Distribution of Trace Elements Controlled by Sector and Growth Zonings in Zircon from Feldspathic Pegmatites (Ilmen Mountains, the Southern Urals)
Abstract
1. Introduction
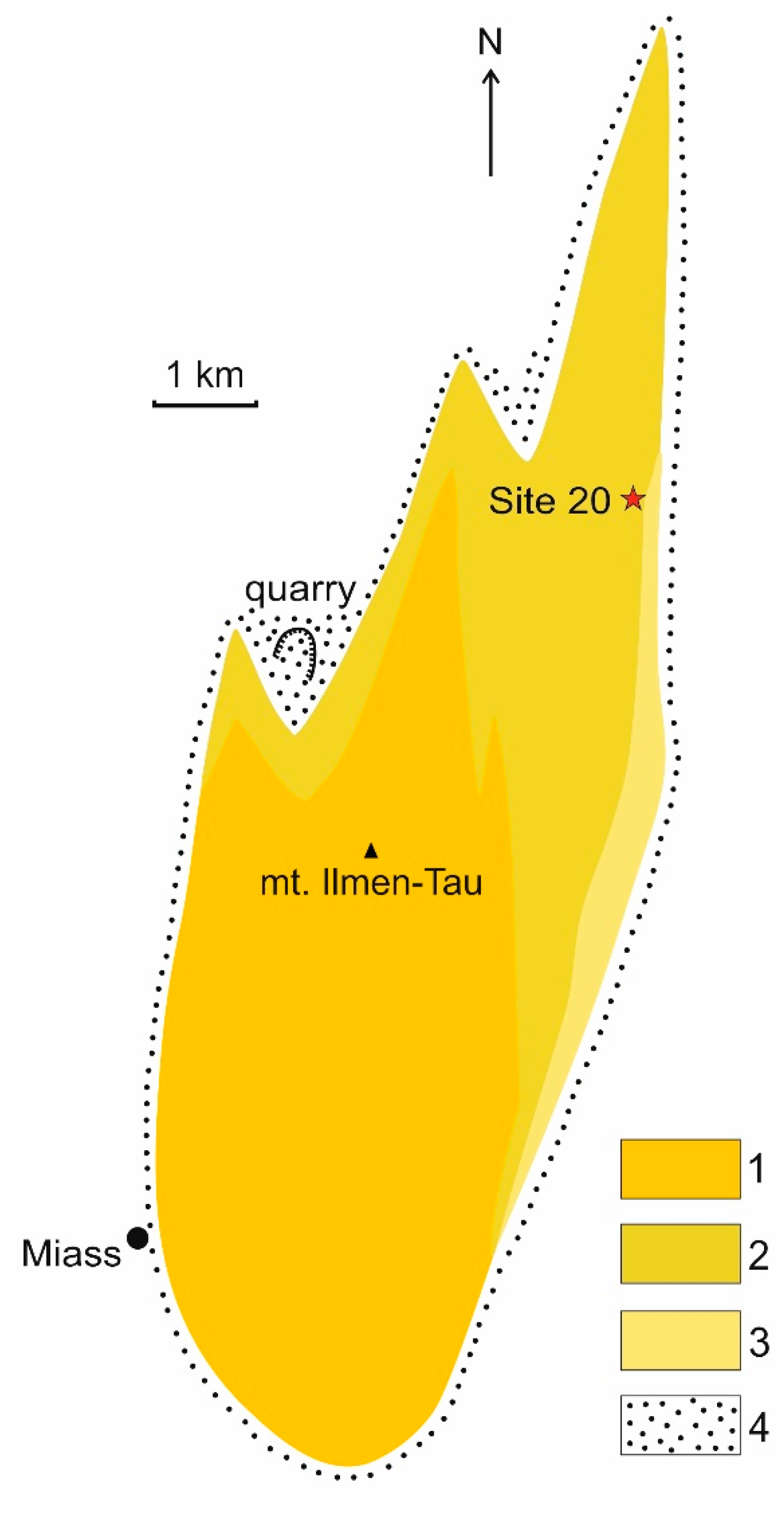
2. Geological Setting
3. Materials and Methods
4. Results
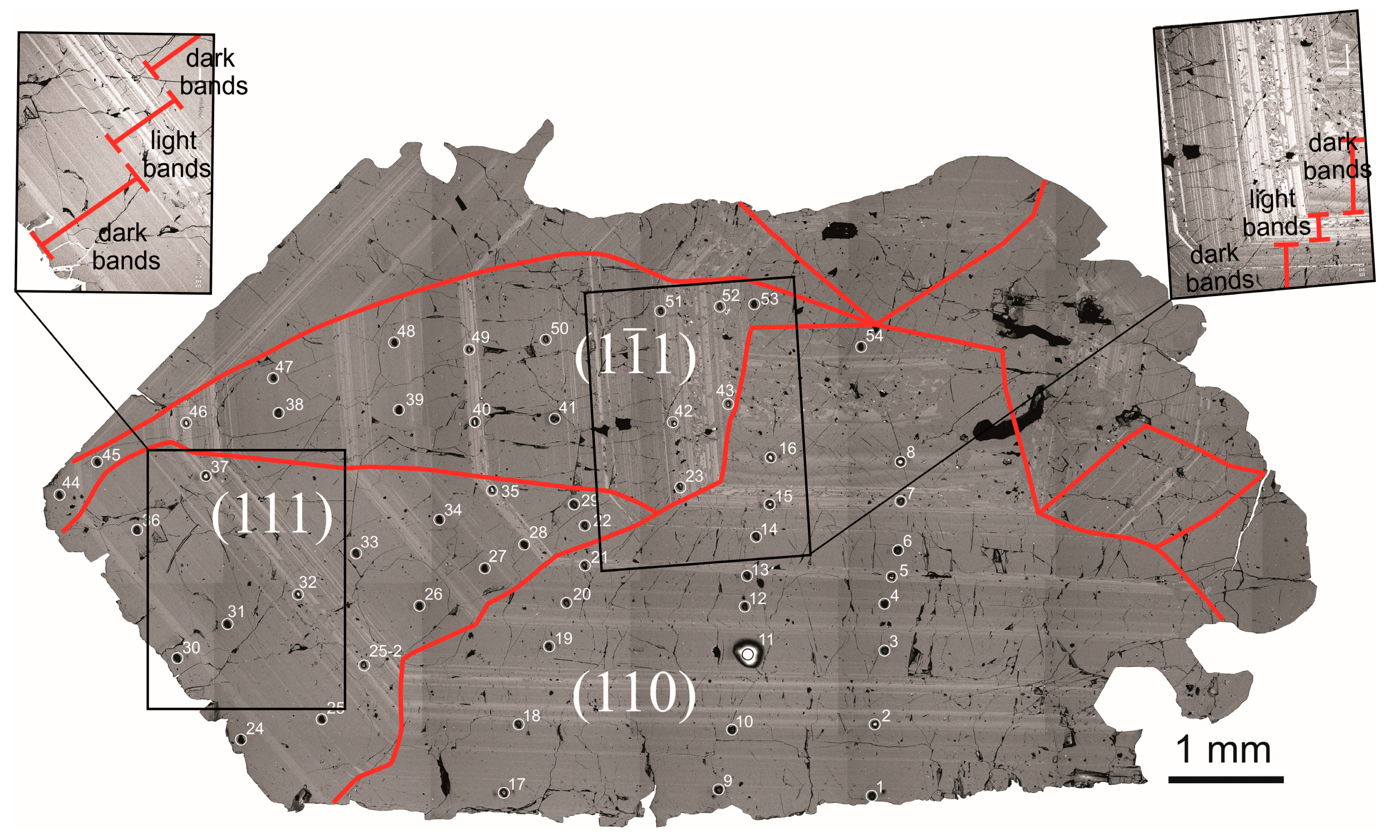
4.1. Internal Zircon Structure
4.2. General REE Distribution Pattern
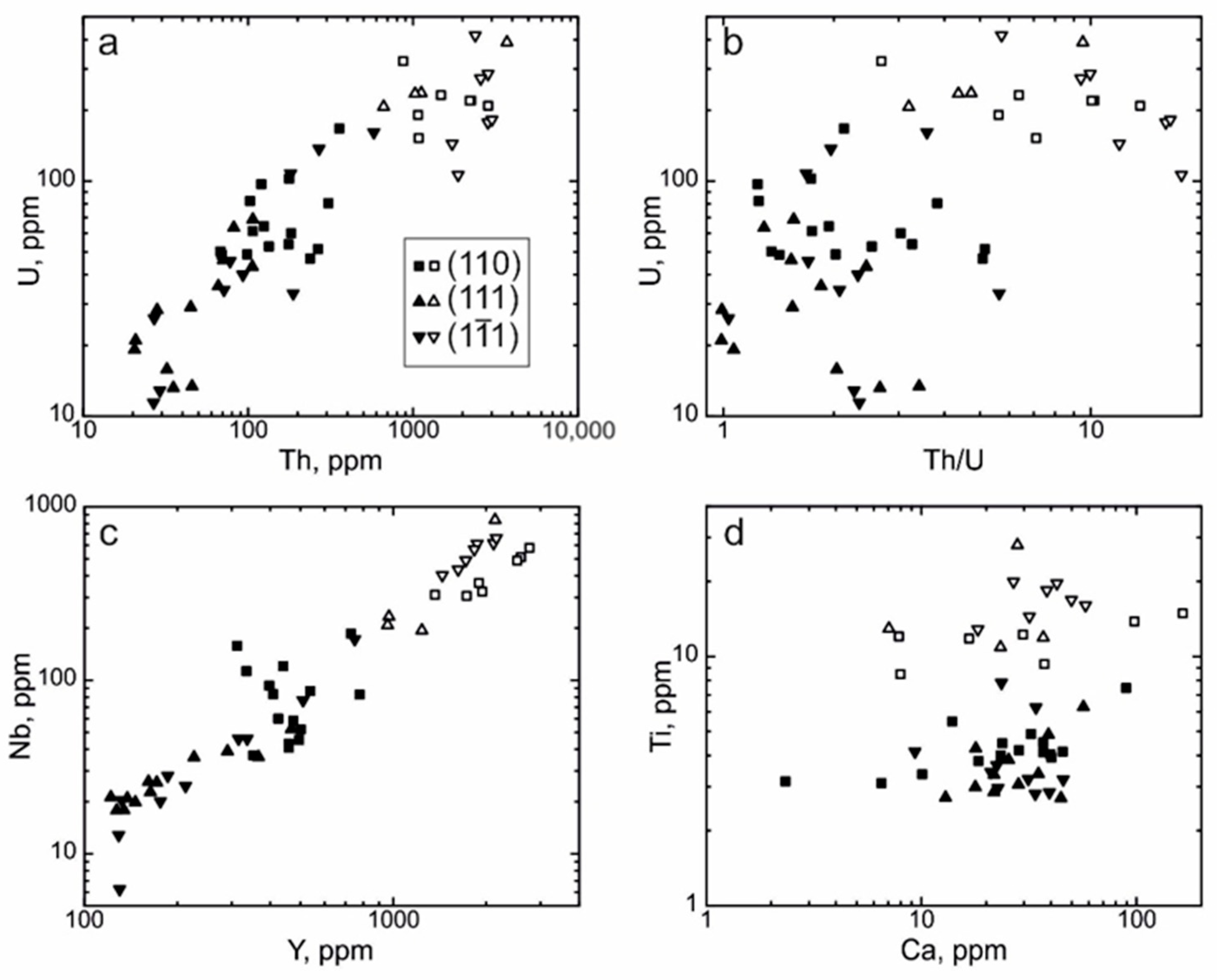
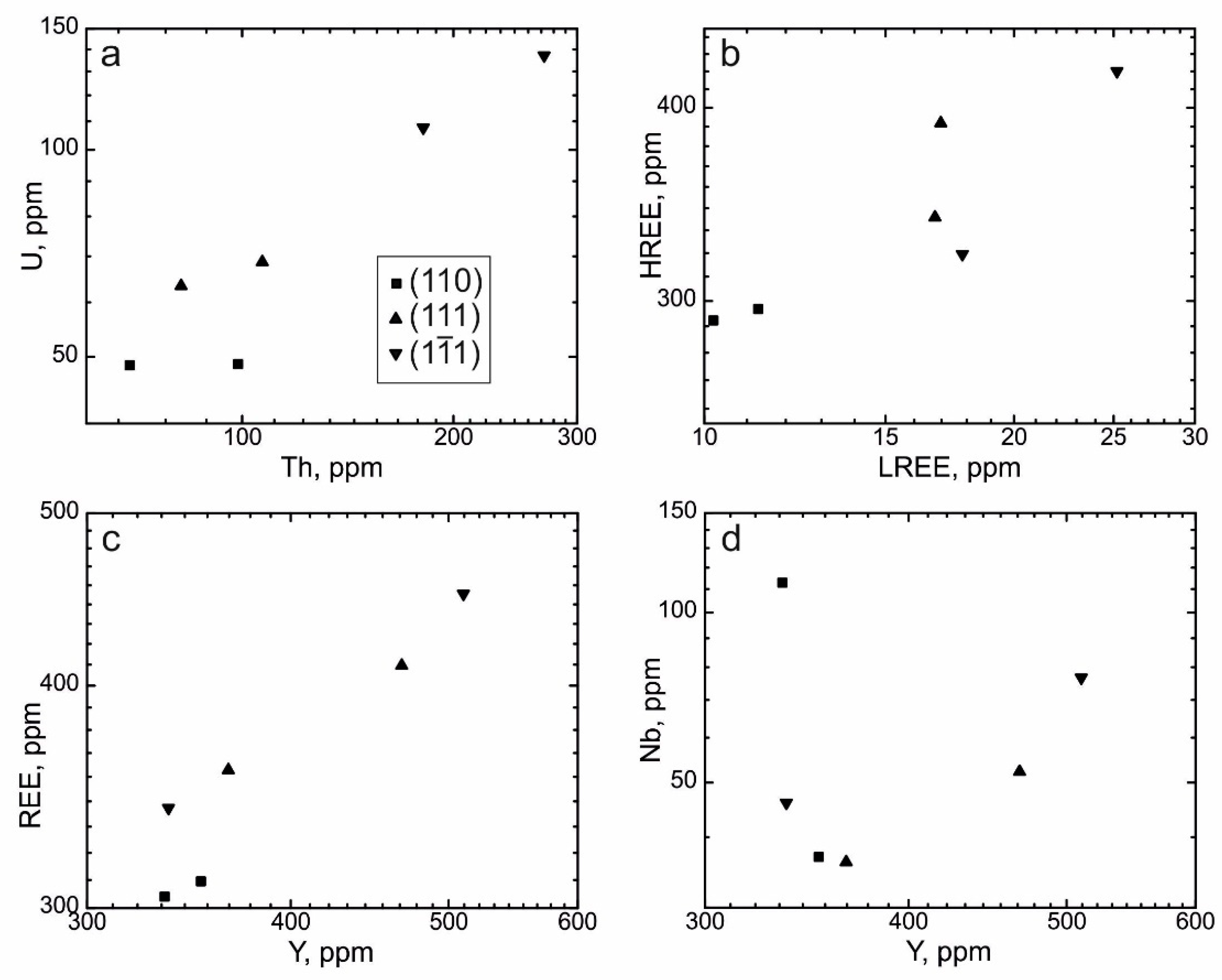
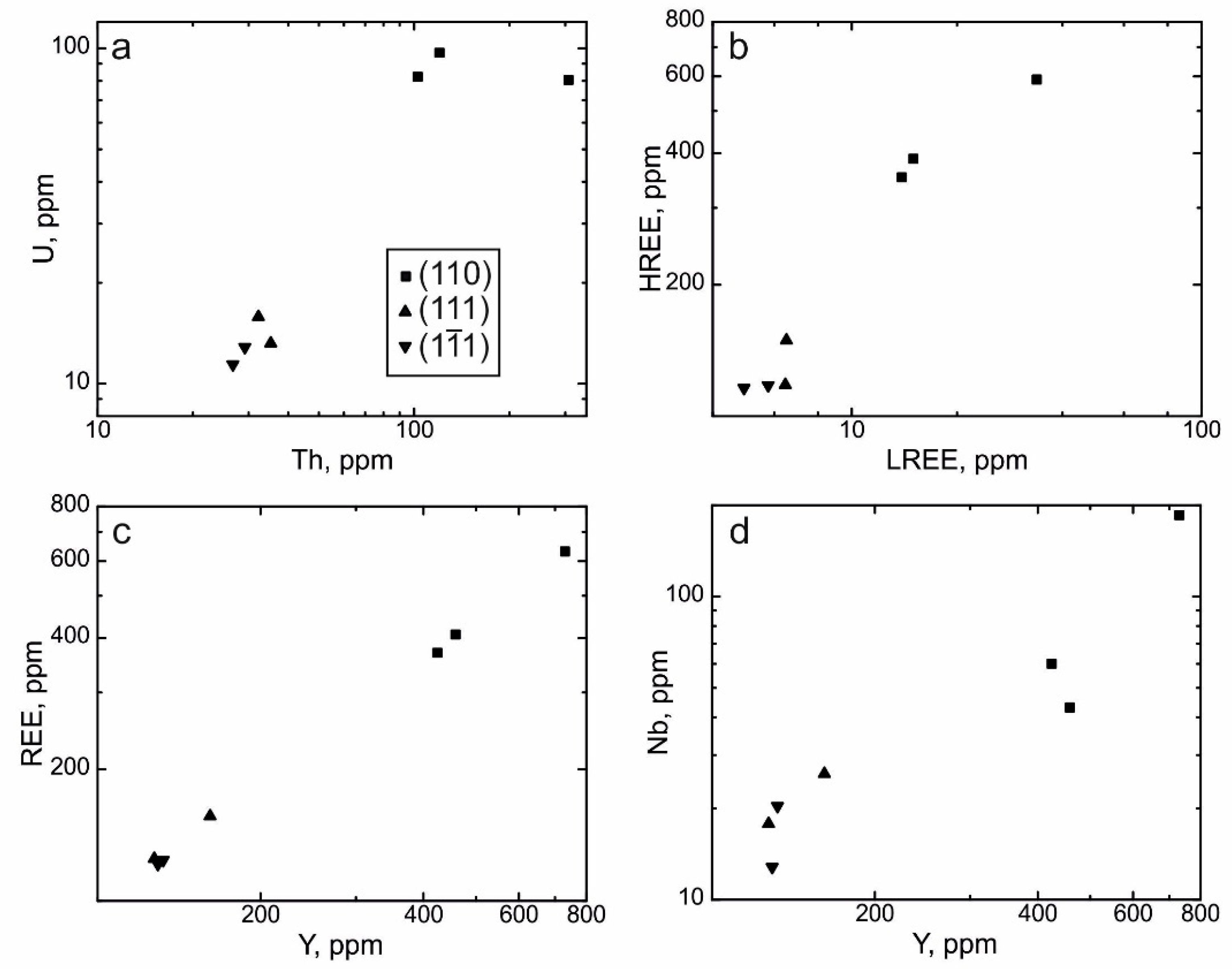
4.3. REE Distribution in the Separate Growth Sectors of the Dipyramid {111} and the Prism {110}
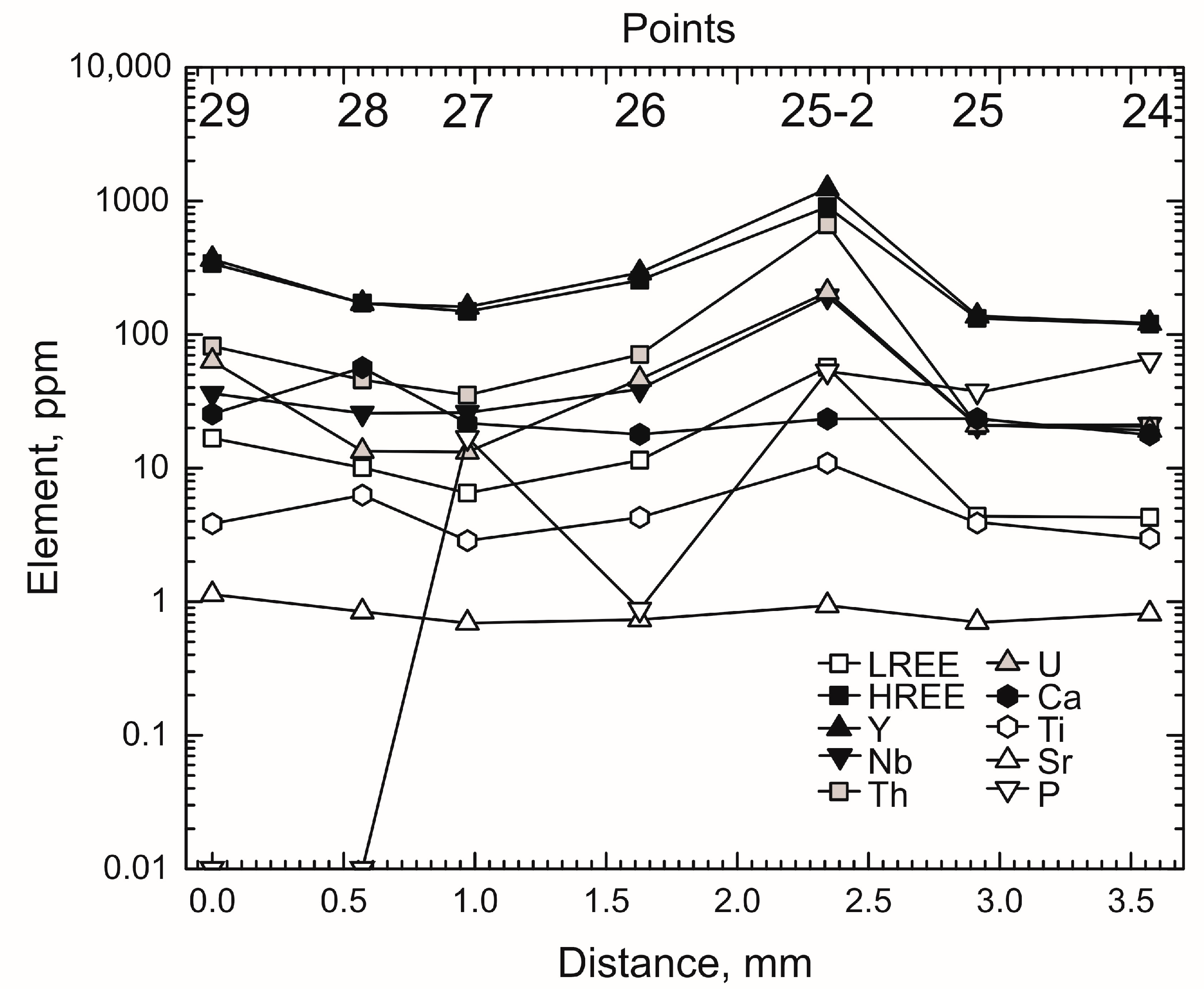
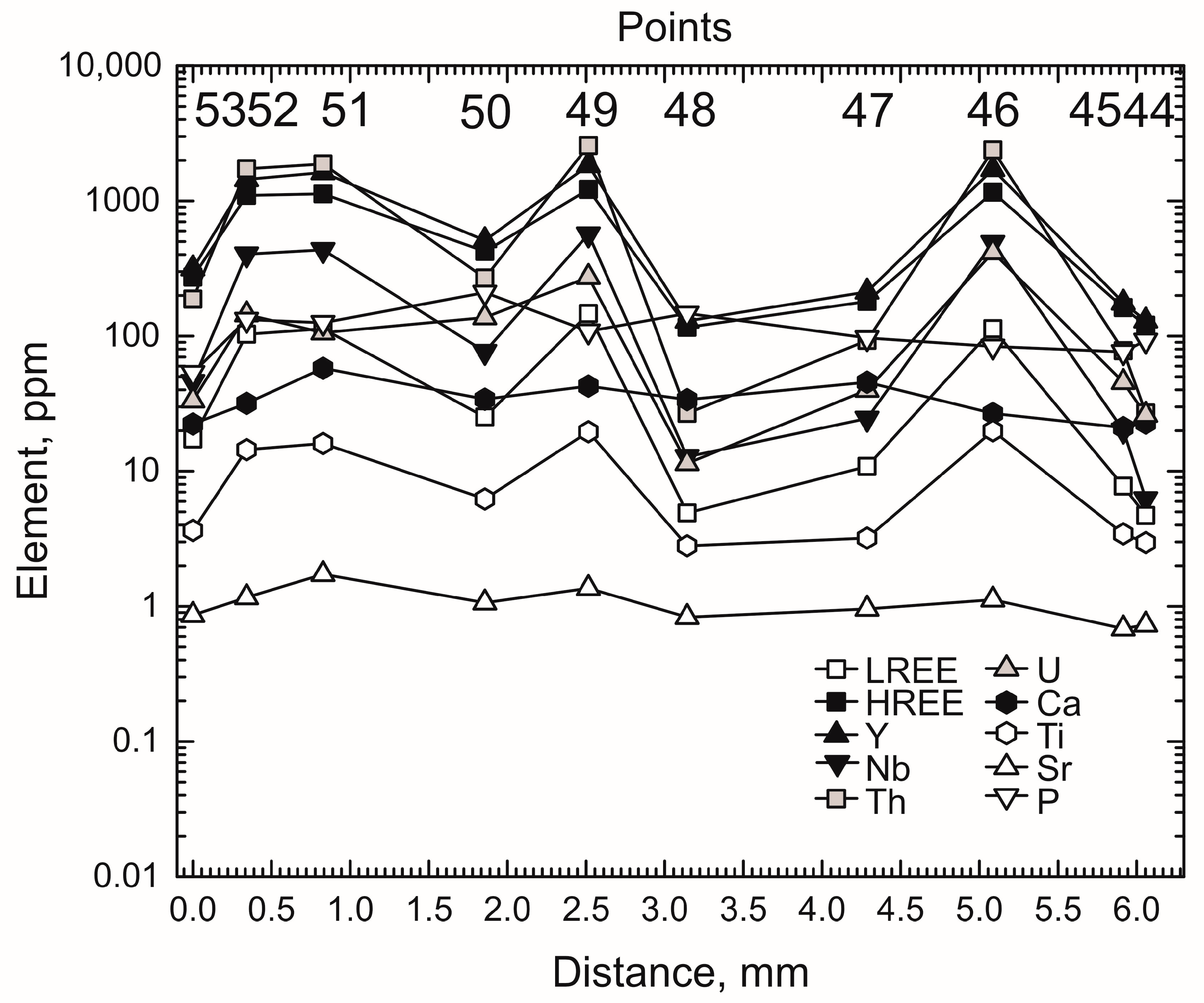
4.4. Trace Elements Distribution in Growth Zones
5. Discussion
6. Conclusions
- The zircon studied displays zoning formed of alternating wide BSE-dark and thin BSE-light bands. The last ones contain elevated Th, U, REE, Y, Nb, and Ti concentrations, Th/U ratio and Ce/Ce*. The zoning is most probably due to combined crystallization of minerals that concentrate trace elements, e.g., apatite and monazite, and the lack of equilibrium between zircon and fluid (melt).
- Zircon from the growth sector of the prism {110} generally contains more Th, U, REE, Y, and Nb and exhibits a more gently sloping HREE distribution pattern and a steeper LREE distribution pattern, in contrast to zircon from the growth sector of the dipyramid {111}. Concentrations of other elements in zircon from these two forms are roughly identical.
- Such a sector zoning pattern was formed at a late stage in crystal growth, when the prism {110} began to prevail over the dipyramid {111}. At an early crystallization stage, the prism and the dipyramid grew at comparable speed, but zircon from the prism contained less Th, U, REE, and Y.
- Considerable variations in Th, U, and Ti concentrations and Th/U ratio, the characteristics widely used for assessment of the conditions of formation of zircon and capable of affecting age dating, were revealed within one zircon crystal. Therefore, the possible growth and sector zoning of zircon should be taken into account in U-Pb dating studies and in the designing of geothermometers, geobarometers, and other indicators.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Watson, E.B.; Liang, Y. A simple model for sector zoning in slowly grown crystals: Implications for growth rate and lattice diffusion, with emphasis on accessory minerals in crystal rocks. Am. Mineral. 1995, 80, 1179–1187. [Google Scholar] [CrossRef]
- Watson, E.B. Surface enrichment and trace-element uptake during crystal growth. Geochim. Cosmochim. Acta 1996, 60, 5013–5020. [Google Scholar] [CrossRef]
- Dowty, E. Crystal structure and crystal growth: II. Sector zoning in minerals. Am. Mineral. 1976, 61, 460–469. [Google Scholar]
- Reeder, R.J.; Rakovan, J. Surface structural controls on trace element incorporation during crystal growth. In Growth, Dissolution and Pattern-Formation in Geosystems; Jamtveit, B., Meakin, P., Eds.; Kluwer Academic Publishers: Norwell, MA, USA, 1999; pp. 143–162. [Google Scholar]
- Hughes, J.M.; Cameron, M.; Mariano, A.N. Rare-earth-element ordering and structural variations in natural rare-earth-bearing apatites. Am. Mineral. 1991, 76, 1165–1173. [Google Scholar]
- Rakovan, J.; Reeder, R.I. Differential incorporation of trace elements and dissymmetrization in apatite: The role of surface structure during growth. Am. Mineral. 1994, 79, 892–903. [Google Scholar]
- Rakovan, J.; Reeder, R.J. Intracrystalline rare earth element distributions in apatite: Surface structural influences on zoning during growth. Geochim. Cosmochim. Acta 1996, 60, 4435–4445. [Google Scholar] [CrossRef]
- Rakovan, J.; McDaniel, D.K.; Reeder, R.J. Use of surface-controlled REE sectoral zoning in apatite from Llallagua, Bolivia, to determine a single-crystal Sm-Nd age. Earth Planet. Sci. Lett. 1997, 146, 329–336. [Google Scholar] [CrossRef]
- Rakovan, J.; Luo, Y.; Borkiewicz, O. Synchrotron microanalytical methods in the study of trace and minor elements in apatite. Mineralogia 2008, 39, 31–40. [Google Scholar] [CrossRef]
- Paterson, B.A.; Stephens, W.E.; Herd, D.A. Zoning in granitoid accessory minerals as revealed by backscattered electron imagery. Mineral. Mag. 1989, 53, 55–61. [Google Scholar] [CrossRef]
- Bosze, S.; Rakovan, J. Surface structure controlled sectoral zoning of the rare-earth elements in fluorite from Long Lake, N.Y. and Bingham, N.M. Geochim. Cosmochim. Acta 2002, 66, 997–1009. [Google Scholar] [CrossRef]
- Repina, S.A. Fractionation of REE in the xenotime and florencite paragenetic association from Au-REE mineral occurrences of the near-Polar Urals. Geochem. Int. 2011, 49, 868–887. [Google Scholar] [CrossRef]
- Repina, S.A.; Khiller, V.V.; Makagonov, E.P. Microheterogeneity of crystal growth zones as a result of REE fractionation. Geochem. Int. 2014, 52, 1057–1071. [Google Scholar] [CrossRef]
- Vavra, G. On the kinematics of zircon growth and its petrogenetic significance: A cathodoluminescence study. Contrib. Mineral. Petrol. 1990, 106, 90–99. [Google Scholar] [CrossRef]
- Vavra, G.; Gebauer, D.; Schmid, R.; Compston, W. Multiple zircon growth and recrystallization during polyphase Late Carboniferous to Triassic metamorphism in granulites of the Ivrea Zone (Southern Alps): An ion microprobe (SHRIMP) study. Contrib. Mineral. Petrol. 1996, 122, 337–358. [Google Scholar] [CrossRef]
- Sturm, R. Morphology and growth trends of accessory zircons from various granitoids of the South-western Bohemian Massif (Moldanubicum, Austria). Geochemistry 2010, 70, 185–196. [Google Scholar] [CrossRef]
- Sturm, R. Internal morphology and crystal growth of accessory zircon from igneous rocks. In Zircon and Olivine; van Dijk, G., van den Berg, V., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2012; pp. 37–65. [Google Scholar]
- Paquette, I.; Reeder, R.I. Relationship between surface structure, growth mechanism, and trace element incorporation in calcite. Geochim. Cosmochim. Acta 1995, 59, 735–749. [Google Scholar] [CrossRef]
- Kukui, A.L.; Skublov, S.G. Geochemistry of rare earth elements in Iceland spars from deposits in the Siberian Craton. Dokl. Earth Sci. 2008, 418, 109–113. [Google Scholar] [CrossRef]
- Schwandt, C.S.; McKay, G.A. Minor- and trace-element sector zoning in synthetic enstatite. Am. Mineral. 2006, 91, 1607–1615. [Google Scholar] [CrossRef]
- Lofgren, G.E.; Huss, G.R.; Wasserburg, G.J. An experimental study of trace-element partitioning between Ti-Al-clinopyroxene and melt: Equilibrium and kinetic effects including sector zoning. Am. Mineral. 2006, 91, 1596–1606. [Google Scholar] [CrossRef]
- Stowell, H.; Zuluaga, C.S.; Boyle, A.; Bulman, G. Garnet sector and oscillatory zoning linked with changes in crystal morphology during rapid growth, North Cascades, Washington. Am. Mineral. 2011, 96, 1354–1362. [Google Scholar] [CrossRef]
- Trail, D. Heterogeneous distribution of trace elements in zircon. In Proceedings of the Goldschmidt 2014, Sacramento, CA, USA, 8–13 June 2014; p. 2510. [Google Scholar]
- Benisek, A.; Finge, F. Factors controlling the development of prism faces in granite zircons: A microprobe study. Contrib. Mineral. Petrol. 1993, 114, 441–451. [Google Scholar] [CrossRef]
- Pupin, J.P. Zircon and granite petrology. Contrib. Mineral. Petrol. 1980, 73, 207–220. [Google Scholar] [CrossRef]
- Pupin, J.P. Granites as indicators in paleogeodynamics. Rend. Soc. Ital. Mineral. Petrol. 1988, 43, 237–262. [Google Scholar]
- De Hoog, J.C.M.; Lissenberg, C.J.; Brooker, R.A.; Hinton, R.; Trail, D.; Hellebrand, E. Hydrogen incorporation and charge balance in natural zircon. Geochim. Cosmochim. Acta 2014, 141, 472–486. [Google Scholar] [CrossRef]
- Burnham, A.D. Key concepts in interpreting the concentrations of the rare earth elements in zircon. Chem. Geol. 2020, 551, 119765. [Google Scholar] [CrossRef]
- Kapustin, Y.L. Distribution of trace elements in accessory zircon of various generations from pegmatites. Geochemistry 1985, 4, 514–528. (In Russian) [Google Scholar]
- Popova, V.I. Identification of elemental and isotopic zoning and sectoriality of large crystals of minerals using neutron radiography. Mineralogy 2015, 1, 25–33. (In Russian) [Google Scholar]
- Nedosekova, I.L.; Vladykin, N.V.; Pribavkin, S.V.; Bayanova, T.B. The Il’mensky-Vishnevogorsky miaskite-carbonatite complex, the Urals, Russia: Origin, ore resource potential, and sources. Geol. Ore Depos. 2009, 51, 139–162. [Google Scholar] [CrossRef]
- Popov, V.A.; Popova, V.I. Mineralogy of pegmatites of the Ilmen mountains. In Mineralogical Almanac; ECOST Association: Moscow, Russia, 2006; Volume 9, p. 152. (In Russian) [Google Scholar]
- Levin, V.Y.; Ronenson, B.M.; Samkov, V.S.; Levina, I.A.; Sergeev, N.S.; Kiselev, A.P. Alkaline-Carbonatite Complexes of the Urals; Uralgeolkom: Ekaterinburg, Russia, 1997; p. 274. (In Russian) [Google Scholar]
- Popova, V.I.; Chesnokov, B.V. Anatomy of zircon crystals from alkaline pegmatites of the Vishnevye mountains. In Ontogeny of Pegmatites of the Urals; USC USSR Academy of Sciences: Sverdlovsk, Russia, 1980; pp. 91–101. (In Russian) [Google Scholar]
- Popova, V.I. Zircons of the Ilmen mountains: Touches of history and the new data. In Scientific Relations between Germany and Russia for the Study of Ilmen Mountains; Institute of Mineralogy Uralian Branch of RAS: Miass, Russia, 2002; pp. 46–54. (In Russian) [Google Scholar]
- Krasnobaev, A.A.; Valizer, P.M.; Anfilogov, V.N.; Nemova, A.B.; Busharina, S.V. Zirconology of pegmatites of the Ilmeny Mountains. Dokl. Earth Sci. 2014, 457, 960–964. [Google Scholar] [CrossRef]
- Nedosekova, I.L.; Belyatsky, B.V. Age and substance sources of the Ilmeno-Vishnevogorsky alkaline complex (South Urals): Rb-Sr, Sm-Nd, U-Pb, and Lu-Hf isotope data. Dokl. Earth Sci. 2012, 446, 1071–1076. [Google Scholar] [CrossRef]
- Sturm, R. Imaging of growth banding of minerals using 2-stage sectioning: Application to accessory zircon. Micron 2004, 35, 681–684. [Google Scholar] [CrossRef] [PubMed]
- Hinton, R.W.; Upton, B.G.J. The chemistry of zircon: Variations within and between large crystals from syenite and alkali basalt xenoliths. Geochim. Cosmochim. Acta 1991, 55, 3287–3302. [Google Scholar] [CrossRef]
- Portnyagin, M.V.; Simakin, S.G.; Sobolev, A.V. Fluorine in primitive magmas of the Troodos Ophiolite complex, Cyprus: Analytical methods and main results. Geochem. Int. 2002, 40, 625–632. [Google Scholar]
- Fedotova, A.A.; Bibikova, E.V.; Simakin, S.G. Ion-microprobe zircon geochemistry as an indicator of mineral genesis during geochronological studies. Geochem. Int. 2008, 46, 912–927. [Google Scholar] [CrossRef]
- Dokukina, K.A.; Kaulina, T.V.; Konilov, A.N.; Mints, M.V.; Van, K.V.; Natapov, L.; Belousova, E.; Simakin, S.G.; Lepekhina, E.N. Archaean to Palaeoproterozoic high-grade evolution of the Belomorian eclogite province in the Gridino area, Fennoscandian Shield: Geochronological evidence. Gondwana Res. 2014, 25, 585–613. [Google Scholar] [CrossRef]
- Jochum, K.P.; Dingwell, D.B.; Rocholl, A.; Stoll, B.; Hofmann, A.W.; Becker, S.; Besmehn, A.; Bessette, D.; Dietze, H.-J.; Dulski, P.; et al. The preparation and preliminary characterisation of eight geological MPI-DING reference glasses for in-situ microanalysis. Geostand. Newsl. 2000, 24, 87–133. [Google Scholar] [CrossRef]
- Jochum, K.P.; Stoll, B.; Herwig, K.; Willbold, M.; Hofmiann, A.W.; Amini, M.; Aarburg, S.; Abouchami, W.; Hellebrand, E.; Mocek, B.; et al. MPI-DING reference glasses for in situ microanalysis: New reference values for element concentrations and isotope ratios. Geochem. Geophys. Geosyst. 2006, 7, 44. [Google Scholar] [CrossRef]
- Rocholl, A.B.E.; Simon, K.; Jochum, K.P.; Bruhn, F.; Gehann, R.; Kramar, U.; Luecke, W.; Molzahn, M.; Pernicka, E.; Seufert, M.; et al. Chemical characterisation of NIST silicate glass certified reference material SRM 610 by ICP-MS, TIMS, LIMS, SSMS, INAA, AAS and PIXE. Geostand. Newsl. 1997, 21, 101–114. [Google Scholar] [CrossRef]
- Bottazzi, P.; Ottolini, R.; Vannucci, A.; Zanetti, A. An accurate procedure for the quantification of rare earth elements in silicates. In SIMS IX Proceedings; Wileys: New York, NY, USA, 1994; pp. 927–930. [Google Scholar]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Watson, E.B.; Wark, D.A.; Thomas, J.B. Crystallization thermometers for zircon and rutile. Contrib. Mineral. Petrol. 2006, 151, 413–433. [Google Scholar] [CrossRef]
- Skublov, S.G.; Berezin, A.V.; Berezhnaya, N.G. General relations in the trace-element composition of zircons from eclogites with implications for the age of eclogites in the Belomorian Mobile Belt. Petrology 2012, 20, 427–449. [Google Scholar] [CrossRef]
- Corfu, F.; Hanchar, J.M.; Hoskin, P.W.O.; Kinny, P. Atlas of zircon textures. Rev. Mineral. Geochem. 2003, 53, 469–500. [Google Scholar] [CrossRef]
- Hoskin, P.W.O.; Schaltegger, U. The composition of zircon and igneous and metamorphic petrogenesis. Rev. Mineral. Geochem. 2003, 53, 27–62. [Google Scholar] [CrossRef]
- Nedosekova, I.L.; Belousova, E.A.; Belyatsky, B.V. Hf isotopes and trace elements as indicators of zircon genesis in the evolution of the alkaline-carbonatite magmatic system (Il’meno-Vishnevogorskii Complex, Urals, Russia). Dokl. Earth Sci. 2015, 461, 384–389. [Google Scholar] [CrossRef]
- Geisler, T.; Schaltegger, U.; Tomaschek, F. Re-equilibration of zircon in aqueous fluids and melts. Elements 2007, 3, 43–50. [Google Scholar] [CrossRef]
- Gagnevin, D.; Daly, J.S.; Kronz, A. Zircon texture and chemical composition as a guide to magmatic processes and mixing in a granitic environment and coeval volcanic system. Contrib. Mineral. Petrol. 2010, 159, 579–596. [Google Scholar] [CrossRef]
- Hoffman, J.F.; Long, J.V.P. Unusual sector zoning in Lewisian zircons. Mineral. Mag. 1984, 48, 513–517. [Google Scholar] [CrossRef]
- Chamberlain, K.J.; Wilson, C.J.N.; Wooden, J.L.; Charlier, B.L.A.; Ireland, T.R. New perspectives on the Bishop Tuff from zircon textures, ages and trace elements. J. Petrol. 2014, 55, 395–426. [Google Scholar] [CrossRef]
- Finch, R.J.; Hanchar, J.M.; Hoskin, P.W.O.; Burns, P.C. Rare-earth elements in synthetic zircon: Part 2. A singlecrystal X-ray study of xenotime substitution. Am. Mineral. 2001, 86, 681–689. [Google Scholar] [CrossRef]
- Burnham, A.D.; Berry, A.J. Formation of Hadean granites by melting of igneous crust. Nat. Geosci. 2017, 10, 457–461. [Google Scholar] [CrossRef]
- Skublov, S.G.; Marin, Y.B.; Galankina, O.L.; Simakin, S.G.; Myskova, T.M.; Astaf’ev, B.Y. The first discovery of abnormal (Y+REE)-enriched zircons in rocks of the Baltic Shield. Dokl. Earth Sci. 2011, 441, 1724–1731. [Google Scholar] [CrossRef]
- Hofmann, A.E.; Valley, J.W.; Watson, E.B.; Cavosie, A.J.; Eiler, J.M. Sub-micron scale distributions of trace elements in zircon. Contrib. Mineral. Petrol. 2009, 158, 317–335. [Google Scholar] [CrossRef]
- Hofmann, A.E.; Baker, M.B.; Eiler, J.M. Sub-micron-scale trace-element distributions in natural zircons of known provenance: Implications for Ti-in-zircon thermometry. Contrib. Mineral. Petrol. 2014, 168, 1057. [Google Scholar] [CrossRef]





| Dark Color | Light Color | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Prism | Dipyramid | (111) | All Points | Prism | Dipyramid | (111) | All Points | |||
| n = 15 | n = 22 | n = 12 | n = 10 | n = 37 | n = 7 | n = 11 | n = 4 | n = 7 | n = 18 | |
| La | 0.09 | 0.08 | 0.08 | 0.05 | 0.08 | 0.42 | 0.36 | 0.18 | 0.49 | 0.39 |
| Ce | 12.9 | 7.68 | 6.44 | 9.59 | 11.0 | 149 | 102 | 55.5 | 132 | 130 |
| Pr | 0.17 | 0.04 | 0.04 | 0.04 | 0.11 | 1.35 | 0.94 | 0.41 | 1.05 | 1.08 |
| Nd | 1.90 | 0.29 | 0.26 | 0.36 | 1.18 | 16.1 | 11.0 | 4.91 | 13.0 | 13.0 |
| Sm | 2.38 | 0.59 | 0.46 | 0.63 | 1.37 | 18.1 | 12.4 | 6.48 | 13.8 | 14.4 |
| Eu | 1.25 | 0.30 | 0.26 | 0.35 | 0.69 | 6.92 | 6.06 | 3.34 | 6.98 | 6.65 |
| Gd | 9.82 | 3.09 | 2.32 | 3.17 | 6.37 | 49.3 | 44.2 | 25.3 | 51.9 | 47.6 |
| Dy | 39.0 | 13.5 | 12.2 | 15.3 | 28.9 | 186 | 156 | 97.6 | 172 | 172 |
| Er | 89.9 | 35.4 | 33.2 | 39.2 | 68.6 | 357 | 302 | 204 | 309 | 327 |
| Yb | 207 | 94.3 | 84.0 | 97.4 | 159 | 719 | 568 | 427 | 585 | 667 |
| Lu | 35.7 | 16.9 | 15.1 | 17.5 | 27.3 | 116 | 94.0 | 71.0 | 94.8 | 108 |
| Li | 0.04 | 0.03 | 0.03 | 0.04 | 0.04 | 0.12 | 0.18 | 0.12 | 0.20 | 0.13 |
| P | 66.1 | 58.4 | 25.3 | 78.0 | 44.7 | 60.0 | 56.2 | 49.8 | 83.8 | 58.1 |
| Ca | 28.4 | 24.5 | 24.4 | 27.5 | 25.4 | 29.7 | 31.8 | 25.6 | 38.3 | 30.7 |
| Ti | 4.12 | 3.37 | 3.37 | 3.33 | 3.84 | 12.0 | 16.0 | 12.4 | 16.9 | 13.4 |
| Sr | 0.82 | 0.84 | 0.78 | 0.86 | 0.83 | 1.23 | 1.17 | 0.95 | 1.36 | 1.20 |
| Y | 458 | 174 | 163 | 199 | 335 | 1945 | 1718 | 1104 | 1831 | 1850 |
| Nb | 82.6 | 25.2 | 24.2 | 26.4 | 40.9 | 364 | 491 | 220 | 568 | 462 |
| Ba | 1.91 | 1.76 | 1.70 | 1.79 | 1.78 | 2.18 | 2.36 | 2.10 | 2.65 | 2.35 |
| Hf | 6867 | 7063 | 7124 | 7054 | 7004 | 6918 | 7381 | 7323 | 7381 | 7284 |
| Th | 134 | 68.4 | 45.3 | 85.3 | 98.7 | 1479 | 2385 | 1075 | 2569 | 2043 |
| U | 60.0 | 33.9 | 28.7 | 37.2 | 48.6 | 220 | 235 | 236 | 183 | 220 |
| Th/U | 2.02 | 1.90 | 1.55 | 2.17 | 1.96 | 7.11 | 9.52 | 4.55 | 12.0 | 9.46 |
| Eu/Eu* | 0.78 | 0.73 | 0.72 | 0.74 | 0.76 | 0.79 | 0.79 | 0.79 | 0.79 | 0.79 |
| Ce/Ce* | 26.4 | 38.5 | 24.8 | 47.6 | 29.9 | 34.0 | 46.0 | 47.4 | 43.5 | 45.9 |
| ΣREE | 405 | 173 | 154 | 184 | 304 | 1915 | 1291 | 893 | 1382 | 1507 |
| ΣLREE | 15.0 | 8.52 | 6.75 | 10.1 | 12.0 | 168 | 113 | 60.3 | 147 | 152 |
| ΣHREE | 382 | 164 | 147 | 173 | 291 | 1428 | 1160 | 825 | 1214 | 1302 |
| LuN/LaN | 3128 | 2559 | 2318 | 4041 | 2634 | 3605 | 2609 | 3470 | 2136 | 2784 |
| LuN/GdN | 29.1 | 47.0 | 51.9 | 44.9 | 40.5 | 19.0 | 16.5 | 22.3 | 15.3 | 17.8 |
| SmN/LaN | 38.1 | 14.9 | 10.8 | 18.9 | 19.0 | 78.0 | 61.0 | 63.4 | 61.0 | 63.7 |
| T(Ti), °C | 670 | 655 | 655 | 654 | 665 | 760 | 786 | 762 | 791 | 769 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levashova, E.V.; Skublov, S.G.; Popov, V.A. Distribution of Trace Elements Controlled by Sector and Growth Zonings in Zircon from Feldspathic Pegmatites (Ilmen Mountains, the Southern Urals). Geosciences 2021, 11, 7. https://doi.org/10.3390/geosciences11010007
Levashova EV, Skublov SG, Popov VA. Distribution of Trace Elements Controlled by Sector and Growth Zonings in Zircon from Feldspathic Pegmatites (Ilmen Mountains, the Southern Urals). Geosciences. 2021; 11(1):7. https://doi.org/10.3390/geosciences11010007
Chicago/Turabian StyleLevashova, Ekaterina V., Sergey G. Skublov, and Vladimir A. Popov. 2021. "Distribution of Trace Elements Controlled by Sector and Growth Zonings in Zircon from Feldspathic Pegmatites (Ilmen Mountains, the Southern Urals)" Geosciences 11, no. 1: 7. https://doi.org/10.3390/geosciences11010007
APA StyleLevashova, E. V., Skublov, S. G., & Popov, V. A. (2021). Distribution of Trace Elements Controlled by Sector and Growth Zonings in Zircon from Feldspathic Pegmatites (Ilmen Mountains, the Southern Urals). Geosciences, 11(1), 7. https://doi.org/10.3390/geosciences11010007







