Population Health Screening after Environmental Pollution
Abstract
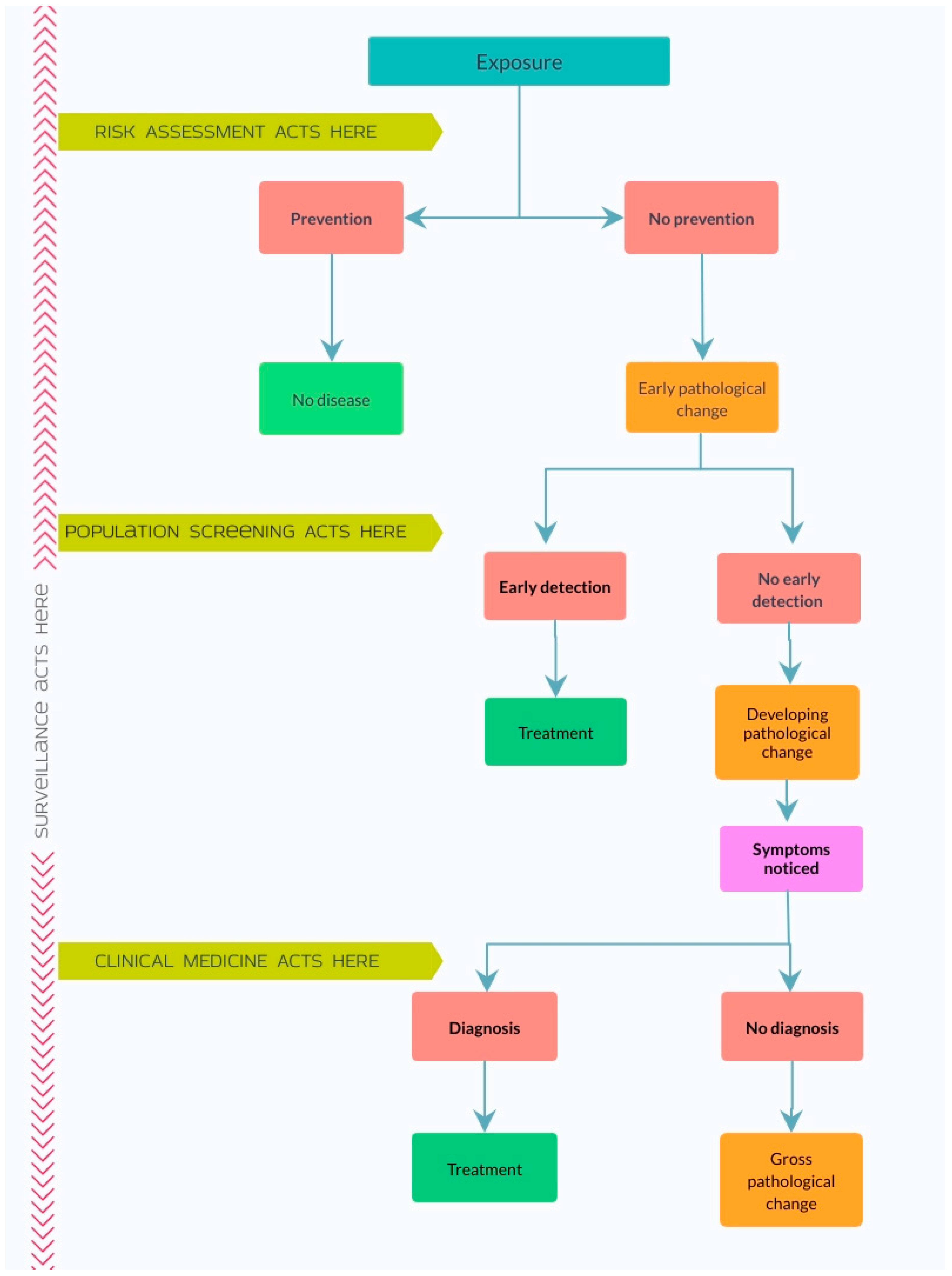
1. Introduction
2. Background
3. Methods
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.; Baldé, A.B.; Bertollini, R.; Bose-O’Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2017, 391, 462–512. [Google Scholar] [CrossRef]
- Lelieveld, J.; Evans, J.S.; Fnais, M.; Giannadaki, D.; Pozzer, A. The contribution of outdoor air pollution sources to premature mortality on a global scale. Nature 2015, 525, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, L.; Kan, H. Air pollution: A global problem needs local fixes. Nature 2019, 570, 437–439. [Google Scholar] [CrossRef] [PubMed]
- Finch, C. The Role of Global Air Pollution in Aging and Disease, 1st ed.; Academic Press: London, UK, 2018; ISBN 978-0-12-813102-2. [Google Scholar]
- Pure Earth and Green Cross Switzerland. The World’s Worst Pollution Problems 2016: The Toxics Beneath our Feet; Pure Earth and Green Cross Switzerland: New York, NY, USA; Zurich, Switzerland, 2016; p. 29. [Google Scholar]
- Whitmee, S.; Haines, A.; Beyrer, C.; Boltz, F.; Capon, A.G.; de Dias, B.F.S.; Ezeh, A.; Frumkin, H.; Gong, P.; Head, P.; et al. Safeguarding human health in the Anthropocene epoch: Report of The Rockefeller Foundation–Lancet Commission on planetary health. Lancet 2015, 386, 1973–2028. [Google Scholar] [CrossRef]
- Prüss-Ustün, A.; Vickers, C.; Haefliger, P.; Bertollini, R. Knowns and unknowns on burden of disease due to chemicals: A systematic review. Environ. Health 2011, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Chillarón, J.C.; Nijland, M.J.; Ascensão, A.A.; Sardão, V.A.; Magalhães, J.; Hitchler, M.J.; Domann, F.E.; Oliveira, P.J. Back to the future: Transgenerational transmission of xenobiotic-induced epigenetic remodeling. Epigenetics 2015, 10, 259–273. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Sadler-Riggleman, I.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenet. 2018, 4, dvy016. [Google Scholar] [CrossRef]
- Stewart, A.G.; Hursthouse, A.S. Environment and Human Health: The Challenge of Uncertainty in Risk Assessment. Geosciences 2018, 8, 24. [Google Scholar] [CrossRef]
- WHO. Childhood Lead Poisoning; World Health Organisation: Geneva, Switzerland, 2010; ISBN 978-92-4-150033-3. [Google Scholar]
- Nevin, R. How lead exposure relates to temporal changes in IQ, violent crime, and unwed pregnancy. Environ. Res. 2000, 83, 1–22. [Google Scholar] [CrossRef]
- European Chemicals Agency. Understanding REACH. Available online: https://echa.europa.eu/regulations/reach/understanding-reach (accessed on 12 January 2020).
- Gallacher, J.; Bronstering, K.; Palmer, S.; Fone, D.; Lyons, R. Symptomatology attributable to psychological exposure to a chemical incident: A natural experiment. J. Epidemiol. Commun. Health 2007, 61, 506–512. [Google Scholar] [CrossRef]
- Stewart, A.G.; Luria, P.; Reid, J.; Lyons, M.; Jarvis, R. Real or Illusory? Case Studies on the Public Perception of Environmental Health Risks in the North West of England. Int. J. Environ. Res. Public Health 2010, 7, 1153–1173. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Holland, W.W. Screening in Disease Prevention: What Works; Nuffield Trust: London, UK, 2005; pp. 1–16. ISBN 1-85775-770-X. [Google Scholar]
- Wilson, J.M.G.; Jungner, G. Principles and Practice of Screening for Disease; Public Health Papers; World Health Organization: Geneva, Switzerland, 1968; p. 168. [Google Scholar]
- Stange, B.; McInerney, J.; Golden, A.; Benade, W.; Neill, B.; Mayer, A.; Witter, R.; Tenney, L.; Stinson, K.; Cragle, D.; et al. Integrated approach to health screening of former department of energy workers detects both occupational and non-occupational illness. Am. J. Ind. Med. 2016, 59, 200–211. [Google Scholar] [CrossRef] [PubMed]
- NHS Screening. Available online: https://www.nhs.uk/conditions/nhs-screening/ (accessed on 24 August 2020).
- Dobrow, M.J.; Hagens, V.; Chafe, R.; Sullivan, T.; Rabeneck, L. Consolidated principles for screening based on a systematic review and consensus process. Can. Med. Assoc. J. 2018, 190, E422–E429. [Google Scholar] [CrossRef] [PubMed]
- Andermann, A.; Blancquaert, I.; Beauchamp, S.; Déry, V. Revisiting Wilson and Jungner in the genomic age: A review of screening criteria over the past 40 years. Bull. World Health Organ. 2008, 86, 317–319. [Google Scholar] [CrossRef]
- Cragun, D.; DeBate, R.D.; Pal, T. Applying Public Health Screening Criteria: How Does Universal Newborn Screening Compare to Universal Tumor Screening for Lynch Syndrome in Adults with Colorectal Cancer? J. Genet. Couns. 2015, 24, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Pollitt, R.J. Principles and performance: Assessing the evidence. Acta Paediatr. 1999, 88, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Boyle, C.A.; Kenneson, A.; Khoury, M.J.; Wilfond, B.S. From Public Health Emergency to Public Health Service: The Implications of Evolving Criteria for Newborn Screening Panels. Pediatrics 2006, 117, 923–929. [Google Scholar] [CrossRef]
- Lindegren, M.L.; Kobrynski, L.; Rasmussen, S.A.; Moore, C.A.; Grosse, S.D.; Vanderford, M.L.; Spira, T.J.; McDougal, J.S.; Vogt, R.F.; Hannon, W.H.; et al. Applying public health strategies to primary immunodeficiency diseases: A potential approach to genetic disorders. MMWR Recomm. Rep. 2004, 53, 1–29. [Google Scholar]
- Harris, R.; Sawaya, G.F.; Moyer, V.A.; Calonge, N. Reconsidering the Criteria for Evaluating Proposed Screening Programs: Reflections From 4 Current and Former Members of the U.S. Preventive Services Task Force. Epidemiol. Rev. 2011, 33, 20–35. [Google Scholar] [CrossRef]
- UK National Screening Committee. First Report of the National Screening Committee; Health Departments of the United Kingdom: London, UK, 1998; p. 45. [Google Scholar]
- Mahoney, G.; Stewart, A.G.; Kennedy, N.; Whitely, B.; Turner, L.; Wilkinson, E. Achieving attainable outcomes from good science in an untidy world: Case studies in land and air pollution. Environ. Geochem. Health 2015, 37, 689–706. [Google Scholar] [CrossRef]
- NHS Screening. Available online: http://www.nsc.nhs.uk/uk_nsc/uk_nsc_ind.htm (accessed on 1 August 2005).
- Ghebrehewet, S.; Stewart, A.G.; Baxter, D.; Shears, P.; Conrad, D.; Kliner, M. (Eds.) Health Protection: Principles and Practice; OUP Oxford: Oxford, UK, 2016; ISBN 978-0-19-874547-1. [Google Scholar]
- Hsiao, L.-L. Raising awareness, screening and prevention of chronic kidney disease: It takes more than a village. Nephrology 2018, 23, 107–111. [Google Scholar] [CrossRef]
- Trasande, L.; Ziebold, C.; Schiff, J.S.; Wallinga, D.; McGovern, P.; Oberg, C.N. The role of the environment in pediatric practice in Minnesota: Attitudes, beliefs, and practices. Minn. Med. 2008, 91, 36–39. [Google Scholar] [PubMed]
- Bernard, S.M. Should the Centers for Disease Control and Prevention’s Childhood Lead Poisoning Intervention Level Be Lowered? Am. J. Public Health 2003, 93, 1253–1260. [Google Scholar] [CrossRef]
- Jackson, R.J.; Cummins, S.K.; Tips, N.M.; Rosenblum, L.S. Preventing Childhood Lead Poisoning: The Challenge of Change. Am. J. Prev. Med. 1998, 14, 84–86. [Google Scholar] [CrossRef]
- Etchevers, A.; Glorennec, P.; Le Strat, Y.; Lecoffre, C.; Bretin, P.; Le Tertre, A. Screening for Elevated Blood Lead Levels in Children: Assessment of Criteria and a Proposal for New Ones in France. Int. J. Environ. Res. Public Health 2015, 12, 15366–15378. [Google Scholar] [CrossRef] [PubMed]
- Neuwirth, L.S. Resurgent lead poisoning and renewed public attention towards environmental social justice issues: A review of current efforts and call to revitalize primary and secondary lead poisoning prevention for pregnant women, lactating mothers, and children within the U.S. Int. J. Occup. Environ. Health 2018, 24, 86–100. [Google Scholar] [PubMed]
- Bruce, S.A.; Christensen, K.Y.; Coons, M.J.; Havlena, J.A.; Meiman, J.G.; Walsh, R.O. Using Medicaid Data to Improve Childhood Lead Poisoning Prevention Program Outcomes and Blood Lead Surveillance. J. Public Health Manag. Pract. 2019, 25, S51. [Google Scholar] [CrossRef] [PubMed]
- Weitzman, M. Blood Lead Screening and the Ongoing Challenge of Preventing Children’s Exposure to Lead. JAMA Pediatr. 2019, 173, 517–519. [Google Scholar] [CrossRef] [PubMed]
- Kaplowitz, S.A.; Perlstadt, H.; D’Onofrio, G.; Melnick, E.R.; Baum, C.R.; Kirrane, B.M.; Post, L.A. The Predictive Value of Self-Report Questions in a Clinical Decision Rule for Pediatric Lead Poisoning Screening. Public Health Rep. 2012, 127, 375–382. [Google Scholar] [CrossRef]
- Rooney, B.L.; Allen, B.K.; Strutt, P.J.; Hayes, E.B. Development of a Screening Tool for Prediction of Children at Risk for Lead Exposure in a Midwestern Clinical Setting. Pediatrics 1994, 93, 183–187. [Google Scholar]
- Tejeda, D.M.; Wyatt, D.D.; Rostek, B.R.; Solomon, W.B. Do Questions About Lead Exposure Predict Elevated Lead Levels? Pediatrics 1994, 93, 192–194. [Google Scholar]
- Burns, M.S.; Shah, L.H.; Marquez, E.R.; Denton, S.L.; Neyland, B.A.; Vereschzagin, D.; Gremse, D.A.; Gerstenberger, S.L. Efforts to Identify At-Risk Children for Blood Lead Screening in Pediatric Clinics—Clark County, Nevada. Clin. Pediatr. 2012, 51, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Grindler, N.M.; Allshouse, A.A.; Jungheim, E.; Powell, T.L.; Jansson, T.; Polotsky, A.J. OBGYN screening for environmental exposures: A call for action. PLoS ONE 2018, 13, e0195375. [Google Scholar] [CrossRef] [PubMed]
- Thompson, N.J.; Youngman, M.J.; Moody, J.; McColl, N.P.; Cos, D.R.; Astbury, J.; Webb, S.; Prosser, S.L. Radiation Monitoring Units: Planning and Operational Guidance; Health Protection Agency, Centre for Radiation, Chemicals and Environmental Hazards: Chilton, UK, 2011; p. 98. [Google Scholar]
- Rojas-Palma, C.; Liland, A.; Jerstad, A.N.; Etherington, G.; del Rosario Perez, M.; Rahola, T.; Smith, K. (Eds.) TMT Handbook. Triage, Monitoring and Treatment of People Exposed to Ionising Radiation Following a Malevolent Act; NRPA: Østerås, Norway, 2009; ISBN 978-82-90362-27-5. [Google Scholar]
- Kotaki, K.; Ikeda, H.; Fukuda, T.; Yuki, F.; Hasuo, K.; Kawano, Y.; Kawasaki, M. Effectiveness of diagnostic screening tests in mass screening for COPD using a cooperative regional system in a region with heavy air pollution: A cross-sectional study. BMJ Open 2017, 7, e012923. [Google Scholar] [CrossRef] [PubMed]
- Vearrier, D.; Greenberg, M.I. The implementation of medical monitoring programs following potentially hazardous exposures: A medico-legal perspective. Clin. Toxicol. 2017, 55, 956–969. [Google Scholar] [CrossRef]
- Gaskin, J.; Rennie, C.; Coyle, D. Reducing Periconceptional Methylmercury Exposure: Cost–Utility Analysis for a Proposed Screening Program for Women Planning a Pregnancy in Ontario, Canada. Environ. Health Perspect. 2015, 123, 1337–1344. [Google Scholar] [CrossRef]
- Garty, G.; Karam, P.A.; Brenner, D.J. Infrastructure to Support Ultra High Throughput Biodosimetry Screening after a Radiological Event. Int. J. Radiat. Biol. 2011, 87, 754–765. [Google Scholar] [CrossRef]
- Vaidyanathan, A.; Staley, F.; Shire, J.; Muthukumar, S.; Kennedy, C.; Meyer, P.A.; Brown, M.J. Screening for Lead Poisoning: A Geospatial Approach to Determine Testing of Children in At-Risk Neighborhoods. J. Pediatr. 2009, 154, 409–414. [Google Scholar] [CrossRef]
- Nieder, A.M.; MacKinnon, J.A.; Fleming, L.E.; Kearney, G.; Hu, J.J.; Sherman Recinda, L.; Huang, Y.; Lee David, J. Bladder Cancer Clusters in Florida: Identifying Populations at Risk. J. Urol. 2009, 182, 46–51. [Google Scholar] [CrossRef]
- Emery, R.J.; Sprau, D.D.; Morecook, R.C. Risk communication considerations to facilitate the screening of mass populations for potential contamination with radioactive material. Health Phys. 2008, 95, S168–S174. [Google Scholar] [CrossRef]
- Kardamanidis, K.; Lyle, D.M.; Boreland, F. Addressing decreasing blood lead screening rates in young children in Broken Hill, NSW. NSW Public Health Bull. 2008, 19, 180–182. [Google Scholar] [CrossRef] [PubMed]
- Hays, S.M.; Becker, R.A.; Leung, H.W.; Aylward, L.L.; Pyatt, D.W. Biomonitoring equivalents: A screening approach for interpreting biomonitoring results from a public health risk perspective. Regul. Toxicol. Pharmacol. 2007, 47, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Glorennec, P.; Declercq, C. Performance of several decision support tools for determining the need for systematic screening of childhood lead poisoning around industrial sites. Eur J. Public Health 2007, 17, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Holisaz, M.T.; Rayegani, S.M.; Hafezy, R.; Khedmat, H.; Motamedi, M.H.K. Screening for peripheral neuropathy in chemical warfare victims. Int. J. Rehabil. Res. 2007, 30, 71–74. [Google Scholar] [CrossRef]
- Howell, E.M.; Russette, L. An innovative blood lead screening program for Indian children. Public Health Rep. 2004, 119, 141–143. [Google Scholar] [CrossRef]
- Dignam, T.A.; Evens, A.; Eduardo, E.; Ramirez, S.M.; Caldwell, K.L.; Kilpatrick, N.; Noonan, G.P.; Flanders, W.D.; Meyer, P.A.; McGeehin, M.A. High-Intensity Targeted Screening for Elevated Blood Lead Levels Among Children in 2 Inner-City Chicago Communities. Am. J. Public Health 2004, 94, 1945–1951. [Google Scholar] [CrossRef]
- Grivas, T.B.; Samelis, P.; Polyzois, B.D.; Giourelis, B.; Polyzois, D. School screening in the heavily industrialized area—Is there any role of industrial environmental factors in idiopathic scoliosis prevalence? Stud. Health Technol. Inform. 2002, 91, 76–80. [Google Scholar]
- Kitamura, K.; Yoshikawa, K.; Iwama, M.; Nagao, M. Justification of measurement of eight congeners levels instead of twenty congeners of dioxins for mass screening of human exposure. J. Toxicol. Sci. 2001, 26, 163–168. [Google Scholar] [CrossRef]
- Karp, R.; Abramson, J.; Clark-Golden, M.; Mehta, S.; Daniele, R.M.; Homel, P.; Deutsch, J. Should we screen for lead poisoning after 36 months of age? Experience in the inner city. Ambul. Pediatr. 2001, 1, 256–258. [Google Scholar] [CrossRef]
- Rolnick, S.J.; Nordin, J.; Cherney, L.M. A comparison of costs of universal versus targeted lead screening for young children. Environ. Res. 1999, 80, 84–91. [Google Scholar] [CrossRef]
- Fletcher, A.M.; Gelberg, K.H.; Marshall, E.G. Reasons for Testing and Exposure Sources Among Women of Childbearing Age with Moderate Blood Lead Levels. J. Commun. Health 1999, 24, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Parsons, P.J.; Reilly, A.A.; Esernio-Jenssen, D. Screening children exposed to lead: An assessment of the capillary blood lead fingerstick test. Clin. Chem. 1997, 43, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Binder, S.; Matte, T.D.; Kresnow, M.; Houston, B.; Sacks, J.J. Lead testing of children and homes: Results of a national telephone survey. Public Health Rep. 1996, 111, 342–346. [Google Scholar] [PubMed]
- Wallace, R.B.; Murray, R.F. Workshop 1: High-risk populations—screening and prevention research strategies. Prevent. Med. 1994, 23, 569–570. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.W. Pediatric lead level screening. Alaska Med. 1993, 35, 173. [Google Scholar]
- Wartenberg, D. Screening for lead exposure using a geographic information system. Environ. Res. 1992, 59, 310–317. [Google Scholar] [CrossRef]
- Webb, K.B. The pilot Missouri health effect study. Bull. Environ. Contam. Toxicol. 1984, 33, 662–672. [Google Scholar] [CrossRef]
- Shigematsu, I.; Minowa, M.; Yoshida, T.; Miyamoto, K. Recent results of health examinations on the general population in cadmium-polluted and control areas in Japan. Environ. Health Perspect. 1979, 28, 205–210. [Google Scholar] [CrossRef]
- Hursthouse, A.; Kowalczyk, G. Transport and dynamics of toxic pollutants in the natural environment and their effect on human health: Research gaps and challenge. Environ. Geochem. Health 2009, 31, 165–187. [Google Scholar] [CrossRef]
- Bommarito, P.A.; Beck, R.; Douillet, C.; Razo, L.M.D.; Garcia-Vargas, G.-G.; Valenzuela, O.L.; Sanchez-Peña, L.C.; Styblo, M.; Fry, R.C. Evaluation of plasma arsenicals as potential biomarkers of exposure to inorganic arsenic. J. Expo. Sci. Environ. Epidemiol. 2019, 29, 718–729. [Google Scholar] [CrossRef]
- Public Health England Evidence Review Criteria: National Screening Programmes. Available online: https://www.gov.uk/government/publications/evidence-review-criteria-national-screening-programmes (accessed on 20 May 2020).
- Gray, J.A.M.; Patnick, J.; Blanks, R.G. Maximising benefit and minimising harm of screening. BMJ 2008, 336, 480–483. [Google Scholar] [CrossRef] [PubMed]
- Swaine, Z. Medical Model. In Encyclopedia of Clinical Neuropsychology; Kreutzer, J.S., DeLuca, J., Caplan, B., Eds.; Springer: New York, NY, USA, 2011; pp. 1542–1543. ISBN 978-0-387-79948-3. [Google Scholar]
- Hosseini Shokouh, S.M.; Arab, M.; Emamgholipour, S.; Rashidian, A.; Montazeri, A.; Zaboli, R. Conceptual Models of Social Determinants of Health: A Narrative Review. Iran. J. Public Health 2017, 46, 435–446. [Google Scholar] [PubMed]
- Galama, T.J.; van Kippersluis, H. A Theory of Socio-economic Disparities in Health over the Life Cycle. Econ. J. 2019, 129, 338–374. [Google Scholar] [CrossRef] [PubMed]
- CDC. The Social-Ecological Model: A Framework for Prevention. Available online: https://www.cdc.gov/violenceprevention/publichealthissue/social-ecologicalmodel.html (accessed on 18 August 2020).
- Olvera Alvarez, H.A.; Appleton, A.A.; Fuller, C.H.; Belcourt, A.; Kubzansky, L.D. An Integrated Socio-Environmental Model of Health and Well-Being: A Conceptual Framework Exploring the Joint Contribution of Environmental and Social Exposures to Health and Disease Over the Life Span. Curr. Environ. Health Rep. 2018, 5, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Vandenberg, L.N.; Colborn, T.; Hayes, T.B.; Heindel, J.J.; Jacobs, D.R., Jr.; Lee, D.-H.; Shioda, T.; Soto, A.M.; vom Saal, F.S.; Welshons, W.V.; et al. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocr. Rev. 2012, 33, 378–455. [Google Scholar] [CrossRef] [PubMed]
- Stewart, A.G.; Carter, J. Towards the development of a multidisciplinary understanding of the effects of toxic chemical mixtures on health. Environ. Geochem. Health 2009, 31, 239–251. [Google Scholar] [CrossRef]
- Hernández, A.F.; Gil, F.; Tsatsakis, A.M. Chapter 33—Biomarkers of Chemical Mixture Toxicity. In Biomarkers in Toxicology, 2nd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 569–585. ISBN 978-0-12-814655-2. [Google Scholar]
- Ross, L.F. Screening for conditions that do not meet the Wilson and Jungner criteria: The case of Duchenne muscular dystrophy. Am. J. Med. Genet. Part. A 2006, 140, 914–922. [Google Scholar] [CrossRef]
- Pienaar, K.; Petersen, A.; Bowman, D.M. Matters of fact and politics: Generating expectations of cancer screening. Soc. Sci. Med. 2019, 232, 408–416. [Google Scholar] [CrossRef]
- Gray, J.A.M. Evidence based policy making. BMJ 2004, 329, 988–989. [Google Scholar] [CrossRef]
- Petros, M. Revisiting the Wilson-Jungner criteria: How can supplemental criteria guide public health in the era of genetic screening? Genet. Med. 2012, 14, 129–134. [Google Scholar] [CrossRef]

| Date | Total Principles | Condition | Test | Program | Treatment | Source |
|---|---|---|---|---|---|---|
| 1968 | 10 | 3 | 2 | 3 | 2 | Original formulation [17] |
| 1998 | 19 | 3 | 4 | 9 | 3 | UK general screening principles [27] |
| 2004 | 20 | 3 | 5 | 8 | 4 | Workshop on genetic immune-deficiencies [25] |
| 2008 | 20 | 3 | 2 | 13 | 2 | Synthesis of 50 lists covering 40 years [21] |
| 2018 | 12 | 3 | 2 | 6 | 1 | Qualitative review to consolidate 40 lists [20] |
| Year | Focus of Paper | Condition | Test | Program | Treatment | Number of Criteria Groups | Total Criteria Quoted | Source |
|---|---|---|---|---|---|---|---|---|
| 2019 | Review of Pb program. USA | 1 | 1 | 2 | 2 | [38] | ||
| 2018 | Obstetrics screening for environmental exposure. USA | 3 | 1 | 3 | [43] | |||
| 2018 | CKD screening. International | 3 | 2 | 3 | 2 | 4 | 10 | [31] |
| 2017 | COPD. Japan | 1 | 1 | 1 | [46] | |||
| 2017 | Legal; harm/benefit. USA | 3 | 1 | 2 | 4 | [47] | ||
| 2015 | Pre-pregnancy CH3Hg. Canada | 1 | 1 | 1 | [48] | |||
| 2015 | Predicting BLL >50. France | 1 | 1 | 1 | [35] | |||
| 2012 | Predicting BLL. NV, USA | 1 | 1 | 1 | [42] | |||
| 2012 | Predicting BLL and costs. MI, USA | 1 | 2 | 2 | 3 | [39] | ||
| 2011 | Rapid radiation test. NYC, USA | 1 | 1 | 1 | [49] | |||
| 2009 | Determining Pb exposed population. GA, USA | 1 | 1 | 1 | [50] | |||
| 2009 | Bladder Cancer. USA | 2 | 1 | 2 | [51] | |||
| 2008 | Radiation risk communications. TX, USA | 1 | 1 | 1 | [52] | |||
| 2008 | Pediatricians’ attitudes & beliefs. MN, USA | 1 | 1 | 1 | 1 | 4 | 4 | [32] |
| 2008 | Not attending for BLL, NSW, Australia | 1 | 1 | 2 | 2 | [53] | ||
| 2007 | Biomonitoring concentrations. USA | 1 | 1 | 1 | [54] | |||
| 2007 | Predicting BLL >100. France | 1 | 1 | 1 | [55] | |||
| 2007 | Health outcomes of war. Iran | 1 | 1 | 1 | [56] | |||
| 2004 | Indian children’s lead. USA | 1 | 1 | 1 | [57] | |||
| 2004 | Pb epidemiology in children. Chicago, USA | 1 | 1 | 2 | 2 | [58] | ||
| 2003 | BLL levels. USA | 1 | 1 | 1 | 1 | 4 | 4 | [33] |
| 2002 | Scoliosis screening. Greece | 2 | 1 | 2 | [59] | |||
| 2001 | Dioxin. Japan | 1 | 1 | 1 | [60] | |||
| 2001 | Screening age >36 m ineffective; Pb. NY, USA | 2 | 1 | 2 | 3 | [61] | ||
| 1999 | Pb costs. MN, USA | 1 | 1 | 2 | 2 | [62] | ||
| 1999 | Screening women for Pb. NY, USA | 2 | 1 | 2 | [63] | |||
| 1998 | Comment on CDC Pb guidelines. USA | 0 | 0 | [34] | ||||
| 1997 | Ease of blood testing, Pb. NY, USA | 1 | 1 | 1 | [64] | |||
| 1996 | Telephone review Pb screening. USA | 1 | 1 | 2 | 2 | [65] | ||
| 1994 | Screening tool, Pb. WI, USA | 1 | 1 | 1 | [40] | |||
| 1994 | Predicting BLL. CA, USA | 1 | 1 | 2 | 2 | [41] | ||
| 1994 | Strategies for health screening. USA | 1 | 2 | 2 | 2 | 4 | 7 | [66] |
| 1993 | Pediatric BLL pilot. AK, USA | 1 | 1 | 1 | [67] | |||
| 1992 | GIS for Pb screening. USA | 1 | 1 | 1 | [68] | |||
| 1984 | Dioxin pilot. MO, USA | 2 | 1 | 2 | 3 | [69] | ||
| 1979 | Testing for Cd. Japan | 2 | 1 | 2 | 3 | [70] |
| Criterion [17] | Soil Arsenic | Radiation Exposure | Childhood Lead |
|---|---|---|---|
| Condition | |||
| Natural history of condition adequately understood | Not adequately | Well understood | Yes, clear links between BLL and disease |
| Important health problem | Cancers and disfiguring skin problems can result | Politically, socially, medically important | Lifelong neuropsychological effects |
| Recognizable latent/early stage | No single identifiable early condition; markers sensitive but not specific for environmental source | Main thrust of program is to reassure the “worried well”. Latent stage of disease comes much later than the “screening” | Unclear if low BLL indicates future high BLL or just low exposure |
| Test | |||
| Suitable test or examination | Sensitive, but not specific as to source of As | Yes, but detects contamination by radiation, not disease | BLL |
| Acceptable to population | Blood, urine, hair sampling are variously acceptable | Yes | Blood test; not always acceptable to children |
| Program | |||
| Ongoing case-finding process | Needs commitment and resources | Resource intensive initially | Needs organizing centrally. Needs commitment and resources |
| Case costs economically balanced with expenditure on medical care | Unknown | No, but high level of reassurance can be given | Yes |
| Diagnostic & treatment facilities available | No | Yes, for contamination | Yes |
| Treatment | |||
| Accepted treatment | No | Depends on resulting disease | Limited to high BLL |
| Agreed policy on whom to treat | No | Well worked out for contamination | No; focus is on prevention not treatment |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stewart, A.G.; Wilkinson, E. Population Health Screening after Environmental Pollution. Geosciences 2020, 10, 477. https://doi.org/10.3390/geosciences10120477
Stewart AG, Wilkinson E. Population Health Screening after Environmental Pollution. Geosciences. 2020; 10(12):477. https://doi.org/10.3390/geosciences10120477
Chicago/Turabian StyleStewart, Alex G., and Ewan Wilkinson. 2020. "Population Health Screening after Environmental Pollution" Geosciences 10, no. 12: 477. https://doi.org/10.3390/geosciences10120477
APA StyleStewart, A. G., & Wilkinson, E. (2020). Population Health Screening after Environmental Pollution. Geosciences, 10(12), 477. https://doi.org/10.3390/geosciences10120477






