Trace Metal and Cd Isotope Systematics of the Basal Datangpo Formation, Yangtze Platform (South China) Indicate Restrained (Bio)Geochemical Metal Cycling in Cryogenian Seawater
Abstract
1. Introduction
1.1. The Cryogenian Datangpo Formation and the Emergence of Substantial Algae Growth
1.2. (Bio)Geochemical Cycling of Trace Elements in Precambrian Oceans
- Manganous condition. Under manganous conditions the dissolution of Mn oxy-hydroxides together with a decreased oxidative OM dissolution may ultimately lead to positive Ce anomalies in shale normalized rare earth element (REE) patterns together with enriched Mn concentrations and negative stable C isotope compositions in carbonates [37,38,39]. Authigenic Mn carbonates, i.e., rhodochrosite, form within the sediment body [40].
- Anoxic condition. Under truly anoxic (pO2 <10 µmol) conditions, OM will not be recycled anymore and deposited in OM-rich shales showing common enrichments of the redox sensitive elements U and Mo [41,42]. By contrast, carbonates will be generally enriched in Mn and Ce due to the breakdown of Mn-oxides [30]. Further, redox sensitive trace metals, such as U, Re, V, and Mo but also Cd, have been shown to be relatively enriched in authigenic carbonate under reducing and anoxic conditions possibly due to supressed oxidative biogeochemical metal recycling [43].
- Euxinic condition. Under euxinic (pO2 <10 µmol, free H2S) conditions in the deep water/pore water space authigenic sulphides (e.g., framboidal pyrite grains) or early-diagenetic sulphides (e.g., idiomorphic pyrite grains) can form, binding crucial trace metals such as Sn, Zn, Cd, Ni, and Cu [44,45,46,47].
1.3. Status Quo of Cd Isotopes in Marine Lithologies
2. Materials and Methods
2.1. Geological Sampling Location and Mineralogy
2.2. Sequential Leaching Protocol for Trace Metal Extraction from OM and Mn Carbonate-Rich Shales
- Step 1: Twenty mL of 1 M double distilled HAc was added to the sample powders in 50 mL centrifuge vials, the solution was stirred for 30 min and reacted overnight. Then the samples were placed in an ultrasonic bath for 30 min, centrifuged at 3500 rpm for 15 min, and finally the leachate L1 was pipetted out and dried down. The residual solids of step 1 remained in the vials.
- Step 2: Residual solids of step 1 were treated with 20 mL 2 M double distilled HCl, stirred for 30 min, and placed in an ultrasonic bath for 15 min. The leachate L2 then was centrifuged at 3500 rpm for 15 min, pipetted, and dried down. The residual solids of step 2 remained in the vials.
- Step 3: Twenty mL 1 M Suprapur® NaOH were added to the residual solids of step 2. The solution was then stirred for 30 min and reacted for 4 hrs in a 60 °C water bath. After placing the solution in an ultrasonic bath for 15 min, it was then centrifuged at 3500 rpm for 15 min. Finally, the leachate L3 was pipetted and dried down. The residual solids of step 3 remained in the c vials.
- Step 4: The residual solids of step 3 were treated with 7 mL Aqua Regia (4 mL conc. double distilled HCl and 3 mL conc. double distilled HNO3). The solution reacted for 24 hrs before the reaction vessels were placed in a water bath at 100 °C with slightly opened caps. Then, the leachate L4 was diluted with 20 mL Milli-Q, centrifuged at 3500 rpm for 15 min, pipetted, and dried down. The residual solids of step 4 remained in the vials.
- Step 5: In the final step the residual solids of step 4 were transferred into perfluoroalkoxy alkane (PFA) beakers and reacted with 2 steps of 5 mL of conc. double distilled HF and 1 mL conc. double distilled HNO3, heated to 120 °C for 24 hrs with closed caps, and dried down afterwards. To break down the remaining fluorides, the leachate (full digestion of remaining silicates) L5 was treated with repeated dry-down steps of small volumes of conc. HNO3.
2.3. Stable Cd Isotope Analysis
2.4. Stable C and O Isotope Analyses in Carbonate
3. Results
3.1. Trace Metal Concentrations of Sequential Leachates
3.2. Stable C, O, and Cd Isotope Compositions
4. Discussion
4.1. The Leaching of Mixed Marine Lithologies: Bioavailable Trace Metal Distributions Associated with (In) Organic Phases
4.2. The Redox State and (Bio)Geochemical Metal Cycling in the Cryogenian Yangtze Ocean
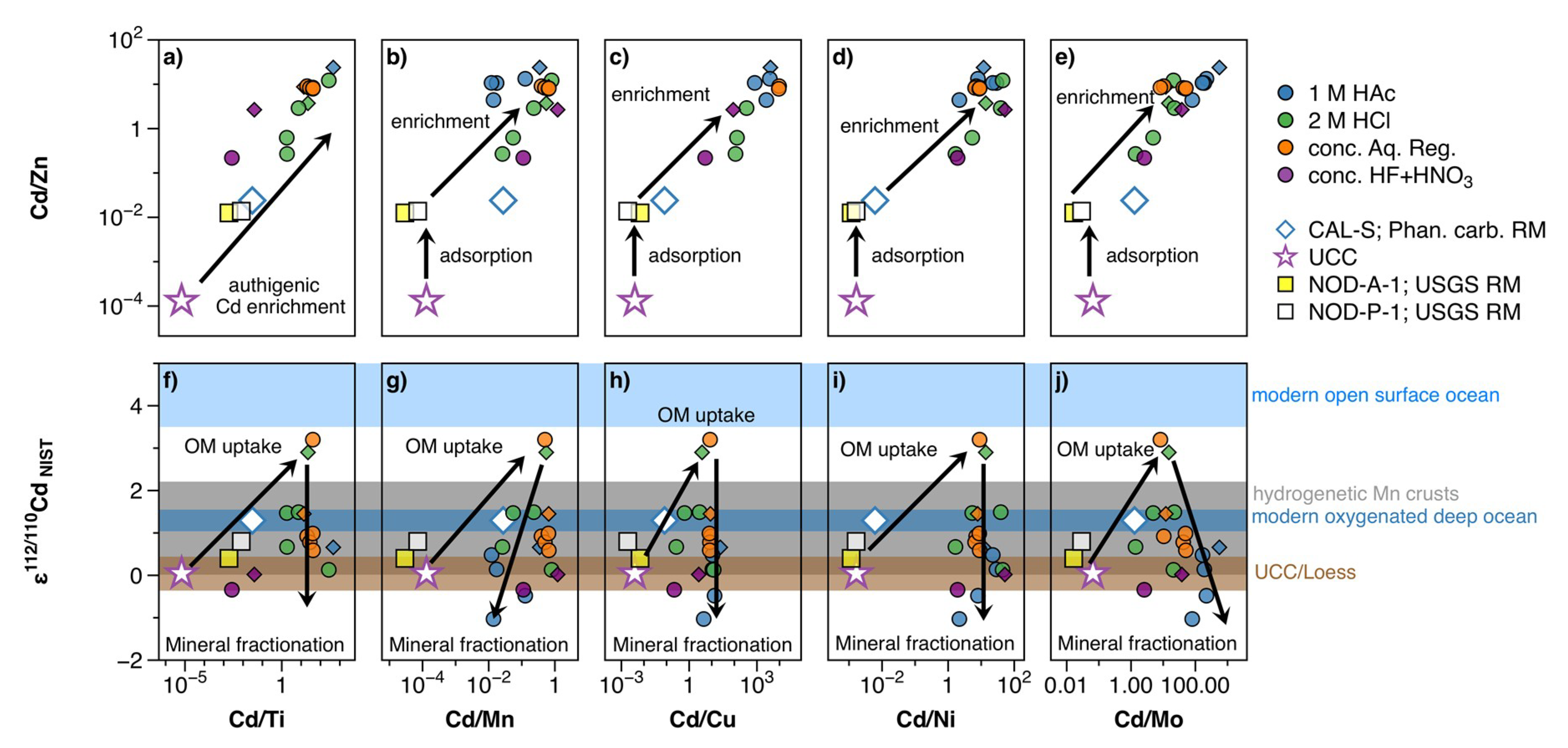
4.3. The Fractionation of Stable Cd Isotopes in Authigenic Phases: Implications on Contemporaneous Cryogenian Nanhua Basin Surface and Deep Waters
4.3.1. The Reliability of Cd Isotopes in Leachates from Mixed Lithology Samples
4.3.2. (Bio)Geochemical Cycling of Cd Isotopes in the Nanhua Basin
4.3.3. Assessing the Cd Isotope Composition of Cryogenian Nanhua Basin Surface and Deep Seawater
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ling, W. Neoproterozoic tectonic evolution of the northwestern Yangtze craton, South China: Implications for amalgamation and break-up of the Rodinia Supercontinent. Precambrian Res. 2003, 122, 111–140. [Google Scholar] [CrossRef]
- Hoffman, P.F. A Neoproterozoic Snowball Earth. Science 1998, 281, 1342–1346. [Google Scholar] [CrossRef]
- Kirschvink, J.L. Late Proterozoic low-latitude global glaciation: The snowball Earth. In The Proterozoic Biosphere: A Multidisciplinary Study; Cambridge University Press: New York, NY, USA, 1992. [Google Scholar]
- Sahoo, S.K.; Planavsky, N.J.; Kendall, B.; Wang, X.; Shi, X. Ocean oxygenation in the wake of the Marinoan glaciation. Nature 2012, 489, 546. [Google Scholar] [CrossRef]
- Canfield, D.E.; Poulton, S.W.; Narbonne, G.M. Late-Neoproterozoic Deep-Ocean Oxygenation and the Rise of Animal Life. Science 2007, 315, 92–95. [Google Scholar] [CrossRef]
- Narbonne, G.M. Ocean Chemistry and Early Animals. Science 2010, 328, 53–54. [Google Scholar] [CrossRef]
- Cai, Y.; Xiao, S.; Li, G.; Hua, H. Diverse biomineralizing animals in the terminal Ediacaran Period herald the Cambrian explosion. Geology 2019, 47, 380–384. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, Y.; Knoll, A.H. Three-dimensional preservation of algae and animal embryos in a Neoproterozoic phosphorite. Nature 1998, 391, 593. [Google Scholar] [CrossRef]
- Lenton, T.M.; Boyle, R.A.; Poulton, S.W.; Shields-Zhou, G.A.; Butterfield, N.J. Co-evolution of eukaryotes and ocean oxygenation in the Neoproterozoic era. Nat. Geosci. 2014, 7, 257–265. [Google Scholar] [CrossRef]
- Li, C.; Love, G.D.; Lyons, T.W.; Scott, C.T.; Feng, L.; Huang, J.; Chang, H.; Zhang, Q.; Chu, X. Evidence for a redox stratified Cryogenian marine basin, Datangpo Formation, South China. Earth Planet. Sci. Lett. 2012, 331, 246–256. [Google Scholar] [CrossRef]
- Zhang, F.; Zhu, X.; Yan, B.; Kendall, B.; Peng, X.; Li, J.; Algeo, T.J.; Romaniello, S. Oxygenation of a Cryogenian ocean (Nanhua Basin, South China) revealed by pyrite Fe isotope compositions. Earth Planet. Sci. Lett. 2015, 429, 11–19. [Google Scholar] [CrossRef]
- Li, C.; Love, G.D.; Lyons, T.W.; Fike, D.A.; Sessions, A.L.; Chu, X. A Stratified Redox Model for the Ediacaran Ocean. Science 2010, 328, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Wood, R.A.; Poulton, S.W.; Prave, A.R.; Hoffmann, K.H.; Clarkson, M.O.; Guilbaud, R.; Lyne, J.W.; Tostevin, R.; Bowyer, F.; Penny, A.M.; et al. Dynamic redox conditions control late Ediacaran metazoan ecosystems in the Nama Group, Namibia. Precambrian Res. 2015, 261, 252–271. [Google Scholar] [CrossRef]
- Wood, R.; Erwin, D.H. Innovation not recovery: Dynamic redox promotes metazoan radiations. Biol. Rev. 2017, 93, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Love, G.D.; Grosjean, E.; Stalvies, C.; Fike, D.A.; Grotzinger, J.P.; Bradley, A.S.; Kelly, A.E.; Bhatia, M.; Meredith, W.; Snape, C.E.; et al. Fossil steroids record the appearance of Demospongiae during the Cryogenian period. Nature 2009, 457, 718. [Google Scholar] [CrossRef] [PubMed]
- Knoll, A.H. Biogeochemistry: Food for early animal evolution. Nature 2017, 548, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Brocks, J.J.; Jarrett, A.J.M.; Sirantoine, E.; Hallmann, C.; Hoshino, Y.; Liyanage, T. The rise of algae in Cryogenian oceans and the emergence of animals. Nature 2017, 548, 578–581. [Google Scholar] [CrossRef]
- Zhou, J.-C.; Wang, X.-L.; Qiu, J.-S. Geochronology of Neoproterozoic mafic rocks and sandstones from northeastern Guizhou, South China: Coeval arc magmatism and sedimentation. Precambrian Res. 2009, 170, 27–42. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, G.; Han, Y. The age of the Nantuo Formation and Nantuo glaciation in South China. Terra Nova 2008, 20, 289–294. [Google Scholar] [CrossRef]
- Condon, D. U-Pb Ages from the Neoproterozoic Doushantuo Formation, China. Science 2005, 308, 95–98. [Google Scholar] [CrossRef]
- Cheng, M.; Li, C.; Chen, X.; Zhou, L.; Algeo, T.J.; Ling, H.-F.; Feng, L.-J.; Jin, C.-S. Delayed Neoproterozoic oceanic oxygenation_ Evidence from Mo isotopes of the Cryogenian Datangpo Formation. Precambrian Res. 2018, 319, 187–197. [Google Scholar] [CrossRef]
- Ye, Y.; Wang, H.; Zhai, L.; Wang, X.; Wu, C.; Zhang, S. Contrasting Mo–U enrichments of the basal Datangpo Formation in South China: Implications for the Cryogenian interglacial ocean redox. Precambrian Res. 2018, 315, 66–74. [Google Scholar] [CrossRef]
- Ma, Z.; Liu, X.; Yu, W.; Du, Y.; Du, Q. Redox conditions and manganese metallogenesis in the Cryogenian Nanhua Basin: Insight from the basal Datangpo Formation of South China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 529, 39–52. [Google Scholar] [CrossRef]
- Xiao, J.; He, J.; Yang, H.; Wu, C. Comparison between Datangpo-type manganese ores and modern marine ferromanganese oxyhydroxide precipitates based on rare earth elements. Ore Geol. Rev. 2017, 89, 1–46. [Google Scholar] [CrossRef]
- Peng, X.; Zhu, X.-K.; Shi, F.; Yan, B.; Zhang, F.; Zhao, N.; Peng, P.; Li, J.; Wang, D.; Shields, G.A. A deep marine organic carbon reservoir in the non-glacial Cryogenian ocean (Nanhua Basin, South China) revealed by organic carbon isotopes. Precambrian Res. 2019, 321, 212–220. [Google Scholar] [CrossRef]
- Wei, W.; Wang, D.; Li, D.; Ling, H.; Chen, X.; Wei, G.; Zhang, F.; Zhu, X.; Yan, B. The marine redox change and nitrogen cycle in the Early Cryogenian interglacial time: Evidence from nitrogen isotopes and Mo contents of the basal Datangpo Formation, northeastern Guizhou, South China. J. Earth Sci. 2016, 27, 233–241. [Google Scholar] [CrossRef]
- Anbar, A.D. Elements and Evolution. Science 2008, 322, 1481–1483. [Google Scholar] [CrossRef]
- Sunda, W.G. Trace Element Nutrients; Elsevier: Amsterdam, The Netherlands, 2001; pp. 75–86. [Google Scholar]
- Whitfield, M. The mean oceanic residence time (MORT) concept—A rationalisation. Mar. Chem. 1979, 8, 101–123. [Google Scholar] [CrossRef]
- Viehmann, S.; Hohl, S.V.; Kraemer, D.; Bau, M.; Walde, D.H.G.; Galer, S.J.G.; Jiang, S.-Y.; Meister, P. Metal cycling in Mesoproterozoic microbial habitats: Insights from trace elements and stable Cd isotopes in stromatolites. Gondwana Res. 2019, 67, 1–14. [Google Scholar] [CrossRef]
- Viehmann, S.; Bau, M.; Hoffmann, J.E.; Münker, C. Decoupled Hf and Nd isotopes in suspended particles and in the dissolved load of Late Archean seawater. Chem. Geol. 2018, 483, 111–118. [Google Scholar] [CrossRef]
- Schier, K.; Bau, M.; Münker, C.; Beukes, N.; Viehmann, S. Trace element and Nd isotope composition of shallow seawater prior to the Great Oxidation Event: Evidence from stromatolitic bioherms in the Paleoproterozoic Rooinekke and Nelani Formations, South Africa. Precambrian Res. 2018, 315, 1–43. [Google Scholar] [CrossRef]
- Viehmann, S.; Hoffmann, J.E.; Münker, C.; Bau, M. Decoupled Hf-Nd isotopes in Neoarchean seawater reveal weathering of emerged continents. Geology 2014, 42, 115–118. [Google Scholar] [CrossRef]
- Viehmann, S.; Bau, M.; Hoffmann, J.E.; Münker, C. Geochemistry of the Krivoy Rog Banded Iron Formation, Ukraine, and the impact of peak episodes of increased global magmatic activity on the trace element composition of Precambrian seawater. Precambrian Res. 2015, 270, 165–180. [Google Scholar] [CrossRef]
- Canfield, D.E. A new model for Proterozoic ocean chemistry. Nature 1998, 396, 450. [Google Scholar] [CrossRef]
- Morse, J.W.; Mackenzie, F.T. Geochemistry of Sedimentary Carbonates; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Hohl, S.V.; Becker, H.; Jiang, S.-Y.; Ling, H.-F.; Guo, Q.; Struck, U. Geochemistry of Ediacaran cap dolostones across the Yangtze Platform, South China: Implications for diagenetic modification and seawater chemistry in the aftermath of the Marinoan glaciation. J. Geol. Soc. 2017, 174, 893–912. [Google Scholar] [CrossRef]
- Tostevin, R.; Shields, G.A.; Tarbuck, G.M.; He, T.; Clarkson, M.O.; Wood, R.A. Effective use of cerium anomalies as a redox proxy in carbonate-dominated marine settings. Chem. Geol. 2016, 438, 146–162. [Google Scholar] [CrossRef]
- Wu, H.-P.; Jiang, S.-Y.; Palmer, M.R.; Wei, H.-Z.; Yang, J.-H. Positive cerium anomaly in the Doushantuo cap carbonates from the Yangtze platform, South China: Implications for intermediate water column manganous conditions in the aftermath of the Marinoan glaciation. Precambrian Res. 2018, 320, 93–110. [Google Scholar] [CrossRef]
- Thomson, J.; Higgs, N.C.; Jarvis, I.; Hydes, D.J. The behaviour of manganese in Atlantic carbonate sediments. Geochim. Et Cosmochim. Acta 1986, 50, 1807–1818. [Google Scholar] [CrossRef]
- Tribovillard, N.; Algeo, T.J.; Lyons, T.; Riboulleau, A. Trace metals as paleoredox and paleoproductivity proxies: An update. Chem. Geol. 2006, 232, 12–32. [Google Scholar] [CrossRef]
- Horner, T.J.; Kinsley, C.W.; Nielsen, S.G. Barium-isotopic fractionation in seawater mediated by barite cycling and oceanic circulation. Earth Planet. Sci. Lett. 2015, 430, 511–522. [Google Scholar] [CrossRef]
- Morford, J.L.; Emerson, S. The geochemistry of redox sensitive trace metals in sediments. Geochim. Et Cosmochim. Acta 1999, 63, 1735–1750. [Google Scholar] [CrossRef]
- John, S.G.; Rouxel, O.J.; Craddock, P.R.; Engwall, A.M.; Boyle, E.A. Zinc stable isotopes in seafloor hydrothermal vent fluids and chimneys. Earth Planet. Sci. Lett. 2008, 269, 17–28. [Google Scholar] [CrossRef]
- Guinoiseau, D.; Galer, S.J.G.; Abouchami, W. Effect of cadmium sulphide precipitation on the partitioning of Cd isotopes: Implications for the oceanic Cd cycle. Earth Planet. Sci. Lett. 2018, 498, 300–308. [Google Scholar] [CrossRef]
- Janssen, D.J.; Conway, T.M.; John, S.G.; Christian, J.R.; Kramer, D.I.; Pedersen, T.F.; Cullen, J.T. Undocumented water column sink for cadmium in open ocean oxygen-deficient zones. Proc. Natl. Acad. Sci. USA 2014, 111, 6888–6893. [Google Scholar] [CrossRef] [PubMed]
- Large, R.R.; Halpin, J.A.; Lounejeva, E.; Danyushevsky, L.V.; Maslennikov, V.V.; Gregory, D.; Sack, P.J.; Haines, P.W.; Long, J.A.; Makoundi, C.; et al. Cycles of nutrient trace elements in the Phanerozoic ocean. Gondwana Res. 2015, 28, 1282–1293. [Google Scholar] [CrossRef]
- Tribovillard, N.; Algeo, T.J.; Baudin, F.; Riboulleau, A. Analysis of marine environmental conditions based onmolybdenum–uranium covariation—Applications to Mesozoic paleoceanography. Chem. Geol. 2012, 324, 46–58. [Google Scholar] [CrossRef]
- Algeo, T.J.; Tribovillard, N. Environmental analysis of paleoceanographic systems based on molybdenum–uranium covariation. Chem. Geol. 2009, 268, 211–225. [Google Scholar] [CrossRef]
- Little, S.H.; Vance, D.; Lyons, T.W.; McManus, J. Controls on trace metal authigenic enrichment in reducing sediments: Insights from modern oxygen-deficient settings. Am. J. Sci. 2015, 315, 77–119. [Google Scholar] [CrossRef]
- Hohl, S.V.; Becker, H.; Herzlieb, S.; Guo, Q. Multiproxy constraints on alteration and primary compositions of Ediacaran deep-water carbonate rocks, Yangtze Platform, South China. Geochim. Et Cosmochim. Acta 2015, 163, 262–278. [Google Scholar] [CrossRef]
- Smrzka, D.; Zwicker, J.; Bach, W.; Feng, D.; Himmler, T.; Chen, D.; Peckmann, J. The behavior of trace elements in seawater, sedimentary pore water, and their incorporation into carbonate minerals: A review. Facies 2019, 65, 1–47. [Google Scholar] [CrossRef]
- Abouchami, W.; Galer, S.J.G.; de Baar, H.J.W.; Alderkamp, A.C.; Middag, R.; Laan, P.; Feldmann, H.; Andreae, M.O. Modulation of the Southern Ocean cadmium isotope signature by ocean circulation and primary productivity. Earth Planet. Sci. Lett. 2011, 305, 83–91. [Google Scholar] [CrossRef]
- Ripperger, S.; Rehkämper, M.; Porcelli, D.; Halliday, A.N. Cadmium isotope fractionation in seawater—A signature of biological activity. Earth Planet. Sci. Lett. 2007, 261, 670–684. [Google Scholar] [CrossRef]
- Lacan, F.; Francois, R.; Ji, Y.; Sherrell, R.M. Cadmium isotopic composition in the ocean. Geochim. Et Cosmochim. Acta 2006, 70, 5104–5118. [Google Scholar] [CrossRef]
- Schmitt, A.-D.; Galer, S.J.G.; Abouchami, W. Mass-dependent cadmium isotopic variations in nature with emphasis on the marine environment. Earth Planet. Sci. Lett. 2009, 277, 262–272. [Google Scholar] [CrossRef]
- Hohl, S.V.; Galer, S.J.G.; Gamper, A.; BECKER, H. Cadmium isotope variations in Neoproterozoic carbonates—A tracer of biologic production? Geochem. Persp. Lett. 2016, 3, 32–44. [Google Scholar] [CrossRef]
- John, S.G.; Kunzmann, M.; Townsend, E.J.; Rosenberg, A.D. Zinc and cadmium stable isotopes in the geological record: A case study from the post-snowball Earth Nuccaleena cap dolostone. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 466, 202–208. [Google Scholar] [CrossRef]
- Price, N.M.; Morel, F.M.M. Cadmium and cobalt substitution for zinc in a marine diatom. Nature 1990, 344, 658–660. [Google Scholar] [CrossRef]
- Xu, Y.; Feng, L.; Jeffrey, P.D.; Shi, Y.; Morel, F.M.M. Structure and metal exchange in the cadmium carbonic anhydrase of marine diatoms. Nature 2008, 452, 56–61. [Google Scholar] [CrossRef]
- Georgiev, S.V.; Horner, T.J.; Stein, H.J.; Hannah, J.L. Cadmium-isotopic evidence for increasing primary productivity during the Late Permian anoxic event. Earth Planet. Sci. Lett. 2015, 410, 84–96. [Google Scholar] [CrossRef]
- Schmitt, A.-D.; Galer, S.J.G.; Abouchami, W. High-precision cadmium stable isotope measurements by double spike thermal ionisation mass spectrometry. J. Anal. At. Spectrom. 2009, 24, 1079–1088. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, H.; Zhu, C.; Fan, H.; Cloquet, C. Cadmium isotopic evidence for the evolution of marine primary productivity and the biological extinction event during the Permian-Triassic crisis from the Meishan section, South China. Chem. Geol. 2018, 481, 110–118. [Google Scholar] [CrossRef]
- Horner, T.J.; Rickaby, R.E.M.; Henderson, G.M. Isotopic fractionation of cadmium into calcite. Earth Planet. Sci. Lett. 2011, 312, 243–253. [Google Scholar] [CrossRef]
- Horner, T.J. Cadmium Isotope Fractionation in Seawater. Ph.D. Thesis, The University of Oxford, Oxford, UK, 2012. [Google Scholar]
- Lambelet, M.; Rehkämper, M.; van de Flierdt, T.; Xue, Z.; Kreissig, K.; Coles, B.; Porcelli, D.; Andersson, P. Isotopic analysis of Cd in the mixing zone of Siberian rivers with the Arctic Ocean—New constraints on marine Cd cycling and the isotope composition of riverine Cd. Earth Planet. Sci. Lett. 2013, 361, 64–73. [Google Scholar] [CrossRef]
- Wei, G.-Y.; Planavsky, N.J.; Tarhan, L.G.; Chen, X.; Wei, W.; Li, D.; Ling, H.-F. Marine redox fluctuation as a potential trigger for the Cambrian explosion. Geology 2018, 46, 1–5. [Google Scholar] [CrossRef]
- Henrique-Pinto, R.; Barnes, S.-J.; Savard, D.D.; Mehdi, S. Quantification of Metals and Semi-Metals in Carbon-Rich Rocks: A New Sequential Protocol Including Extraction from Humic Substances. Geostand. Geoanalytical Res. 2015, 41, 41–62. [Google Scholar] [CrossRef]
- Zhang, Q.R.; Chu, X.L.; Bahlburg, H.; Feng, L.J.; Dobrzinski, N.; Zhang, T.G. Stratigraphic architecture of the neoproterozoic glacial rocks in the “Xiang-Qian-Gui” region of the central Yangtze block, South China. Prog. Nat. Sci. 2003, 13, 783–787. [Google Scholar] [CrossRef]
- Jiang, G.; Shi, X.; Zhang, S.; Wang, Y.; Xiao, S. Stratigraphy and paleogeography of the Ediacaran Doushantuo Formation (ca. 635–551Ma) in South China. Gondwana Res. 2011, 19, 831–849. [Google Scholar] [CrossRef]
- Boguta, P.; Sokołowska, Z. Interactions of Zn(II) Ions with Humic Acids Isolated from Various Type of Soils. Effect of pH, Zn Concentrations and Humic Acids Chemical Properties. PLoS ONE 2016, 11, e015362626. [Google Scholar] [CrossRef]
- Govindaraju, K. 1994 compilation of working values and sample description for 383 Geostandards. Geostand. Newsl. 1994, 18, 1–158. [Google Scholar] [CrossRef]
- Rosman, K.J.R.; De Laeter, J.R.; Gorton, M.P. Cadmium isotope fractionation in fractions of two H3 chondrites. Earth Planet. Sci. Lett. 1980, 48, 166–170. [Google Scholar] [CrossRef]
- Abouchami, W.; Galer, S.J.G.; Horner, T.J.; Rehkämper, M.; Wombacher, F.; Xue, Z.; Lambelet, M.; Gault-Ringold, M.; Stirling, C.H.; Schönbächler, M.; et al. A Common Reference Material for Cadmium Isotope Studies—NIST SRM 3108. Geostand. Geoanal. Res. 2012, 37, 5–17. [Google Scholar] [CrossRef]
- McCrea, J.M. On the Isotopic Chemistry of Carbonates and a Paleotemperature Scale. J. Chem. Phys. 1950, 18, 849–857. [Google Scholar] [CrossRef]
- Coplen, T.B. Guidelines and recommended terms for expression of stable-isotope-ratio and gas-ratio measurement results. Rapid Commun. Mass Spectrom. 2011, 25, 2538–2560. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.R.; McLennan, S.M. The Continental Crust; Wiley-Blackwell: Hoboken, NJ, USA, 1991. [Google Scholar]
- McLennan, S.M. Relationships between the trace element composition of sedimentary rocks and upper continental crust. Geochem. Geophys. Geosystems 2001, 2. [Google Scholar] [CrossRef]
- Abouchami, W.; Galer, S.J.G.; de Baar, H.J.W.; Middag, R.; Vance, D.; Zhao, Y.; Klunder, M.; Mezger, K.; Feldmann, H.; Andreae, M.O. Biogeochemical cycling of cadmium isotopes in the Southern Ocean along the Zero Meridian. Geochim. Et Cosmochim. Acta 2014, 127, 348–367. [Google Scholar] [CrossRef]
- Sieber, M.; Conway, T.M.; de Souza, G.F.; Hassler, C.S.; Ellwood, M.J.; Vance, D. High-resolution Cd isotope systematics in multiple zones of the Southern Ocean from the Antarctic Circumnavigation Expedition. Earth Planet. Sci. Lett. 2019, 527, 115799. [Google Scholar] [CrossRef]
- Conway, T.M.; John, S.G. Biogeochemical cycling of cadmium isotopes along a high-resolution section through the North Atlantic Ocean. Geochim. Et Cosmochim. Acta 2015, 148, 269–283. [Google Scholar] [CrossRef]
- Horner, T.J.; Schönbächler, M.; Rehkämper, M.; Nielsen, S.G.; Williams, H.; Halliday, A.N.; Xue, Z.; Hein, J.R. Ferromanganese crusts as archives of deep water Cd isotope compositions. Geochem. Geophys. Geosyst. 2010, 11, 4. [Google Scholar] [CrossRef]
- Cronan, D.S. Underwater Minerals; Academic Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Comans, R. Adsorption, desorption and isotopic exchange of cadmium on illite: Evidence for complete reversibility. Water Res. 1987, 21, 1573–1576. [Google Scholar] [CrossRef]
- Xie, R.C.; Rehkämper, M.; Grasse, P.; van de Flierdt, T.; Frank, M.; Xue, Z. Isotopic evidence for complex biogeochemical cycling of Cd in the eastern tropical South Pacific. Earth Planet. Sci. Lett. 2019, 512, 134–146. [Google Scholar] [CrossRef]
- Xie, R.C.; Galer, S.J.G.; Abouchami, W.; Rijkenberg, M.J.A.; de Baar, H.J.W.; De Jong, J.; Andreae, M.O. Non-Rayleigh control of upper-ocean Cd isotope fractionation in the western South Atlantic. Earth Planet. Sci. Lett. 2017, 471, 94–103. [Google Scholar] [CrossRef]
- George, E. Marine Biogeochemical Cycling of Cadmium and Its Isotopes: Studies of the South Pacific Ocean, Mediterranean Sea and Black Sea. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2017. Available online: http://hdl.handle.net/10523/7552 (accessed on 8 January 2020).
- George, E.; Stirling, C.H.; Gault-Ringold, M.; Ellwood, M.J.; Middag, R. Marine biogeochemical cycling of cadmium and cadmium isotopes in the extreme nutrient-depleted subtropical gyre of the South West Pacific Ocean. Earth Planet. Sci. Lett. 2019, 514, 84–95. [Google Scholar] [CrossRef]
- Boyle, E.A. Cadmium: Chemical tracer of deepwater paleoceanography. Paleoceanography 1988, 3, 471–489. [Google Scholar] [CrossRef]
- Hohl, S.V.; Jiang, S.-Y.; Wei, H.-Z.; Pi, D.-H.; Liu, Q.; Viehmann, S.; Galer, S.J.G. Cd isotopes trace periodic (bio)geochemical metal cycling at the verge of the Cambrian animal evolution. Geochim. Et Cosmochim. Acta 2019, 263, 1–20. [Google Scholar] [CrossRef]
- Xie, R.C.; Galer, S.J.G.; Abouchami, W.; Frank, M. Limited impact of eolian and riverine sources on the biogeochemical cycling of Cd in the tropical Atlantic. Chem. Geol. 2019, 511, 371–379. [Google Scholar] [CrossRef]
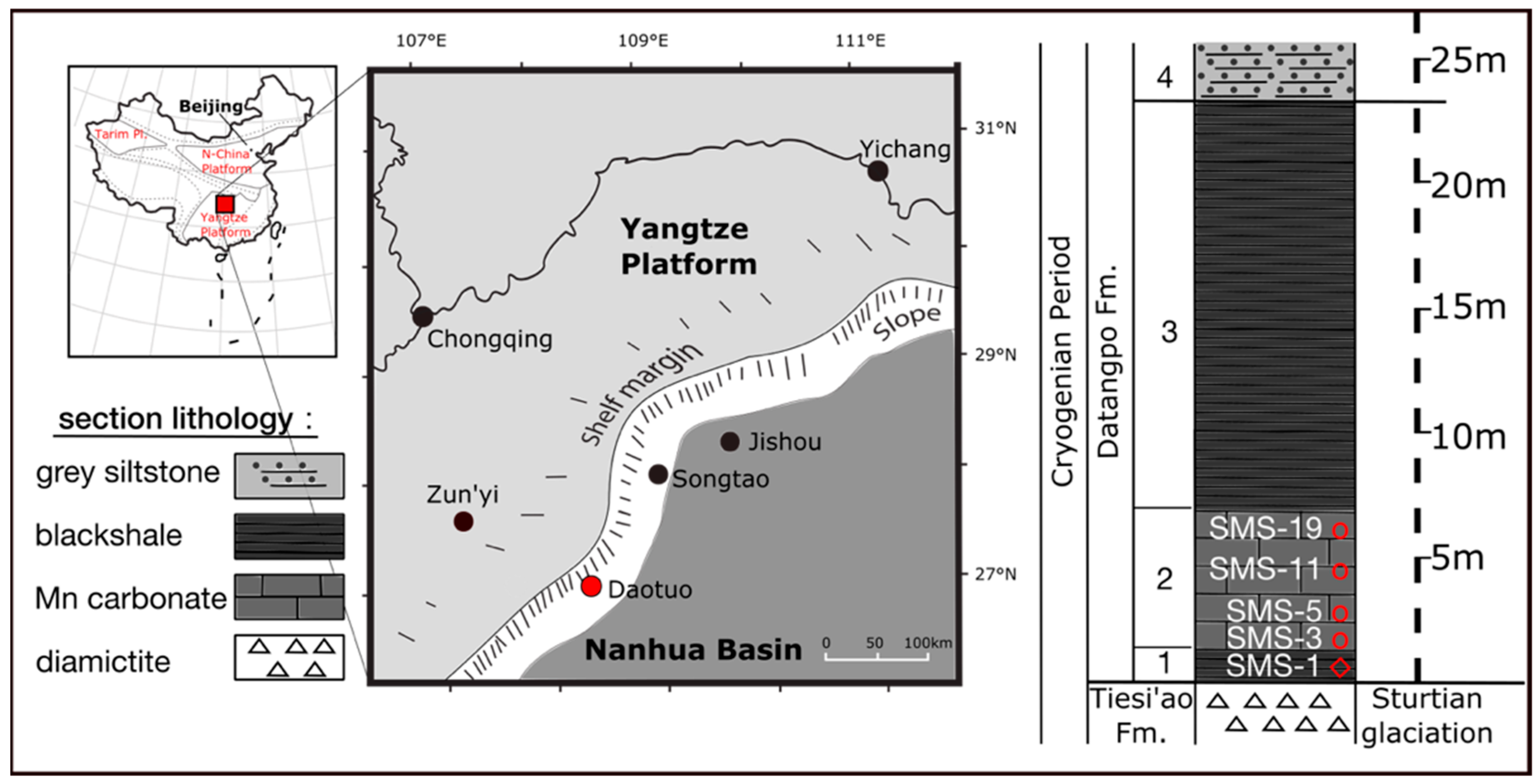
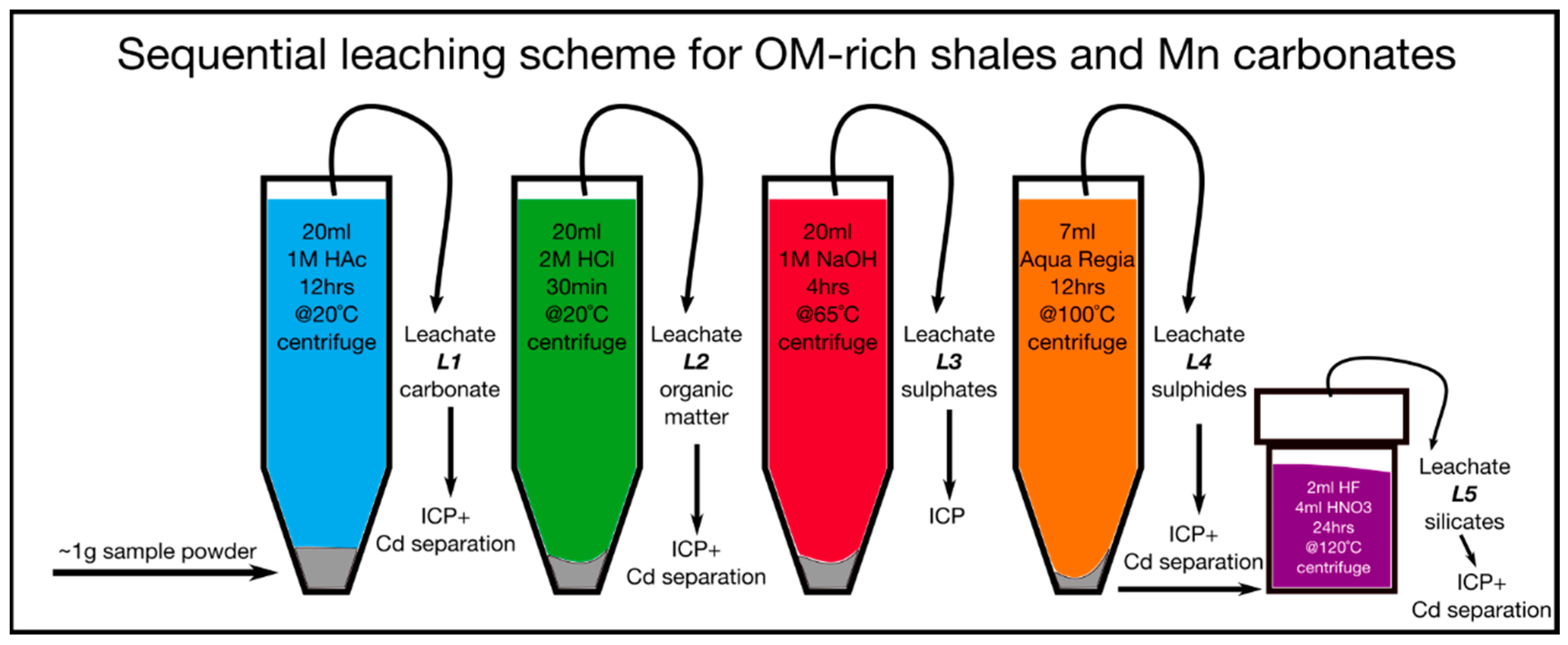
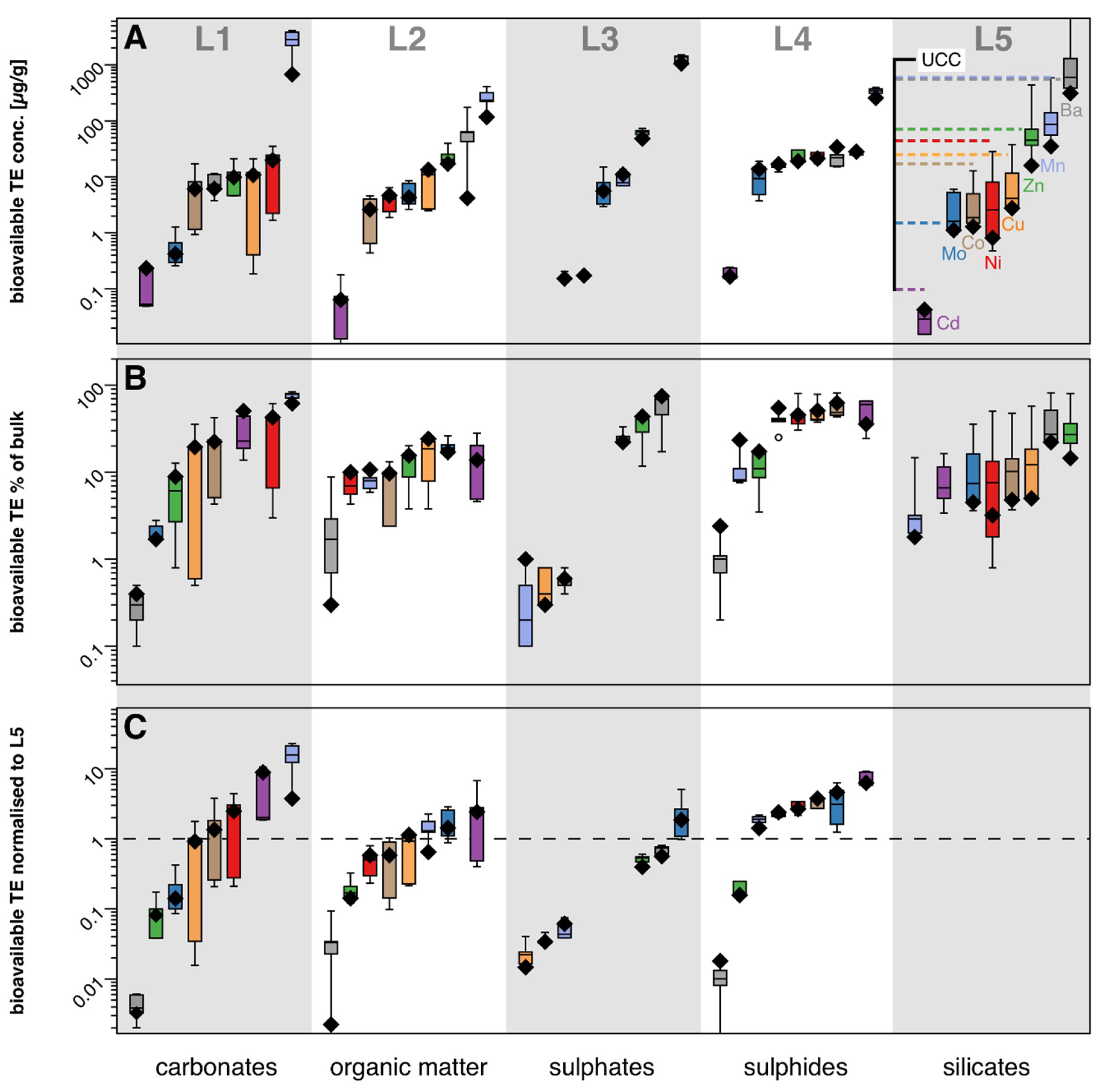
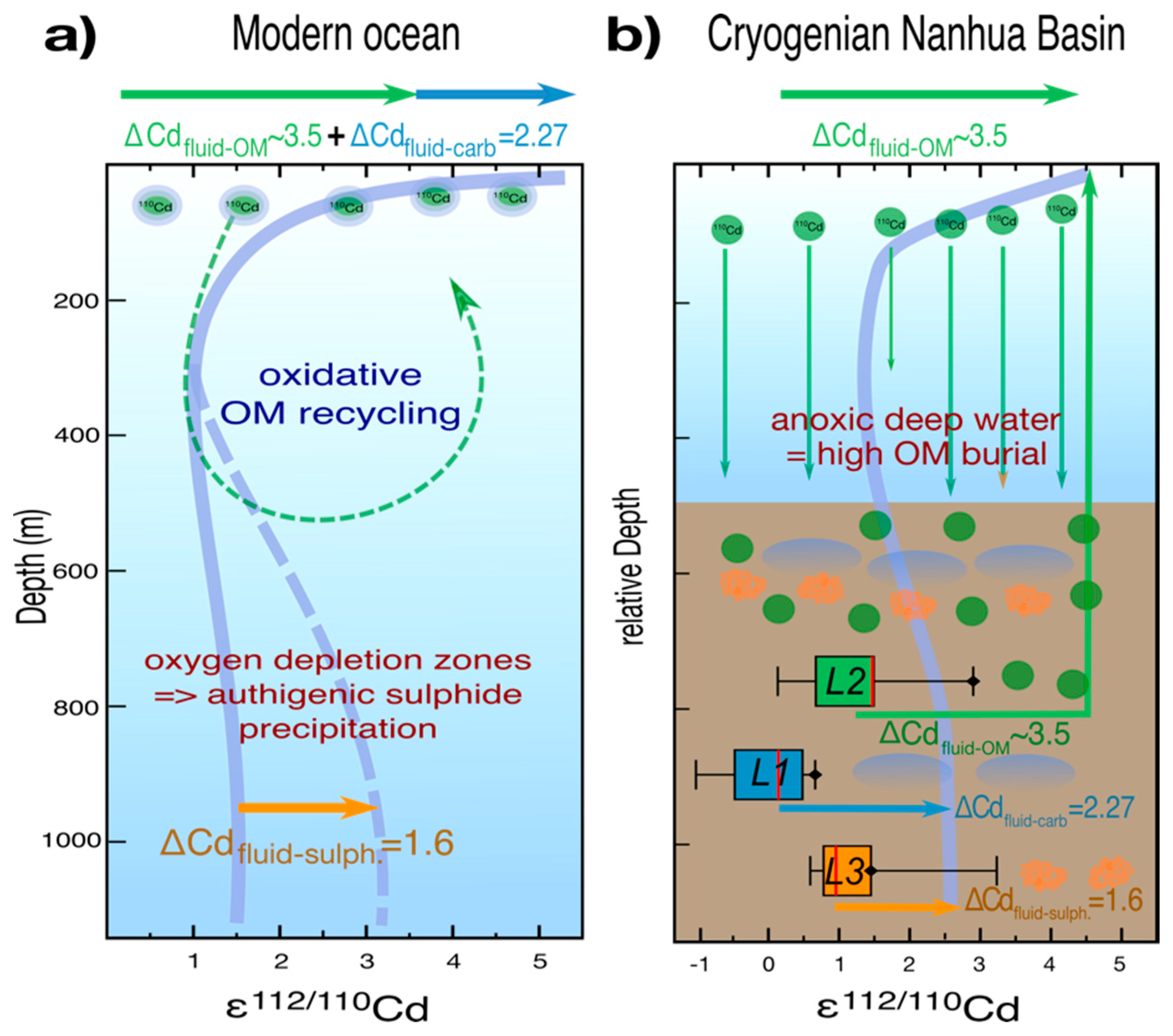
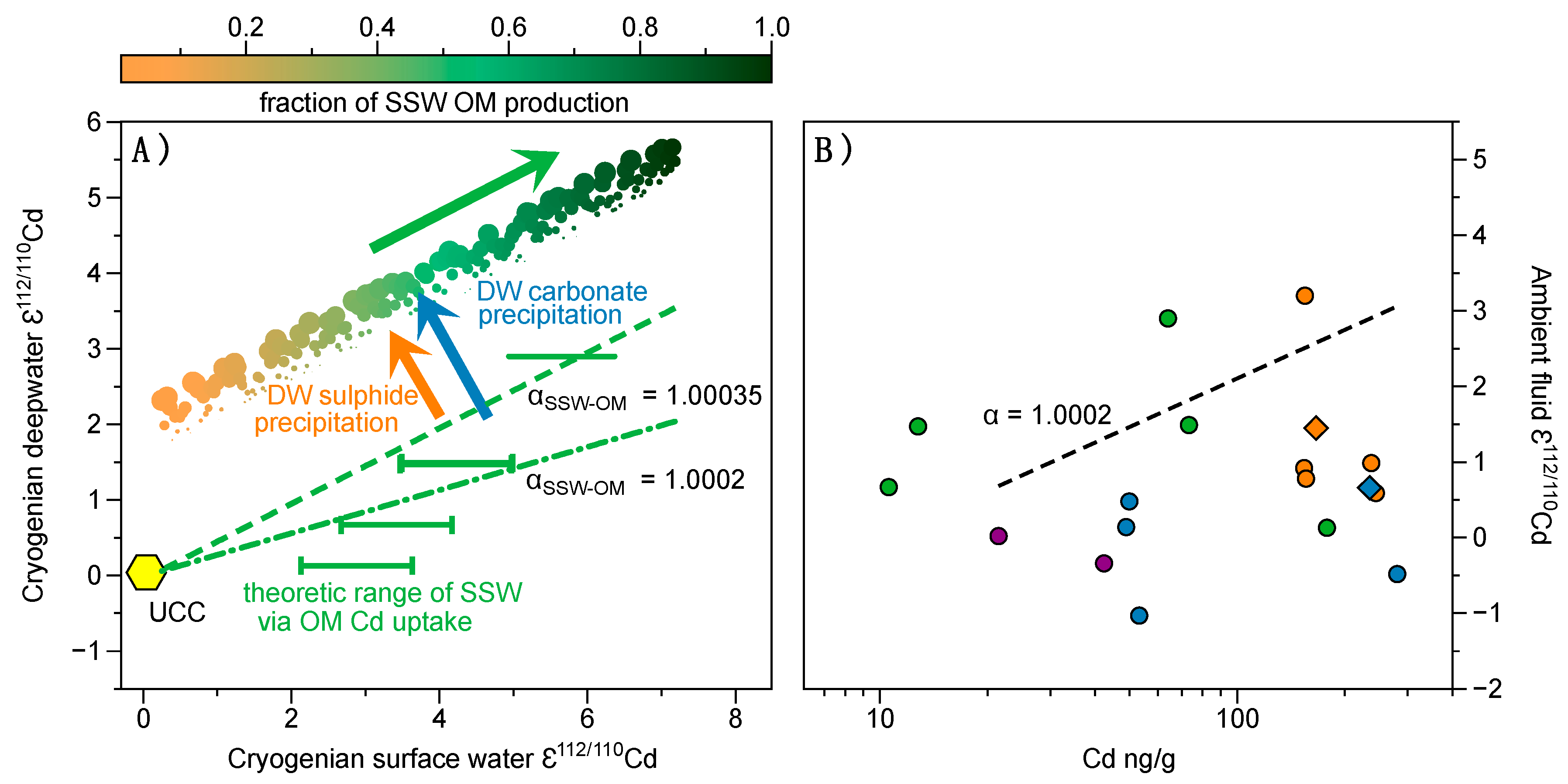
| Sample ID | Depth (m) | Lithology | Leach Sample | Reagent | Leached Phase | Zr (µg/g) | Co (µg/g) | Zn (µg/g) | Ba (µg/g) | V (µg/g) | Cr (µg/g) | Mo (µg/g) | U (µg/g) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SMS-1 | 1481.3 | black shale | L1.1 | 1 M HAc | carbonate | LOD | 6.1 | 9.9 | 6.1 | 1.4 | 0.91 | 0.42 | 0.06 |
| SMS-3 | 1480 | OM-rich Mn carbonate | L1.3 | 1 M HAc | carbonate | LOD | 8.3 | 12 | 7.2 | 3.0 | 1.7 | 0.67 | 0.02 |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L1.5 | 1 M HAc | carbonate | LOD | 17 | 21 | 3.8 | 1.6 | 0.42 | 1.3 | 0.08 |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L1.11 | 1 M HAc | carbonate | LOD | 1.2 | 4.6 | 11 | 3.4 | 1.4 | 0.26 | LOD |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L1.19 | 1 M HAc | carbonate | LOD | 0.9 | 4.7 | 11.0 | 5.3 | 2.1 | 0.30 | 0.063 |
| SMS-1 | 1481.3 | black shale | L2.1 | 2 M HCl | OM | LOD | 2.6 | 17 | 4.2 | 3.0 | 1.7 | 4.3 | LOD |
| SMS-3 | 1480 | OM-rich Mn carbonate | L2.3 | 2 M HCl | OM | LOD | 4.6 | 40 | 42 | 4.3 | 2.1 | 7.7 | 0.094 |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L2.5 | 2 M HCl | OM | LOD | 4 | 15 | 174 | 2.0 | 0.68 | 8.6 | LOD |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L2.11 | 2 M HCl | OM | LOD | 0.65 | 21 | 62 | 1.9 | 1.6 | 2.6 | 0.098 |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L2.19 | 2 M HCl | OM | LOD | 0.44 | 25 | 64 | 2.9 | 1.6 | 3.3 | 0.21 |
| SMS-1 | 1481.3 | black shale | L3.1 | 1M NaOH | sulphate | 16 | 0.15 | 48 | 1053 | 3.4 | 0.9 | 5.6 | LOD |
| SMS-3 | 1480 | OM-rich Mn carbonate | L3.3 | 1M NaOH | sulphate | 4.8 | 0.16 | 57 | 1156 | 3.2 | 1.7 | 8.0 | LOD |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L3.5 | 1M NaOH | sulphate | 27 | 0.21 | 67 | 1383 | 2.4 | 1.7 | 15 | LOD |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L3.11 | 1M NaOH | sulphate | 25 | 0.15 | 65 | 1428 | 1.2 | 1.2 | 2.9 | LOD |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L3.19 | 1M NaOH | sulphate | 27 | 0.15 | 73 | 1509 | 1.5 | 1.3 | 3.3 | LOD |
| SMS-1 | 1481.3 | black shale | L4.1 | Aqua Regia | sulphide | LOD | 17 | 19 | 34 | 2.2 | 4.4 | 13 | LOD |
| SMS-3 | 1480 | OM-rich Mn carbonate | L4.3 | Aqua Regia | sulphide | LOD | 17 | 17 | 25 | 2.4 | 6.5 | 15 | 0.016 |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L4.5 | Aqua Regia | sulphide | LOD | 17 | 18 | 22 | 2.0 | 1.3 | 19 | LOD |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L4.11 | Aqua Regia | sulphide | LOD | 12 | 19 | 15 | 2.3 | 4.9 | 3.7 | LOD |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L4.19 | Aqua Regia | sulphide | LOD | 15 | 30 | 16 | 2.6 | 4.6 | 4.8 | 0.026 |
| SMS-1 | 1481.3 | black shale | L5.1 | conc. HF+HNO3 | silicate | 46 | 1.3 | 16 | 311 | 79 | 20 | 1.2 | 0.48 |
| SMS-3 | 1480 | OM-rich Mn carbonate | L5.3 | conc. HF+HNO3 | silicate | 251 | 5.0 | 71 | 1295 | 359 | 96 | 6.0 | 3.0 |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L5.5 | conc. HF+HNO3 | silicate | 71 | 1.5 | 45 | 387 | 107 | 26 | 1.6 | 0.67 |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L5.11 | conc. HF+HNO3 | silicate | 890 | 13 | 437 | 6765 | 1020 | 307 | 5.3 | 13 |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L5.19 | conc. HF+HNO3 | silicate | 101 | 1.9 | 37 | 600 | 121 | 38 | 0.93 | 1.0 |
| SDO-1.1 | - | OM-rich silt shale | bulk (n = 5) | HF/HNO3 bomb | total digest. | 195 | 47 | 57 | 396 | 172 | 69 | 170 | 45 |
| Govindaraju et al., 1994 | 165 | 47 | 64 | 397 | 160 | 66 | 134 | 49 | |||||
| accuracy (%) | 122 | 109 | 95 | 96 | 119 | 116 | 127 | 82 | |||||
| GBW07107 (GSR-5) | shale | bulk (n = 6) | HF/HNO3 bomb | total digest. | 120 | 24 | 61 | 406 | 130 | 131 | 0.5 | 1.3 | |
| Govindaraju et al., 1994 | 96 | 21 | 50 | 450 | 87 | 99 | 0.4 | 1.5 | |||||
| accuracy (%) | 125 | 117 | 122 | 90 | 150 | 133 | 147 | 86 | |||||
| LOD = below detection limit (blank+ 3x standard deviation of blank) | |||||||||||||
| Sample ID | Depth (m) | Lithology | Leach Sample | Reagent | Leached Phase | Ni (µg/g) | Cu (µg/g) | Mn (µg/g) | Ti (µg/g) | Sr (µg/g) | Nb (µg/g) | Cd (µg/g) | Th (µg/g) |
| SMS-1 | 1481.3 | black shale | L1.1 | 1 M HAc | carbonate | 20 | 11 | 676 | 0.51 | 24 | 0.12 | 0.37 | LOD |
| SMS-3 | 1480 | OM-rich Mn carbonate | L1.3 | 1 M HAc | carbonate | 24 | 12 | 3811 | LOD | 40 | 0.043 | 0.39 | 0.003 |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L1.5 | 1 M HAc | carbonate | 35 | 21 | 2205 | LOD | 20 | 0.063 | 0.41 | 0.20 |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L1.11 | 1 M HAc | carbonate | 1.7 | 0.41 | 2829 | LOD | 40 | 0.034 | 0.070 | 0.081 |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L1.19 | 1 M HAc | carbonate | 2.2 | 0.19 | 4098 | LOD | 48 | 0.032 | 0.066 | 0.099 |
| SMS-1 | 1481.3 | black shale | L2.1 | 2 M HCl | OM | 4.7 | 13 | 117 | 2.8 | 12 | 0.038 | 0.16 | 0.59 |
| SMS-3 | 1480 | OM-rich Mn carbonate | L2.3 | 2 M HCl | OM | 6.4 | 14 | 407 | 5.7 | 20 | 0.038 | 0.30 | 1.0 |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L2.5 | 2 M HCl | OM | 4 | 11 | 224 | 0.67 | 29 | 0.046 | 0.082 | 2.3 |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L2.11 | 2 M HCl | OM | 2.4 | 2.5 | 234 | 7.1 | 1.8 | 0.038 | 0.092 | 1.1 |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L2.19 | 2 M HCl | OM | 1.9 | 2.7 | 319 | 10 | 11 | 0.038 | 0.099 | 1.3 |
| SMS-1 | 1481.3 | black shale | L3.1 | 1M NaOH | sulphate | LOD | 0.17 | 11 | 5.3 | 12 | 0.090 | 0.035 | LOD |
| SMS-3 | 1480 | OM-rich Mn carbonate | L3.3 | 1M NaOH | sulphate | LOD | 0.20 | 7.0 | 2.6 | 9.6 | 0.059 | 0.002 | LOD |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L3.5 | 1M NaOH | sulphate | LOD | 0.48 | 13 | 6.5 | 17 | 0.11 | 0.065 | LOD |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L3.11 | 1M NaOH | sulphate | LOD | 0.26 | 7.8 | 5.6 | 16 | 0.11 | 0.020 | LOD |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L3.19 | 1M NaOH | sulphate | LOD | 0.29 | 7.0 | 6.2 | 17 | 0.11 | 0.016 | LOD |
| SMS-1 | 1481.3 | black shale | L4.1 | Aqua Regia | sulphide | 21 | 28 | 256 | 12 | 3.7 | 0.12 | 0.23 | 1.1 |
| SMS-3 | 1480 | OM-rich Mn carbonate | L4.3 | Aqua Regia | sulphide | 22 | 25 | 392 | 7.6 | 4.4 | 0.075 | 0.25 | 1.6 |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L4.5 | Aqua Regia | sulphide | 17 | 24 | 309 | 3.9 | 6.3 | 0.046 | 0.22 | 1.1 |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L4.11 | Aqua Regia | sulphide | 24 | 25 | 312 | 5.6 | 2.0 | 0.060 | 0.23 | 2.0 |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L4.19 | Aqua Regia | sulphide | 27 | 27 | 372 | 5.9 | 2.2 | 0.060 | 0.27 | 2.6 |
| SMS-1 | 1481.3 | black shale | L5.1 | conc. HF+HNO3 | silicate | 0.82 | 2.8 | 35 | 1114 | 5.0 | 1.7 | 0.025 | LOD |
| SMS-3 | 1480 | OM-rich Mn carbonate | L5.3 | conc. HF+HNO3 | silicate | 8.0 | 12 | 139 | 5855 | 19 | 6.7 | 0.24 | 0.86 |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L5.5 | conc. HF+HNO3 | silicate | 0.45 | 2.8 | 56 | 1167 | 9.5 | 1.5 | 0.047 | LOD |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L5.11 | conc. HF+HNO3 | silicate | 28 | 38 | 585 | 17049 | 88 | 8.9 | 0.82 | 7.9 |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L5.19 | conc. HF+HNO3 | silicate | 2.6 | 4.1 | 87 | 2773 | 6.9 | 9.8 | 0.084 | LOD |
| SDO-1.1 | OM-rich silt shale | bulk (n = 5) | HF/HNO3 bomb | total digest. | 107 | 52 | 313 | 4506 | 87 | 15 | 0.51 | 5.9 | |
| Govindaraju et al., 1994 | 100 | 60 | 300 | 4260 | 75 | 11 | 0.30 | 10 | |||||
| accuracy (%) | 108 | 87 | 104 | 106 | 116 | 127 | 170 | 59 | |||||
| GBW07107 (GSR-5) | shale | bulk (n = 6) | HF/HNO3 bomb | total digest. | 45 | 42 | 180 | 1158 | 103 | 15 | 0.030 | 7.0 | |
| Govindaraju et al., 1994 | 37 | 42 | 173 | 4719 | 90 | 14 | 0.033 | 13 | |||||
| accuracy (%) | 123 | 101 | 104 | 25 | 115 | 102 | 92 | 54 | |||||
| LOD = below detection limit (blank+ 3x standard deviation of blank) | |||||||||||||
| Sample ID | Lithology | Core Depth (m) | δ13Ccarb | 2 SD | δ18Ocarb | 2 SD | δ13Corg | 2 SD | Total Organic Carbon (wt.%) |
|---|---|---|---|---|---|---|---|---|---|
| SMS-1 | black shale | 1481.3 | −12.21 | 0.34 | −11.03 | 0.66 | −31.76 | 0.03 | 3.78 |
| SMS-3 | OM-rich Mn carbonate | 1480 | −12.54 | 0.64 | −9.31 | 0.51 | −31.43 | 0.88 | 3.97 |
| SMS-5 | OM-rich Mn carbonate | 1479.3 | −10.98 | 0.55 | −8.35 | 0.77 | −31.59 | 0.03 | 3.2 |
| SMS-11 | OM-rich Mn carbonate | 1477.3 | −8.15 | 0.15 | −9.01 | 0.11 | −31.51 | 0.17 | 3.33 |
| SMS-19 | OM-rich Mn carbonate | 1475.1 | −6.7 | 0.12 | −9.17 | 0.08 | −31.49 | 0.01 | 2.95 |
| Sample ID | Depth (m) | Lithology | Leach Sample | Reagent | Leached Phase | ε112/110CdNIST | 2SE | Cd ID (ng) |
|---|---|---|---|---|---|---|---|---|
| SMS-1 | 1481.3 | black shale | L1.1 | 1 M HAc | carbonate | 0.66 | 1.1 | 235 |
| SMS-3 | 1480 | OM-rich Mn carbonate | L1.3 | 1 M HAc | carbonate | −1.0 | 2.9 | 53.2 |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L1.5 | 1 M HAc | carbonate | −0.48 | 0.5 | 280 |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L1.11 | 1 M HAc | carbonate | 0.14 | 0.3 | 48.9 |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L1.19 | 1 M HAc | carbonate | 0.48 | 0.2 | 49.9 |
| SMS-1 | 1481.3 | black shale | L2.1 | 2 M HCl | OM | 2.9 | 1.4 | 64.0 |
| SMS-3 | 1480 | OM-rich Mn carbonate | L2.3 | 2 M HCl | OM | 0.67 | 0.6 | 10.6 |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L2.5 | 2 M HCl | OM | 0.13 | 0.9 | 178 |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L2.11 | 2 M HCl | OM | 1.5 | 0.2 | 12.8 |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L2.19 | 2 M HCl | OM | 1.5 | 0.2 | 73.3 |
| SMS-1 | 1481.3 | black shale | L3.1 | 1M NaOH | sulphate | n.a. | n.a. | |
| SMS-3 | 1480 | OM-rich Mn carbonate | L3.3 | 1M NaOH | sulphate | n.a. | n.a. | |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L3.5 | 1M NaOH | sulphate | n.a. | n.a. | |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L3.11 | 1M NaOH | sulphate | n.a. | n.a. | |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L3.19 | 1M NaOH | sulphate | n.a. | n.a. | |
| SMS-1 | 1481.3 | black shale | L4.1 | Aqua Regia | sulphide | 1.5 | 0.4 | 166 |
| SMS-3 | 1480 | OM-rich Mn carbonate | L4.3 | Aqua Regia | sulphide | 0.92 | 0.8 | 154 |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L4.5 | Aqua Regia | sulphide | 3.2 | 1.7 | 155 |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L4.11 | Aqua Regia | sulphide | 0.8 | 0.3 | 156 |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L4.19 | Aqua Regia | sulphide | 1.0 | 0.1 | 237 |
| SMS-19# | 1475.1 | OM-rich Mn carbonate | L4.19# | Aqua Regia | sulphide | 0.6 | 0.8 | 244 |
| SMS-1 | 1481.3 | black shale | L5.1 | conc. HF+HNO3 | silicate | 0.02 | 0.8 | 42.5 |
| SMS-3 | 1480 | OM-rich Mn carbonate | L5.3 | conc. HF+HNO4 | silicate | −0.34 | 1 | 15.5 |
| SMS-5 | 1479.3 | OM-rich Mn carbonate | L5.5 | conc. HF+HNO5 | silicate | n.a. | n.a. | n.a. |
| SMS-11 | 1477.3 | OM-rich Mn carbonate | L5.11 | conc. HF+HNO6 | silicate | n.a. | n.a. | n.a. |
| SMS-19 | 1475.1 | OM-rich Mn carbonate | L5.19 | conc. HF+HNO7 | silicate | n.a. | n.a. | n.a. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohl, S.V.; Jiang, S.-Y.; Viehmann, S.; Wei, W.; Liu, Q.; Wei, H.-Z.; Galer, S.J.G. Trace Metal and Cd Isotope Systematics of the Basal Datangpo Formation, Yangtze Platform (South China) Indicate Restrained (Bio)Geochemical Metal Cycling in Cryogenian Seawater. Geosciences 2020, 10, 36. https://doi.org/10.3390/geosciences10010036
Hohl SV, Jiang S-Y, Viehmann S, Wei W, Liu Q, Wei H-Z, Galer SJG. Trace Metal and Cd Isotope Systematics of the Basal Datangpo Formation, Yangtze Platform (South China) Indicate Restrained (Bio)Geochemical Metal Cycling in Cryogenian Seawater. Geosciences. 2020; 10(1):36. https://doi.org/10.3390/geosciences10010036
Chicago/Turabian StyleHohl, Simon V., Shao-Yong Jiang, Sebastian Viehmann, Wei Wei, Qian Liu, Hai-Zhen Wei, and Stephen J.G. Galer. 2020. "Trace Metal and Cd Isotope Systematics of the Basal Datangpo Formation, Yangtze Platform (South China) Indicate Restrained (Bio)Geochemical Metal Cycling in Cryogenian Seawater" Geosciences 10, no. 1: 36. https://doi.org/10.3390/geosciences10010036
APA StyleHohl, S. V., Jiang, S.-Y., Viehmann, S., Wei, W., Liu, Q., Wei, H.-Z., & Galer, S. J. G. (2020). Trace Metal and Cd Isotope Systematics of the Basal Datangpo Formation, Yangtze Platform (South China) Indicate Restrained (Bio)Geochemical Metal Cycling in Cryogenian Seawater. Geosciences, 10(1), 36. https://doi.org/10.3390/geosciences10010036





