Silification of the Mesozoic Rocks Accompanying the Bełchatów Lignite Deposit, Central Poland
Abstract
1. Introduction
2. Geological Setting
3. Materials and Methods
4. Results
4.1. Diffraction
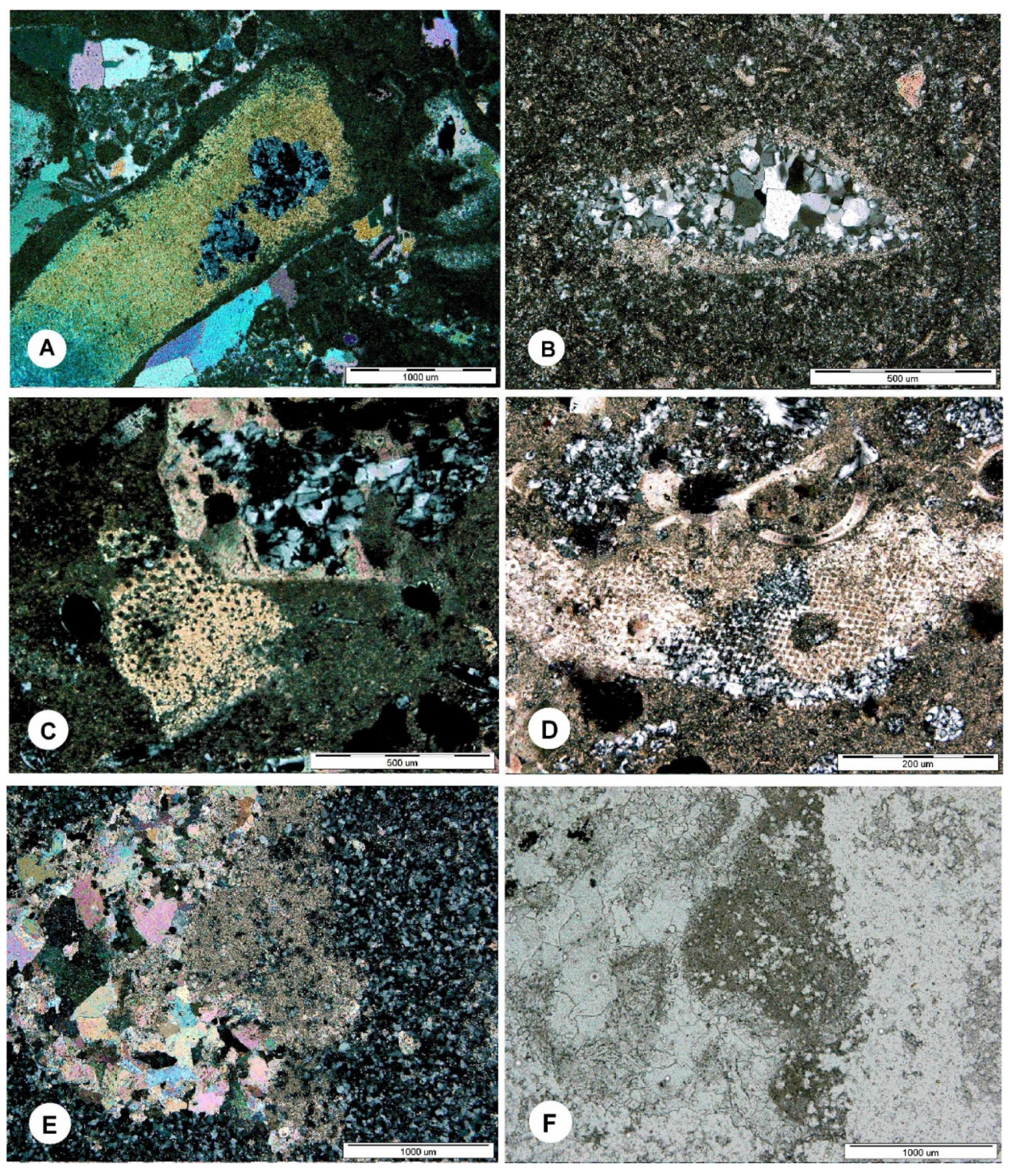
4.2. Microscopy
5. Silification Pathway
- ichnofauna channels, as oxygen-rich water makes contact with the reducing sediment of the channel walls;
- the boundary of bioturbed, oxidized and nondisturbed sediment;
- the seabed, in places where erosion reaches the reduction zone;
- in the oxidative zone, where there are local concentrations of organic matter.
- interaction of volcanic fluids and rocks producing silica-rich fluids;
- high CO2 concentrations accelerating calcite dissolution;
- an increased geothermal gradient associated with fluid and heat transport;
- fast flow, for example, along faults or fault zones;
- cooling, for example, by infiltrating surface waters;
- the presence of silica in primary sediments.
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Haldar, S.K.; Tišljar, J. Introduction to Mineralogy and Petrology; Elsevier: Amsterdam, The Netherlands, 2014; eBook; ISBN 9780124167100. [Google Scholar]
- Butts, S.H. Reading and Writing of the Fossil Record: Preservational Pathways to Exceptional Fossilization; LaFlamme, M., Schiffbauer, J.D., Darroch, S.A.F., Eds.; Paleontological Society: Bethesda, MD, USA, 2014; pp. 15–34. Available online: https://www.researchgate.net/publication/283711238_Silicification (accessed on 11 April 2020).
- Lee, D.R. Characterisation and the Diagenetic Transformation of Non and Micro-crystalline Silica Minerals. University of Liverpool, UK. Mesci. Geol. 2007. Available online: https://www.scribd.com/document/261814378/Micro-Silica (accessed on 11 April 2020).
- Laschet, C. On the origin of cherts. Facies 1984, 10, 257–290. [Google Scholar] [CrossRef]
- Hesse, R. Diagenesis 13. Origin of chert: Diagenesis of biogenic siliceous sediments. Geosci. Can. 1988, 15, 171–192. [Google Scholar]
- Kwiatkowski, S. Diageneza nie detrytycznych osadów krzemionkowych. Przegląd Geol. 1996, 44, 612–618. [Google Scholar]
- Vine, M.; Dietrich, D.; Mustoe, G.; Link, P.; Lampke, T.; Götze, J.; Rößler, R. Multi-Stage Silicification of PlioceneWood: Re-Examination of an 1895 Discovery from Idaho, USA. Geosciences 2016, 6, 21. [Google Scholar] [CrossRef]
- Mustoe, G.E. Late Tertiary Petrified Wood from Nevada, USA: Evidence of Multiple Silicification Pathways. Geosciences 2015, 5, 286–309. [Google Scholar] [CrossRef]
- Mustoe, G.E. Wood Petrifaction: A New View of Permineralization and Replacement. Geosciences 2017, 7, 119. [Google Scholar] [CrossRef]
- Trümper, S.; Rößler, R.; Götze, J. Deciphering Silicification Pathways of Fossil Forests: Case Studies from the Late Paleozoic of Central Europe. Minerals 2018, 8, 432. [Google Scholar] [CrossRef]
- Kasiński, J.R. Resource lignite potential in Poland and its usability. Biul. Państw. Inst. Geol. 2010, 439, 87–98. [Google Scholar]
- Widera, M.; Kasztelewicz, Z.; Ptak, M. Lignite mining and electricity generation in Poland: The current state and future prospects. Energy Policy 2016, 92, 151–157. [Google Scholar] [CrossRef]
- Widera, M. An overview of lithotype associations of Miocene lignite seams exploited in Poland. Geologos 2016, 22, 213–225. [Google Scholar] [CrossRef]
- Kasiński, J.R. Geological Atlas of the Tertiary Brown—Carbon Association in the Polish Part of the Żytawska Basin; 1:50 000; Polish Geological Institute: Warsaw, Poland, 2002. [Google Scholar]
- Widera, M. Changes of the lignite seam architecture—A case study from Polish lignite deposits. Int. J. Coal Geol. 2013, 114, 60–73. [Google Scholar] [CrossRef]
- Widera, M. Genetic classification of Polish lignite deposits: A review. Int. J. Coal Geol. 2016, 158, 107–118. [Google Scholar] [CrossRef]
- Ciuk, E.; Piwocki, M. Tertiary geology in the Kleszczów fault trench and its surroundings. In Guide of the LII Congress of the Polish Geological Society; Wydawnictwo Geologiczne: Warszawa, Poland, 1980. [Google Scholar]
- Czarnecki, L.; Frankowski, R.; Kuszneruk, J. Syntetyczny profil litostratygraficzny utworów trzeciorzędu złoża Bełchatów. In Sympozium: “Geologia Formacji Węglonośnych Polski”; Lipiarski, I., Ed.; Wydawnictwo Akademii Górniczo-Hutniczej: Kraków, Poland, 1992. [Google Scholar]
- Gotowała, R.; Hałuszczak, A. The Late Alpinie structural development of the Kleszczów Graben (Central Poland) as a result of a reactivation of the pre-existing, regional dislocation. Stephan Mueller Spec. Pub. Ser. (Egu) 2002, 1, 137–150. [Google Scholar] [CrossRef]
- Widera, M.; Hałuszczak, A. Stages of the Cenozoic tectonics in central Poland: Examples from selected grabens. Z. Der Dtsch. Ges. Für Geowiss. 2011, 162, 203–214. [Google Scholar] [CrossRef]
- Zadurski, Z. Occurrence of light opoka–rock in the Bełchatów area. Geol. Rev. 1971, 1, 8–9. [Google Scholar]
- Simczyjew, P.; Wiśniewski, W. Własności surowcowe węglanowych skał i zwietrzelin z odkrywki Bełchatów. Górnictwo Odkryw. 1997, 39, 1–2. [Google Scholar]
- Wyrwicki, R. Charact. Opoka–Rocks Kwb Bełchatów. Górnictwo Odkryw. 2001, 43, 2–3. [Google Scholar]
- Ratajczak, T.; Kosk, I.; Pabis, J. Weathered Sediments from the Mesozoic-Tertiary Contact Zone in the Bełchatów Lignite Deposit—Their Lithology, Raw Material Character and Possibility of Use; IGSMiE PAN: Kraków, Poland, 2002; Volume 56. [Google Scholar]
- Gilarska, A.; Stachura, E. Mineralogical and petrographic characteristic of the siliceous rocks of the Tertiary-Mesozoic contact zone in the Belchatów lignite deposit. Zesz. Nauk. Politech. ŚląskiejSer. Górnictwo 2005, 269, 97–107. [Google Scholar]
- Gilarska, A.; Hycnar, E. Influence weahtering processes over mineralogy and petrographic characteristic of rocks from the Teritiary—Mezosoic zone in the Bałchatów deposit. Górnictwo Odkryw. 2007, 49, 24–29. [Google Scholar]
- Hycnar, E.; Gilarska, A.; Wisła-Walsh, E.; Zych, Ł.; Sikorska, M. Wapienie ze strefy kontaktu trzeciorzęd—mezozoik w złożu węgla brunatnego „Bełchatów” i możliwości ich wykorzystania jako sorbentów do obniżania emisji SO2. [Limestone from the tertiary—Mesozoic contact zone in the Bełchatów lignite deposit and the possibility of their use as sorbents to reduce SO2 emission.]. Górnictwo Odkryw. 2007, 49, 30–36. [Google Scholar]
- Hycnar, E. Structural-textural nature and sorption properties of limestones from the Mesozoic-Neogene contact zone in the Bełchatów deposit. Miner. Resour. Manag. 2015, 3, 75–94. [Google Scholar] [CrossRef]
- Pękala, A. Mineralogical—geochemical study of the transitional rocks from the Mesozoic—Neogene contact zone in the “Bełchatów” lignite deposit. Min. Geol. 2012, 7, 187–205. [Google Scholar]
- Pękala, A. The mineral character and geomechanical properties of the transitional rocks from the Mesozoic —Neogene contact zone in the Bełchatów lignite. J. Sustain. Min. 2014, 13, 3–9. [Google Scholar] [CrossRef][Green Version]
- Hycnar, E.; Pękala, A. Opoka-rock from the Bełchatów lignite deposit and the possibilitoes of it’ s practical use. J. Civ. Eng. Environ. Arch. 2011, 58, 2–11. [Google Scholar]
- Pękala, A. The Opoka-Rock from the Mesozoic/Neogene Contact Zone in the Bełchatów Lignite Deposit—Characteristics of a Petrographic Nature and as a Raw Material. J. Ecol. Eng. 2019, 20, 232–237. [Google Scholar] [CrossRef]
- Dapples, E.C. Silica as an Agent in Diagenesis. In Diagenesis in Sediments; Larson, G., Chilingar, G.V., Eds.; Dev. Sedimentol.; Elsevier: Amsterdam, The Netherlands, 1967; Volume 8, pp. 323–342. [Google Scholar]
- Bąk, M.; Górny, Z.; Bąk, K.; Stożek, B.M. Successive stages of calcitization and silicification of Cenomanian spicule-bearing turbidites based on microfacies analysis, Polish Outer Carpathians. Ann. Soc. Geol. Pol. 2015, 85, 187–203. [Google Scholar] [CrossRef][Green Version]
- Jurkowska, A.; Bąk, M.; Kowalik, S. The role of biogenic silica in the formation of Upper Cretaceous pelagic carbonates and its palaeoecological implications. Cretac. Res. 2019, 93, 170–187. [Google Scholar] [CrossRef]
- Götz, A.E.; Montenari, M.; Costin, G. Silicification and organic matter preservation in the Anisian Muschelkalk: Implications for the basin dynamics of the central European Muschelkalk Sea. Cent. Eur. Geol. 2017, 60, 35–52. [Google Scholar] [CrossRef]
- Flörke, O.W.; Graetsch, H.; Martin, B.; Röller, K.; Wirth, R. Nomenclature of micro- and non-crystalline silica minerals, based on structure and microstructure. Neues Jahrbuch für Mineralogie, Abhandlungen 1991, 163, 4–19. [Google Scholar]
- Dόdony, I.; Nemeth, T.; Kovács, K. Crystal—chemical study of smectite—tridymite/cristobalite association in chloropal. In Proceedings of the Mid-European Clay Conference, Stará Lesná, Slovakia, 9–14 September 2001. Book of Abstracts. [Google Scholar]
- Pękala, A. The influence of siliceous mineral phases on the mechanical properties of transitional rocks in the Bełchatów lignite deposit. Arch. Civ. Eng. 2015, 61, 47–58. [Google Scholar] [CrossRef][Green Version]
- Murata, K.J.; Norman, M.B. An index of crystallinity for quartz. Am. J. Sci. 1976, 276, 1120–1130. [Google Scholar] [CrossRef]
- Zijlstra, H.J.P. Early diagenetic silica precipitation, in relation to redox boundaries and bacterial metabolism in late Cretaceous chalk of the Maastrichtian type locality. Geol. En Mijnb. 1987, 66, 345–355. [Google Scholar]
- Machajová, Z.; Verbich, F.; Sýkorová, I. The geology, petrography and mineralogy composition of coal from the Nováky deposit. Acta Montan. Slovaca 2002, 7, 28–33. [Google Scholar]
- Carson, G.A. Silicification of fossils. In Taphonomy: Releasing the Data Locked in the Fossil Record; Allison, P.A., Briggs, D.E.G., Eds.; Kluwer Academic; Plenum Press Publishers: New York, NY, USA, 1991; Volume 9, pp. 455–499. [Google Scholar]
- Tucker, M.E. Sedimentary Petrology. An Introduction to the Origin of Sedimentary Rocks; Blackwell Science: Hoboken, NJ, USA, 1991; p. 260. [Google Scholar]
- Garcia-Fresca, B.; Gabellone, T.; Whitake, F.F. Reactive Transport Modeling Approach to Studying Silicification of Carbonates. In Proceedings of the AAPG Annual Convention and Exhibition, San Antonio, TX, USA, 19–22 May 2019. [Google Scholar] [CrossRef]
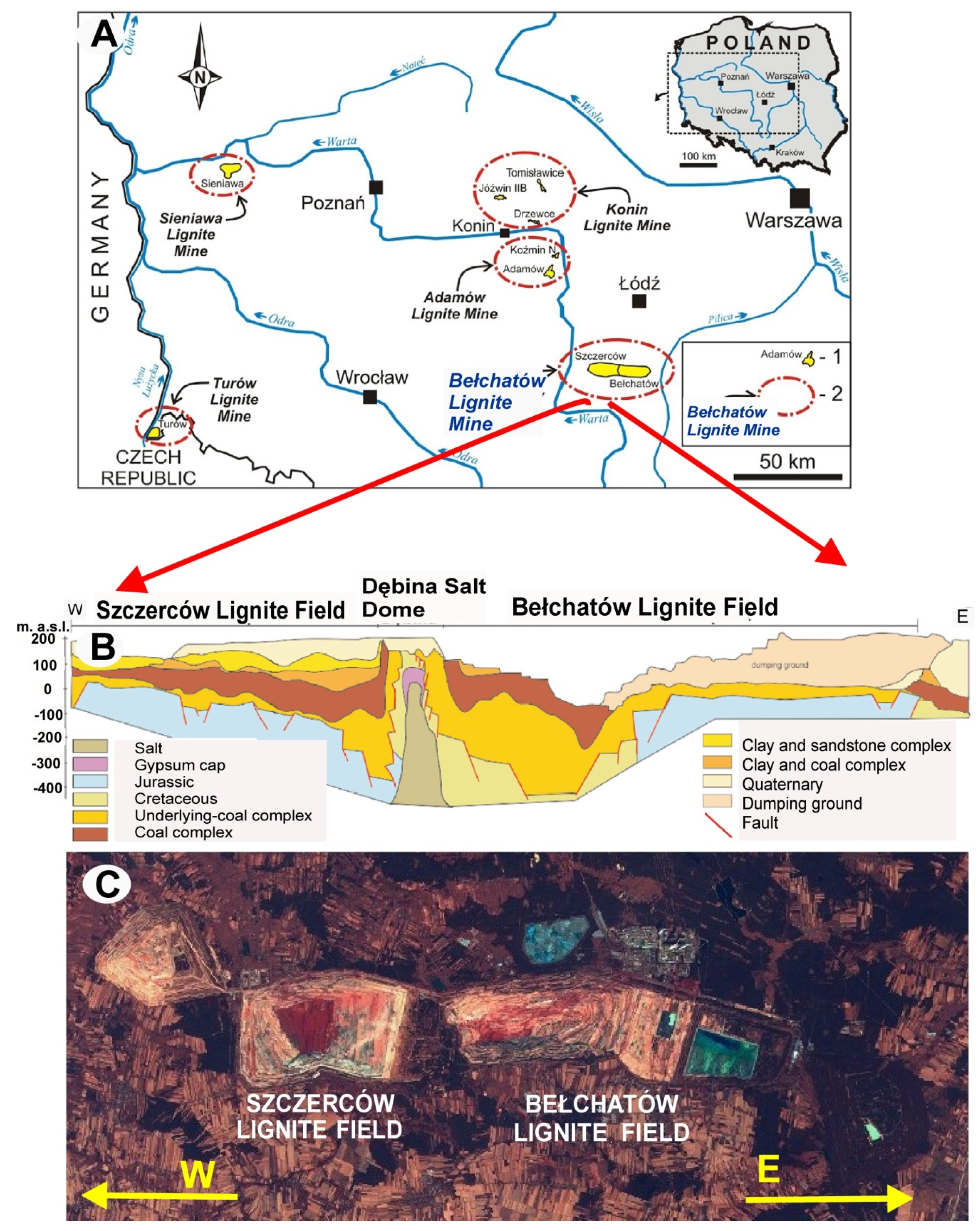
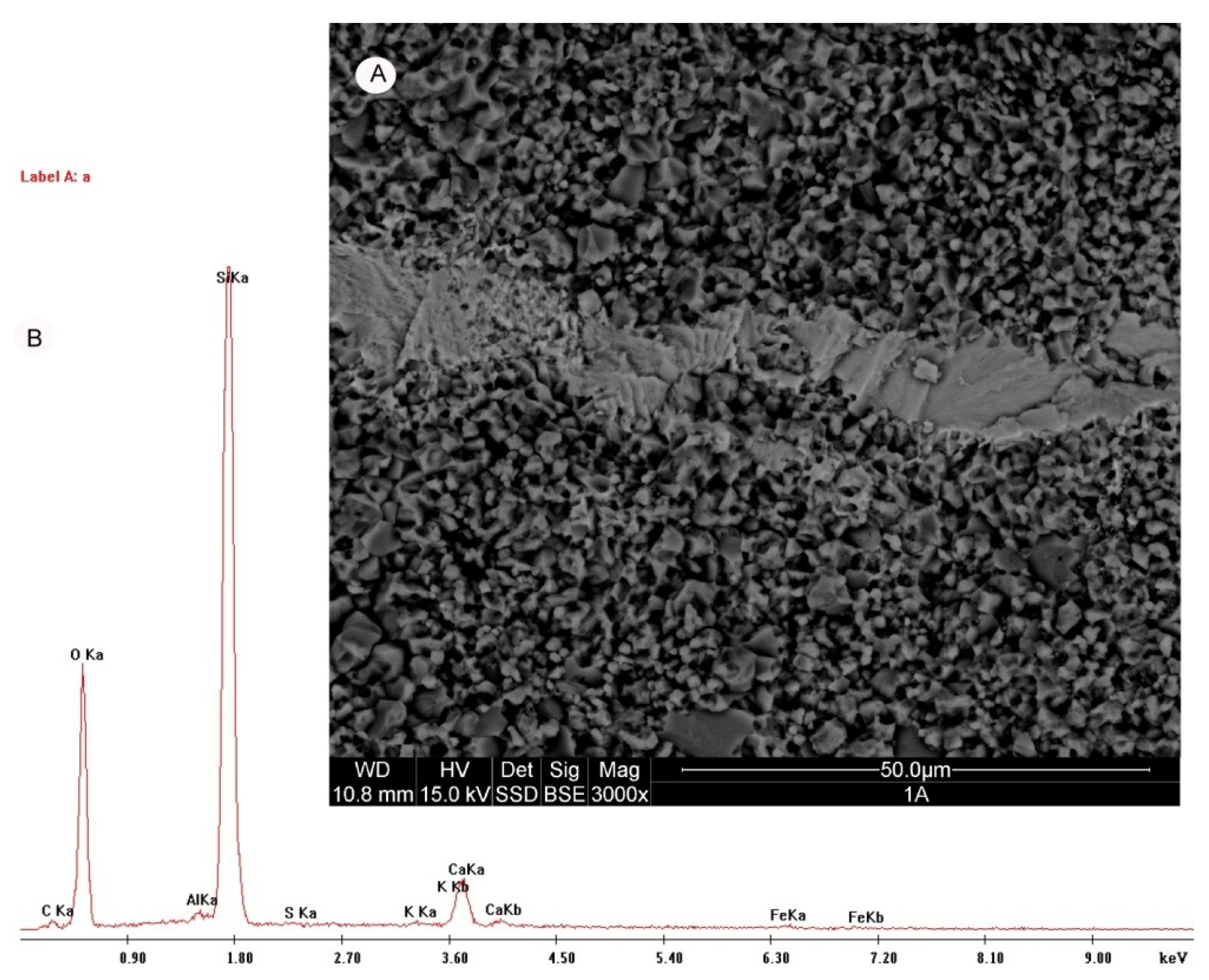
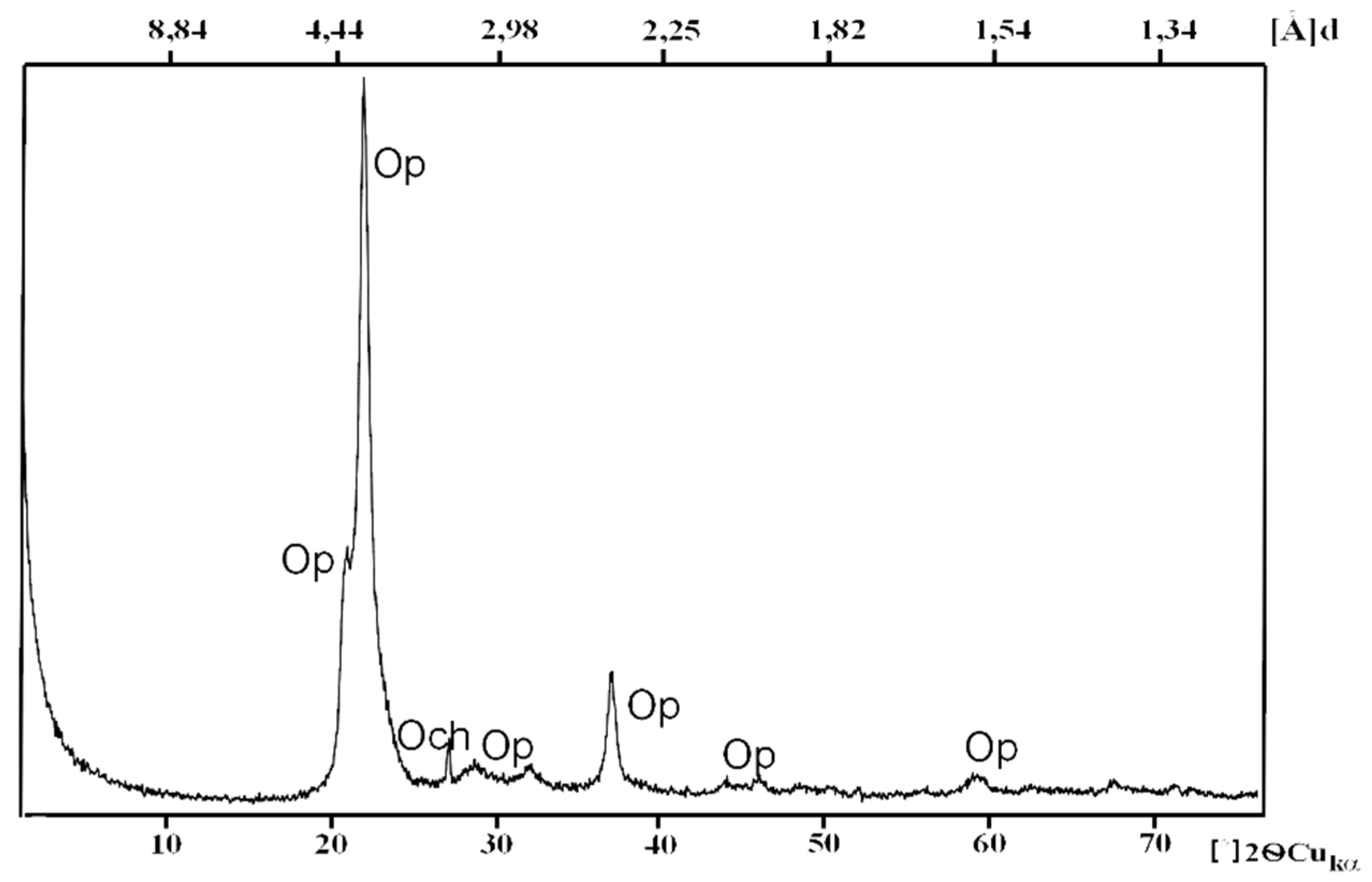

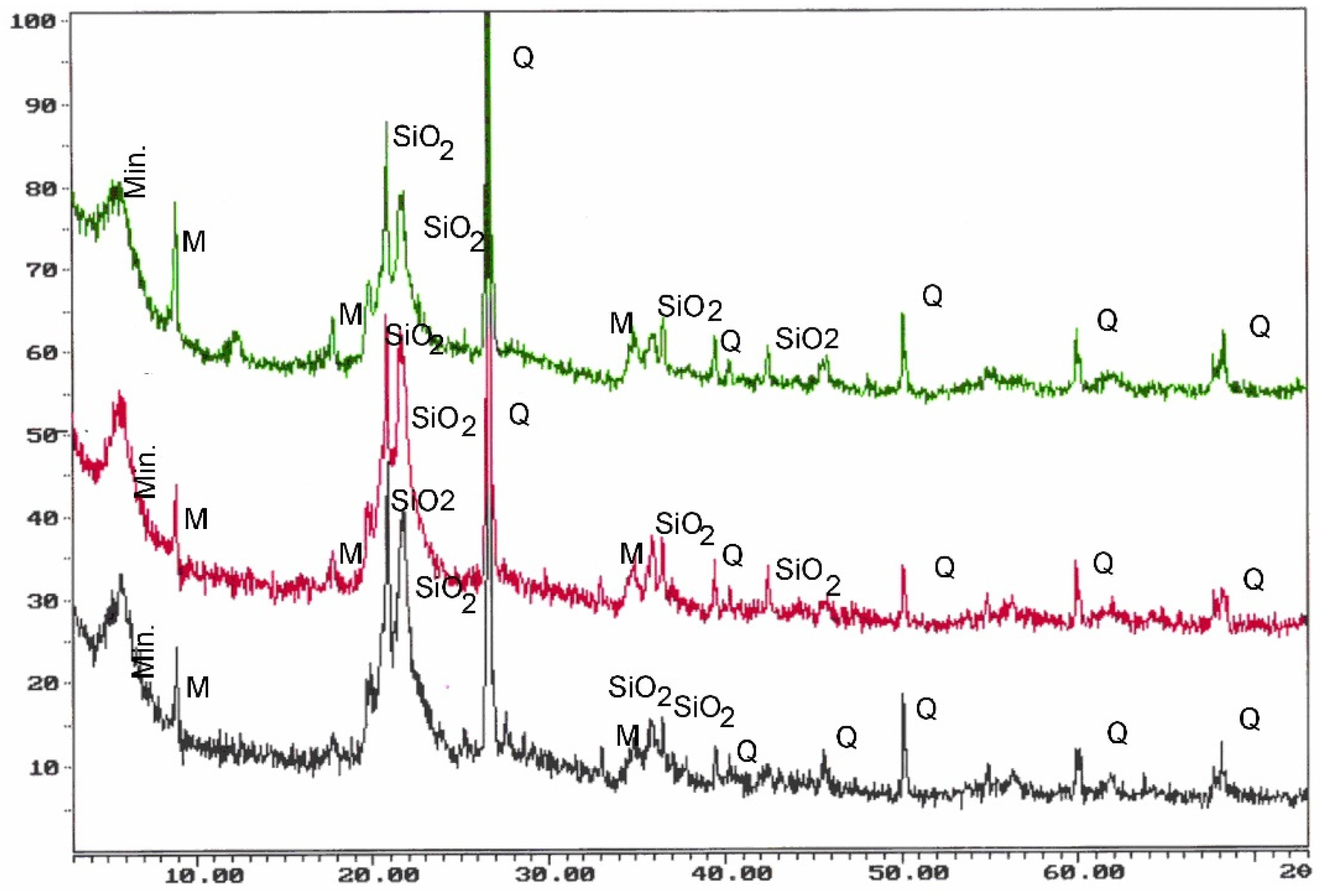


| Parameters | Opoka-rocks (Opal Type A and CT) | Silicified Opoka-rocks (Opal Type CT Relative to Opal Type C) | Gaize (Chalcedony–Quartz) |
|---|---|---|---|
| Porosity (vol.%) DECREASE | 44.5 | 24.3 | 10 |
| Density (g/cm3) INCREASE | 1.32–1.41 | 1.9 | n.d. |
| Compressive strength (MPa) INCREASE | 31.9 | 52 | 55 |
| Crystallinity index* INCREASE | 1.8 | 2.17 | 7.8 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pękala, A. Silification of the Mesozoic Rocks Accompanying the Bełchatów Lignite Deposit, Central Poland. Geosciences 2020, 10, 141. https://doi.org/10.3390/geosciences10040141
Pękala A. Silification of the Mesozoic Rocks Accompanying the Bełchatów Lignite Deposit, Central Poland. Geosciences. 2020; 10(4):141. https://doi.org/10.3390/geosciences10040141
Chicago/Turabian StylePękala, Agnieszka. 2020. "Silification of the Mesozoic Rocks Accompanying the Bełchatów Lignite Deposit, Central Poland" Geosciences 10, no. 4: 141. https://doi.org/10.3390/geosciences10040141
APA StylePękala, A. (2020). Silification of the Mesozoic Rocks Accompanying the Bełchatów Lignite Deposit, Central Poland. Geosciences, 10(4), 141. https://doi.org/10.3390/geosciences10040141





