Investigating the Geochemical Controls on Pb Bioaccessibility in Urban Agricultural Soils to Inform Sustainable Site Management
Abstract
1. Introduction
2. Materials and Methods
3. Results
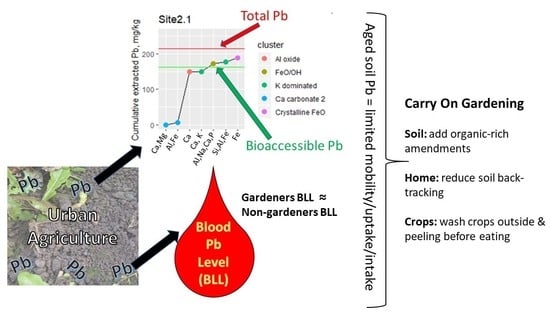
3.1. Pb Fractionation in the Soils
3.1.1. Pb Extraction Profiles
- The method is very fast (ca. 10–15 min per extract);
- The analysis is simple to carry out because there are no reagents with high total dissolved solids (TDS) to cause nebulizer blockages and other analytical problems;
- Rapid extraction and strong acid reagents minimize the potential for elements to be mobilized and redistributed to other phases; and
- The phases identified are a true representation of the natural state of the soil; they are not methodologically defined as in more conventional procedures.
3.1.2. Fractionation of Pb between the CISED Identified Geochemical Components
3.1.3. Interpretation of the Geochemical Clusters
- the extraction step when the geochemical component first appears;
- the extraction step where the maximum amount is extracted (i.e., the extraction peak); and
- the last extraction step where the geochemical component appears.
3.1.4. Soil Substrate Contributions to Pb Extraction Profiles
3.1.5. Relationship between Pb Fractionation and Bioaccessibility
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Van Den Berg, A.E.; Custers, M.H. Gardening promotes neuroendocrine and affective restoration from stress. J. Health Psychol. 2011, 16, 3–11. [Google Scholar] [CrossRef]
- Soga, M.; Gaston, K.J.; Yamaura, Y. Gardening is beneficial for health: A meta-analysis. Prev. Med. Rep. 2017, 5, 92–99. [Google Scholar] [CrossRef]
- Rouillon, M.; Harvey, P.J.; Kristensen, L.J.; George, S.G.; Taylor, M.P. VegeSafe: A community science program measuring soil-metal contamination, evaluating risk and providing advice for safe gardening. Environ. Pollut. 2017, 222, 557–566. [Google Scholar] [CrossRef]
- Entwistle, J.A.; Amaibi, P.M.; Dean, J.R.; Deary, M.E.; Medock, D.; Morton, J.; Rodushkin, I.; Bramwell, L. An apple a day? Assessing gardeners’ lead exposure in urban agriculture sites to improve the derivation of soil assessment criteria. Environ. Int. 2019, 122, 130–141. [Google Scholar] [CrossRef]
- Lanphear, B.P.; Rauch, S.; Auinger, P.; Allen, R.W.; Hornung, R.W. Low-level lead exposure and mortality in US adults: A population-based cohort study. Lancet Public Health 2018, 3, e177–e184. [Google Scholar] [CrossRef]
- Boisa, N.; Bird, G.; Brewer, P.; Dean, J.; Entwistle, J.A.; Kemp, S.; Macklin, M. Potentially harmful elements (PHEs) in scalp hair, soil and metallurgical wastes in Mitrovica, Kosovo: The role of oral bioaccessibility and mineralogy in human PHE exposure. Environ. Int. 2013, 60, 56–70. [Google Scholar] [CrossRef]
- Office for National Statistics, National Records of Scotland, Northern Ireland Statistics and Research Agency. 2011 Census Aggregate Data; UK Data Service (Edition: June 2016); Office for National Statistics: London, UK, 2016. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- BARGE. Bioaccessibility Research Group of Europe. Available online: http://www.bgs.ac.uk/barge/home.html (accessed on 4 October 2020).
- Denys, S.; Caboche, J.; Tack, K.; Rychen, G.; Wragg, J.; Cave, M.; Jondreville, C.; Feidt, C. In vivo validation of the unified BARGE method to assess the bioaccessibility of arsenic, antimony, cadmium, and lead in soils. Environ. Sci. Technol. 2012, 46, 6252–6260. [Google Scholar] [CrossRef]
- ISO. ISO 17924:2018, Soil Quality—Assessment of Human Exposure from Ingestion of Soil and Soil Material—Procedure for the Estimation of the Human Bioaccessibility/Bioavailability of Metals in Soil. Available online: https://www.iso.org/standard/64938.html (accessed on 27 August 2020).
- Amaibi, P.M.; Entwistle, J.A.; Kennedy, N.; Cave, M.; Kemp, S.; Potgieter-Vermaak, S.; Dean, J.R. Mineralogy, solid-phase fractionation and chemical extraction to assess the mobility and availability of arsenic in an urban environment. Appl. Geochem. 2018, 100, 244–257. [Google Scholar] [CrossRef]
- Giacomino, A.; Abollino, O.; Malandrino, M.; Mentasti, E. The role of chemometrics in single and sequential extraction assays: A Review. Part II. Cluster analysis, multiple linear regression, mixture resolution, experimental design and other techniques. Anal. Chim. Acta 2011, 688, 122–139. [Google Scholar] [CrossRef]
- Wragg, J.; Cave, M.; Gregory, S. The solid phase distribution and bioaccessibility of arsenic, chromium, and nickel in natural ironstone soils in the UK. Appl. Environ. Soil Sci. 2014, 2014, 924891. [Google Scholar] [CrossRef]
- Wragg, J.; Cave, M.R. Assessment of a geochemical extraction procedure to determine the solid phase fractionation and bioaccessibility of potentially harmful elements in soils: A case study using the NIST 2710 reference soil. Anal. Chim. Acta 2012, 722, 43–54. [Google Scholar] [CrossRef]
- Cave, M. The Use of Self Modelling Mixture Resolution Methods for the Interpretation of Geochemical Data Sets IR/08/035; British Geological Survey: Keyworth, Nottingham, UK, 2008. [Google Scholar]
- Filgueiras, A.; Lavilla, I.; Bendicho, C. Chemical sequential extraction for metal partitioning in environmental solid samples. J. Environ. Monit. 2002, 4, 823–857. [Google Scholar] [CrossRef]
- Mackey, E.; Christopher, S.; Lindstrom, R.; Long, S.; Marlow, A.; Murphy, K.; Paul, R.; Popelka-Filcoff, R.; Rabb, S.; Sieber, J. Certification of three NIST renewal soil standard reference materials for element content: SRM 2709a San Joaquin Soil, SRM 2710a Montana Soil I, and SRM 2711a Montana Soil II. Nist Spec. Publ. 2010, 260, 1–39. [Google Scholar]
- Sutherland, R.A. BCR®-701: A review of 10-years of sequential extraction analyses. Anal. Chim. Acta 2010, 680, 10–20. [Google Scholar] [CrossRef]
- Wragg, J. BGS Guidance Material 102, Ironstone Soil, Certificate of Analysis; IR/09/006; British Geological Survey: Keyworth, Nottingham, UK, 2009. [Google Scholar]
- Cave, M.R.; Milodowski, A.E.; Friel, E.N. Evaluation of a method for identification of host physico-chemical phases for trace metals and measurement of their solid-phase partitioning in soil samples by nitric acid extraction and chemometric mixture resolution. Geochem. Explor. Environ. Anal. 2004, 4, 71–86. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Cox, S.F.; Rollinson, G.; McKinley, J.M. Mineralogical characterisation to improve understanding of oral bioaccessibility of Cr and Ni in basaltic soils in Northern Ireland. J. Geochem. Explor. 2017, 183, 166–177. [Google Scholar] [CrossRef]
- Gal, J.; Hursthouse, A.; Cuthbert, S. Bioavailability of arsenic and antimony in soils from an abandoned mining area, Glendinning (SW Scotland). J. Environ. Sci. Health Part A 2007, 42, 1263–1274. [Google Scholar] [CrossRef]
- Tardif, S.; Cipullo, S.; So, H.U.; Wragg, J.; Holm, P.E.; Coulon, F.; Brandt, K.K.; Cave, M. Factors governing the solid phase distribution of Cr, Cu and As in contaminated soil after 40 years of ageing. Sci. Total Environ. 2019, 652, 744–754. [Google Scholar] [CrossRef]
- Wragg, J.; Broadway, A.; Cave, M.R.; Fordyce, F.M.; Palumbo-Roe, B.; Beriro, D.J.; Farmer, J.G.; Graham, M.C.; Ngwenya, B.T.; Bewley, R.J.F. Linkage between solid-phase apportionment and bioaccessible arsenic, chromium and lead in soil from Glasgow, Scotland, UK. Earth Environ. Sci. Trans. R. Soc. Edinb. 2019, 108, 217–230. [Google Scholar] [CrossRef]
- Rauret, G.; López-Sánchez, J.F.; Sahuquillo, A.; Rubio, R.; Davidson, C.; Ure, A.; Quevauviller, P. Improvement of the BCR three step sequential extraction procedure prior to the certification of new sediment and soil reference materials. J. Environ. Monit. 1999, 1, 57–61. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential extraction procedure for the speciation of particulate trace metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Reis, A.P.; Patinha, C.; Wragg, J.; Dias, A.C.; Cave, M.; Sousa, A.J.; Costa, C.; Cachada, A.; Ferreira da Silva, E.; Rocha, F.; et al. Geochemistry, mineralogy, solid-phase fractionation and oral bioaccessibility of lead in urban soils of Lisbon. Environ. Geochem. Health 2014, 36, 867–881. [Google Scholar] [CrossRef]
- Fraley, C.; Raftery, A.E. Model-based methods of classification: Using the mclust software in chemometrics. J. Stat. Softw. 2007, 18, 1–13. [Google Scholar] [CrossRef]
- Zhuang, J.; Yu, G.R. Effects of surface coatings on electrochemical properties and contaminant sorption of clay minerals. Chemosphere 2002, 49, 619–628. [Google Scholar] [CrossRef]
- Adams, J.A.; Howarth, D.T.; Campbell, A.S. Plumbogummite minerals in a strongly weathered New Zealand soil. J. Soil Sci. 1973, 24, 224–231. [Google Scholar] [CrossRef]
- Ildefonse, P.; Morin, G.; Juillot, F.; Calas, G.; Samama, J.C.; Brown, G.E.; Chevallier, P.; Populus, P. Weathering of a Pb-mineralized sandstone, Ardeche, France: Lead speciation. In Geochemistry of the Earth’s Surface; Armannsson, H., Ed.; Balema: Rotterdam, The Netherlands, 1999; pp. 385–388. [Google Scholar]
- Morin, G.; Juillot, F.; Ildefonse, P.; Calas, G.; Samama, J.C.; Chevallier, P.; Brown, G.E. Mineralogy of lead in a soil developed on a Pb-mineralized sandstone (Largentiere, France). Am. Mineral. 2001, 86, 92–104. [Google Scholar] [CrossRef]
- Nriagu, J.O. Formation and stability of base metal phosphates in soils and sediments. In Phosphate Minerals; Springer: Berlin/Heidelberg, Germany, 1984; pp. 318–329. [Google Scholar]
- Sarma, V.A.K.; Murti, G.K. Plumbogummite minerals in Indian soils. Geoderma 1970, 3, 321. [Google Scholar] [CrossRef]
- Baker, L.R.; Pierzynski, G.M.; Hettiarachchi, G.M.; Scheckel, K.G.; Newville, M. Micro-X-Ray Fluorescence, Micro-X-Ray Absorption Spectroscopy, and Micro-X-Ray Diffraction Investigation of Lead Speciation after the Addition of Different Phosphorus Amendments to a Smelter-Contaminated Soil. J. Environ. Qual. 2014, 43, 488–497. [Google Scholar] [CrossRef]
- Cornell, R.M.; Schwertmann, U. The Iron Oxides-Structure Properties, Reactions, Occurences and Uses; VCH Publishers: Weinheim, Germany, 1996. [Google Scholar]
- Baranowski, R.; Rybak, A.; Baranowska, I. Speciation analysis of elements in soil samples by XRF. Pol. J. Environ. Stud. 2002, 11, 473–482. [Google Scholar]
- Henry, H.; Naujokas, M.F.; Attanayake, C.; Basta, N.T.; Cheng, Z.; Hettiarachchi, G.M.; Maddaloni, M.; Schadt, C.; Scheckel, K.G. Bioavailability-Based In Situ Remediation To Meet Future Lead (Pb) Standards in Urban Soils and Gardens. Environ. Sci. Technol. 2015, 49, 8948–8958. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, M.A.; Filippelli, G.M.; Brown, S.; Paz-Ferreiro, J.; Reichman, S.M.; Netherway, P.; Truskewycz, A.; Ball, A.S.; Mielke, H.W. Case studies and evidence-based approaches to addressing urban soil lead contamination. Appl. Geochem. 2017, 83, 14–30. [Google Scholar] [CrossRef]
- Attanayake, C.P.; Hettiarachchi, G.M.; Harms, A.; Presley, D.; Martin, S.; Pierzynski, G.M. Field evaluations on soil plant transfer of lead from an urban garden soil. J. Environ. Qual. 2014, 43, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Attanayake, C.P.; Hettiarachchi, G.M.; Martin, S.; Pierzynski, G.M. Potential bioavailability of lead, arsenic, and polycyclic aromatic hydrocarbons in compost-amended urban soils. J. Environ. Qual. 2015, 44, 930–944. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Lee, L.; Dayan, S.; Grinshtein, M.; Shaw, R. Speciation of heavy metals in garden soils: Evidences from selective and sequential chemical leaching. J. Soils Sediments 2011, 11, 628. [Google Scholar] [CrossRef]
- Hettiarachchi, G.M.; Pierzynski, G.M.; Ransom, M.D. In situ stabilization of soil lead using phosphorus and manganese oxide. Environ. Sci. Technol. 2000, 34, 4614–4619. [Google Scholar] [CrossRef]
- Miretzky, P.; Fernandez-Cirelli, A. Phosphates for Pb immobilization in soils: A review. Environ. Chem. Lett. 2008, 6, 121–133. [Google Scholar] [CrossRef]
- Villen-Guzman, M.; Garcia-Rubio, A.; Paz-Garcia, J.M.; Vereda-Alonso, C.; Gomez-Lahoz, C.; Rodriguez-Maroto, J.M. Aging effects on the mobility of Pb in soil: Influence on the energy requirements in electroremediation. Chemosphere 2018, 213, 351–357. [Google Scholar] [CrossRef]
- Li, S.-W.; Liu, X.; Sun, H.-J.; Li, M.-Y.; Zhao, D.; Luo, J.; Li, H.-B.; Ma, L.Q. Effect of phosphate amendment on relative bioavailability and bioaccessibility of lead and arsenic in contaminated soils. J. Hazard. Mater. 2017, 339, 256–263. [Google Scholar] [CrossRef]
- Kastury, F.; Placitu, S.; Boland, J.; Karna, R.R.; Scheckel, K.G.; Smith, E.; Juhasz, A.L. Relationship between Pb relative bioavailability and bioaccessibility in phosphate amended soil: Uncertainty associated with predicting Pb immobilization efficacy using in vitro assays. Environ. Int. 2019, 131, 104967. [Google Scholar] [CrossRef]
- Bramwell, L.; Morton, J.; Harding, A.-H.; Lin, N.; Entwistle, J. Determinants of adult lead body burdens including exposures from urban agricultural sites. (Unpublished report on the Newcastle Allotments Biomonitoring Study [NABS]).
- Brown, S.; Chaney, R.L.; Hallfrisch, J.G.; Xue, Q. Effect of biosolids processing on lead bioavailability in an urban soil. J. Environ. Qual. 2003, 32, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.L.; Chaney, R.L.; Hettiarachchi, G.M. Lead in Urban Soils: A Real or Perceived Concern for Urban Agriculture? J. Environ. Qual. 2016, 45, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ferreiro, J.; Lu, H.; Fu, S.; Méndez, A.; Gascó, G. Use of phytoremediation and biochar to remediate heavy metal polluted soils: A review. Solid Earth 2014, 5, 65–75. [Google Scholar] [CrossRef]













| Extraction Order | Extractant Concentration | Volume of Extractant (mL) | No of Repeat Extractions | Volume of 30 Vol H2O2 (mL) |
|---|---|---|---|---|
| 1–2 | De-ionized water | 10 | 2 | 0 |
| 3–4 | 0.01 M aqua-regia | 10 | 2 | 0 |
| 5–6 | 0.05 M aqua-regia | 10 | 2 | 0 |
| 7–8 | 0.1 M aqua-regia | 9.75 | 2 | 0.25 |
| 9–10 | 0.5 M aqua-regia | 9.50 | 2 | 0.50 |
| 11–12 | 1.0 M aqua-regia | 9.25 | 2 | 0.75 |
| 13–14 | 5.0 M aqua-regia | 9.00 | 2 | 1.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Entwistle, J.; Bramwell, L.; Wragg, J.; Cave, M.; Hamilton, E.; Gardner, A.; Dean, J.R. Investigating the Geochemical Controls on Pb Bioaccessibility in Urban Agricultural Soils to Inform Sustainable Site Management. Geosciences 2020, 10, 398. https://doi.org/10.3390/geosciences10100398
Entwistle J, Bramwell L, Wragg J, Cave M, Hamilton E, Gardner A, Dean JR. Investigating the Geochemical Controls on Pb Bioaccessibility in Urban Agricultural Soils to Inform Sustainable Site Management. Geosciences. 2020; 10(10):398. https://doi.org/10.3390/geosciences10100398
Chicago/Turabian StyleEntwistle, Jane, Lindsay Bramwell, Joanna Wragg, Mark Cave, Elliott Hamilton, Amanda Gardner, and John R Dean. 2020. "Investigating the Geochemical Controls on Pb Bioaccessibility in Urban Agricultural Soils to Inform Sustainable Site Management" Geosciences 10, no. 10: 398. https://doi.org/10.3390/geosciences10100398
APA StyleEntwistle, J., Bramwell, L., Wragg, J., Cave, M., Hamilton, E., Gardner, A., & Dean, J. R. (2020). Investigating the Geochemical Controls on Pb Bioaccessibility in Urban Agricultural Soils to Inform Sustainable Site Management. Geosciences, 10(10), 398. https://doi.org/10.3390/geosciences10100398