ouyang, Z.; Elsworth, D. Evaluation of groundwater flow into mined panels. Int. J. Rock Mech. Min. Sci. 1993, 30, 71–79. [Google Scholar] [CrossRef]
- You, Z.; Badalyan, A.; Yang, Y.; Bedrikovetsky, P.; Hand, M. Fines migration in geothermal reservoirs: Laboratory and mathematical modelling. Geothermics 2019, 77, 344–367. [Google Scholar] [CrossRef]
- Bedrikovetsky, P.; Siqueira, F.D.; Furtado, C.A.; Souza, A.L.S. Modified particle detachment model for colloidal transport in porous media. Transp. Porous Med. 2011, 86, 353–383. [Google Scholar] [CrossRef]
- Dove, P.M. The dissolution kinetics of quartz in aqueous mixed cation solutions. Geochim. Cosmochim. Acta 1999, 63, 3715–3727. [Google Scholar] [CrossRef]
- Dove, P.M.; Crerar, D.A. Kinetics of quartz dissolution in electrolyte solutions using a hydrothermal mixed flow reactor. Geochim. Cosmochim. Acta 1990, 54, 955–969. [Google Scholar] [CrossRef]
- Ruiz-Agudo, E.; Kowacz, M.; Putnis, C.; Putnis, A. The role of background electrolytes on the kinetics and mechanism of calcite dissolution. Geochim. Cosmochim. Acta 2010, 74, 1256–1267. [Google Scholar] [CrossRef]
- Moore, D.E.; Morrow, C.; Byerlee, J. Chemical reactions accompanying fluid flow through granite held in a temperature gradient. Geochim. Cosmochim. Acta 1983, 47, 445–453. [Google Scholar] [CrossRef]
- Morrow, C.; Lockner, D.; Moore, D.; Byerlee, J. Permeability of granite in a temperature gradient. J. Geophys. Res. 1981, 86, 445–453. [Google Scholar] [CrossRef]
- Rimstidt, J.D.; Barnes, H. The kinetics of silica-water reactions. Geochim. Cosmochim. Acta 1980, 44, 1683–1699. [Google Scholar] [CrossRef]
- Brantley, S.L. Kinetics of Mineral Dissolution. In Kinetics of Water-Rock Interaction; Springer: New York, NY, USA, 2008; pp. 151–210. [Google Scholar]


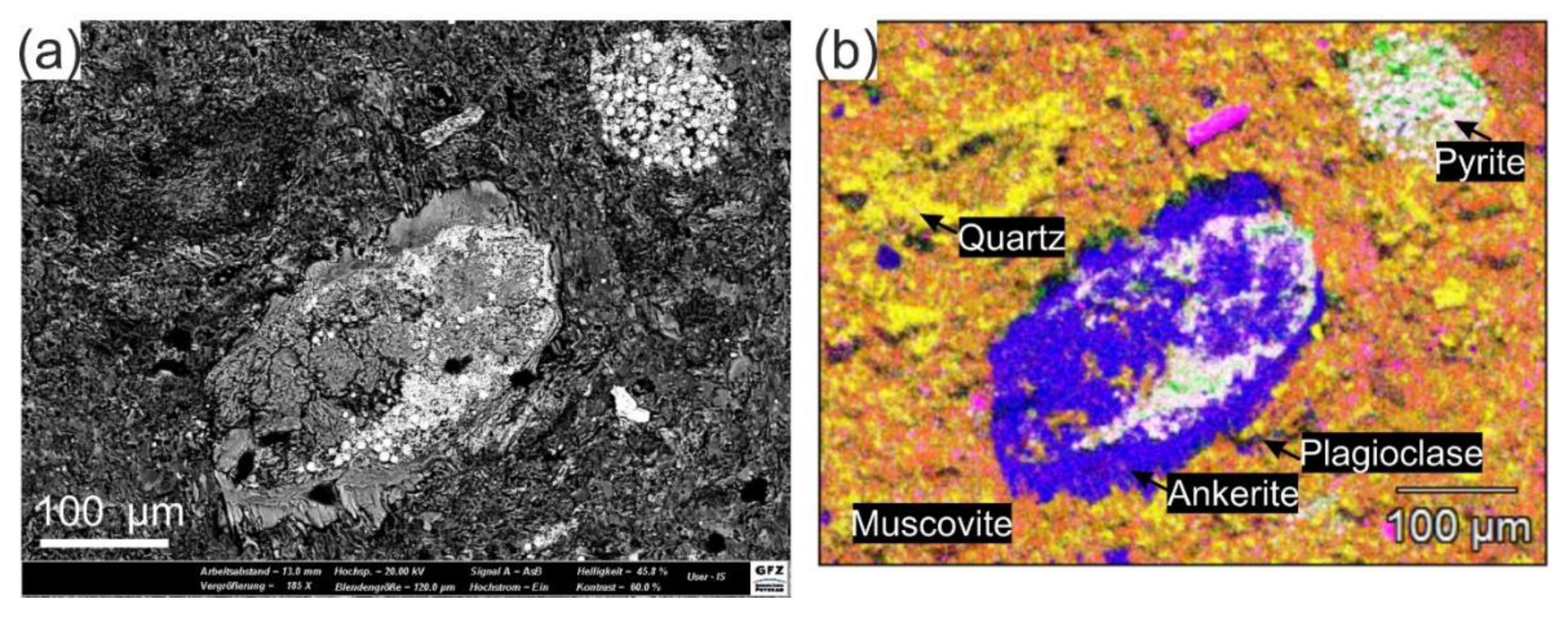

| Mineral Content (wt %) | Quartz | Muscovite | Chlorite | Plagioclase | Chalcocite | Ankerite | Pyrite |
|---|---|---|---|---|---|---|---|
| SM1 | 36 | 33 | 12 | 8 | 5 | 5 | 1 |
| SM2 | 36 | 35 | 11 | 8 | 5 | 4 | 1 |
| Sample | Temperature (°C) | Flow Rate a (mL/min) | Flow Type | Duration b (Days) | Permeant Fluid |
|---|---|---|---|---|---|
| SM1 | Room temperature | 0.3~0.5 | Continuous | ~3 | DI |
| 25 → 50 → 70 → 90 → 70 → 50 → 32 → 90 | 0.3~0.5 | Intermittent | 3 | ||
| 90 | 0.05~0.3 | Intermittent | 34 | ||
| SM2 | Room temperature | 0.3~0.5 | Continuous | <1 | DI |
| 25 → 50 → 70 → 50 → 32 → 70 | 0.3~0.5 | Intermittent | 3 | ||
| 70 | 0.1~0.3 | Intermittent | 34 | ||
| SM3 | Room temperature | 0.02~0.3 | Continuous | ~3 | 0.5 M NaCl |
| 25 → 50 → 70 → 90 → 70 → 50 → 32 → 90 | 0.02~0.1 | Intermittent | 3 | ||
| 90 | 0.1 | Intermittent | 34 |
| Sample | Max T (°C) | kf (Initial) (10−12 m2) | kf (Final) (10−12 m2) | Duration (Days) | Fluid |
|---|---|---|---|---|---|
| SM1 | 90 | 4.76 | 1.02 | 40 | DI |
| SM2 | 70 | 4.98 | 2.66 | 38 | DI |
| SM3 | 90 | 3.49 | 2.01 | 40 | 0.5 M NaCl |
| Parameters | SM1_A | SM1_B | SM2_A | SM2_B | SM3_A | SM3_B | Stage |
|---|---|---|---|---|---|---|---|
| Rp (µm) | 41.18 | 51.08 | 78.13 | 80.97 | 24.00 | 119.26 | Before |
| 65.45 | 70.98 | 78.57 | 116.80 | 52.96 | 106.55 | After | |
| Rm (µm) | 2.35 | 1.82 | 10.99 | 1.46 | 1.56 | 10.31 | Before |
| 3.52 | 1.78 | 10.69 | 3.47 | 1.98 | 9.12 | After | |
| Rrms (µm) | 3.00 | 2.41 | 14.31 | 2.16 | 2.05 | 17.94 | Before |
| 4.33 | 2.44 | 13.69 | 4.93 | 2.60 | 16.27 | After |
| Sample | k0 | α/b0 | n | Adjusted R2 |
|---|---|---|---|---|
| SM1 | 1.95 × 10−15 | 7.3 × 10−2 | 0.43 | 0.99 |
| SM2 | 2.0 × 10−15 | 4.5 × 10−2 | 0.61 | 0.99 |
| SM3 | 1.13 × 10−14 | 0.6 | 0.072 | 0.98 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, C.; Herrmann, J.; Wagner, B.; Leiss, B.; Stammeier, J.A.; Rybacki, E.; Milsch, H. Long-Term Evolution of Fracture Permeability in Slate: An Experimental Study with Implications for Enhanced Geothermal Systems (EGS). Geosciences 2021, 11, 443. https://doi.org/10.3390/geosciences11110443
Cheng C, Herrmann J, Wagner B, Leiss B, Stammeier JA, Rybacki E, Milsch H. Long-Term Evolution of Fracture Permeability in Slate: An Experimental Study with Implications for Enhanced Geothermal Systems (EGS). Geosciences. 2021; 11(11):443. https://doi.org/10.3390/geosciences11110443
Chicago/Turabian StyleCheng, Chaojie, Johannes Herrmann, Bianca Wagner, Bernd Leiss, Jessica A. Stammeier, Erik Rybacki, and Harald Milsch. 2021. "Long-Term Evolution of Fracture Permeability in Slate: An Experimental Study with Implications for Enhanced Geothermal Systems (EGS)" Geosciences 11, no. 11: 443. https://doi.org/10.3390/geosciences11110443
APA StyleCheng, C., Herrmann, J., Wagner, B., Leiss, B., Stammeier, J. A., Rybacki, E., & Milsch, H. (2021). Long-Term Evolution of Fracture Permeability in Slate: An Experimental Study with Implications for Enhanced Geothermal Systems (EGS). Geosciences, 11(11), 443. https://doi.org/10.3390/geosciences11110443






