Laterality as a Tool for Assessing Breed Differences in Emotional Reactivity in the Domestic Cat, Felis silvestris catus
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Food-Reaching Test
2.3. Procedure
2.4. Statistical Analysis
2.5. Ethical Note
3. Results
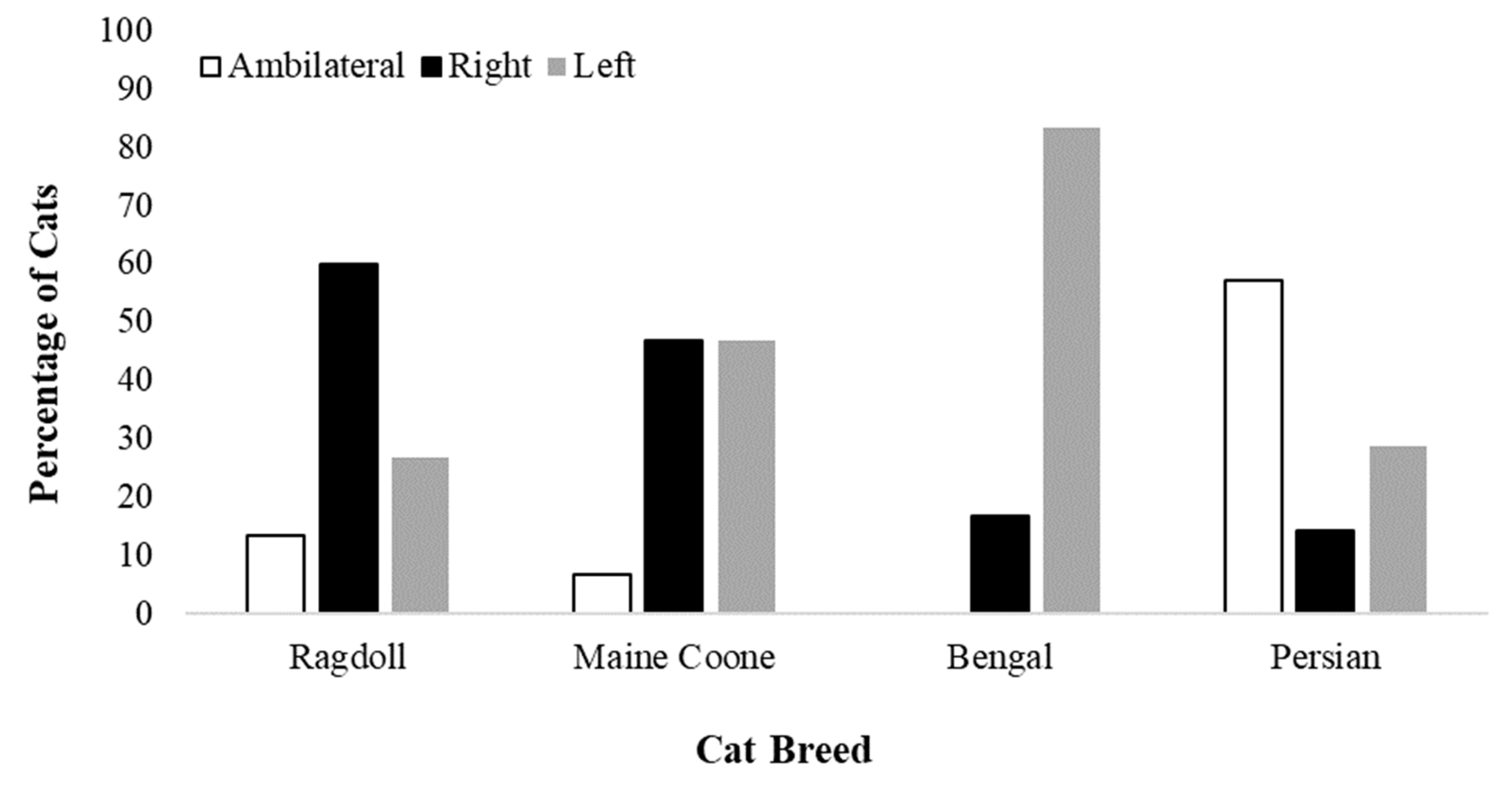
3.1. Distribution of Paw Preference
3.2. Direction of Paw Preference
3.3. Strength of Paw Preference
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harris, L.J. Laterality of Function in the Infant: Historical and Contemporary Trends in Theory and Research. In Manual Specialization and the Developing Brain; Elsevier: British Columbia, Canada, 1983; pp. 177–247. [Google Scholar]
- Springer, S.P.; Deutsch, G. Left Brain, Right Brain; W.H. Freeman: New York, NY, USA, 1989. [Google Scholar]
- Annett, M. Left, Right, Hand and Brain: The Right Shift Theory; Lawrence Erlbaum: New York, NY, USA, 1985. [Google Scholar]
- Porac, C.; Coren, S. Lateral Preferences and Human Behavior; Springer Science and Business Media LLC: Berlin, Germany, 1981. [Google Scholar]
- Frasnelli, E.; Vallortigara, G.; Rogers, L.J. Left–right asymmetries of behaviour and nervous system in invertebrates. Neurosci. Biobehav. Rev. 2012, 36, 1273–1291. [Google Scholar] [CrossRef] [PubMed]
- MacNeilage, P.F.; Rogers, L.J.; Vallortigara, G. Origins of the left and right brain. Sci. Am. 2009, 301, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Lateralization in vertebrates: Its early evolution, general pattern, and development. Adv. Study Behav. 2002, 31, 107–161. [Google Scholar]
- Rogers, L.J.; Vallortigara, G.; Andrew, R.J. Divided Brains: The Biology and Behaviour of Brain Asymmetries; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Vallortigara, G.; Chiandetti, C.; Sovrano, V.A. Brain asymmetry (animal). WIREs Cog. Sci. 2010, 1, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G.; Rogers, L.J. Survival with an asymmetrical brain: Advantages and disadvantages of cerebral lateralization. Behav. Brain Sci. 2005, 28, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Vallortigara, G.; Versace, E. Laterality at the neural, cognitive, and behavioral levels. In APA Handbook of Comparative Psychology: Basic Concepts, Methods, Neural Substrate, and Behavior; Call, J., Burghardt, G.M., Pepperberg, I.M., Snowdon, C.T., Zentall, T., Eds.; American Psychological Association: Washington, DC, USA, 2017; pp. 557–577. [Google Scholar]
- Corballis, M.C. The evolution and genetics of cerebral asymmetry. Phil. Trans. R. Soc. B 2009, 364, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.J. Hand and paw preferences in relation to the lateralized brain. Phil. Trans. R. Soc. B 2009, 364, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Versace, E.; Vallortigara, G. Forelimb preferences in human beings and other species: Multiple models for testing hypotheses on lateralization. Front. Psychol. 2015, 6, 233. [Google Scholar] [CrossRef] [PubMed]
- MacNeilage, P.F.; Studdert-Kennedy, M.G.; Lindblom, B. Primate handedness reconsidered. Behav. Brain Sci. 1987, 10, 247–263. [Google Scholar] [CrossRef]
- Stancher, G.; Sovrano, V.A.; Vallortigara, G. Motor asymmetries in fishes, amphibians, and reptiles. Progress Brain. Res. 2018, 238, 33–56. [Google Scholar]
- Diamond, A.C.; McGrew, W.C. True handedness in the cotton-top tamarin (Saguinus oedipus). Primates 1994, 35, 69–77. [Google Scholar] [CrossRef]
- Laska, M. Manual Laterality in Spider Monkeys (Ateles geoffroyi) Solving Visually and Tactually Guided Food-Reaching Tasks. Cortex 1996, 32, 717–726. [Google Scholar] [CrossRef]
- Clapham, P.J.; Leimkuhler, E.; Gray, B.K.; Mattila, D.K. Do humpback whales exhibit lateralized behaviour? Anim. Behav. 1995, 50, 73–82. [Google Scholar] [CrossRef]
- Brown, C.; Magat, M. The evolution of lateralized foot use in parrots: A phylogenetic approach. Behav. Ecol. 2011, 22, 1201–1208. [Google Scholar] [CrossRef][Green Version]
- Anderson, D.M.; Murray, L.W. Sheep laterality. Laterality 2013, 18, 179–193. [Google Scholar] [CrossRef] [PubMed]
- Morgante, M.; Gianesella, M.; Versace, E.; Contalbrigo, L.; Casella, S.; Cannizzo, C.; Piccione, G.; Stelletta, C. Preliminary study on metabolic profile of pregnant and non-pregnant ewes with high or low degree of behavioural lateralization. Anim. Sci. J. 2010, 81, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Versace, E.; Morgante, M.; Pulina, G.; Vallortigara, G. Behavioural lateralization in sheep (Ovis aries). Behav. Brain Res. 2007, 184, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Austin, N.; Rogers, L. Limb preferences and lateralization of aggression, reactivity and vigilance in feral horses, Equus caballus. Anim. Behav. 2012, 83, 239–247. [Google Scholar] [CrossRef]
- Austin, N.P.; Rogers, L.J. Lateralization and agonistic and vigilance responses in Prewalski horses (Equus przewalskii). Appl. Anim. Behav. Sci. 2014, 151, 43–50. [Google Scholar] [CrossRef]
- Lucidi, P.; Bacco, G.; Sticco, M.; Mazzoleni, G.; Benvenuti, M.; Bernabò, N.; Trentini, R. Assessment of motor laterality in foals and young horses (Equus caballus) through an analysis of derailment at trot. Physiol. Behav. 2013, 109, 8–13. [Google Scholar] [CrossRef]
- Siniscalchi, M.; D’Ingeo, S.; Quaranta, A. Lateralized Functions in the Dog Brain. Symmetry 2017, 9, 71. [Google Scholar] [CrossRef]
- McDowell, L.J.; Wells, D.L.; Hepper, P.G.; Dempster, M. Lateral bias and temperament in the domestic cat (Felis silvestris). J. Comp. Psychol. 2016, 130, 313–320. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.J.; Wells, D.L.; Hepper, P.G. Lateralisation of spontaneous behaviours in the domestic cat, Felis silvestris. Anim. Behav. 2018, 135, 37–43. [Google Scholar] [CrossRef]
- Wells, D.L.; Millsopp, S. Lateralised behaviour in the domestic cat, Felis silvestris catus. Anim. Behav. 2009, 78, 537–541. [Google Scholar] [CrossRef]
- Wells, D.L.; Millsopp, S. The ontogeny of lateralised behaviour in the domestic cat, Felis silvestris catus. J. Comp. Psychol. 2012, 126, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Bell, A.T.; Niven, J.E. Individual-level, context-dependent handedness in the desert locust. Curr. Biol. 2014, 24, R382–R383. [Google Scholar] [CrossRef]
- Ades, C.; Ramires, E.N. Asymmetry of Leg Use During Prey Handling in the Spider Scytodes globula (Scytodidae). J. Insect Behav. 2002, 15, 563–570. [Google Scholar] [CrossRef]
- Barnard, S.; Wells, D.L.; Hepper, P.G.; Milligan, A.D.S. Association between lateral bias and personality traits in the domestic dog. J. Comp. Psychol. 2017, 131, 246–256. [Google Scholar] [CrossRef]
- Gordon, D.J.; Rogers, L.J. Cognitive bias, hand preference and welfare of common marmosets. Behav. Brain Res. 2015, 287, 100–108. [Google Scholar] [CrossRef]
- Wells, D.L.; Hepper, P.G.; Milligan, A.D.S.; Barnard, S. Cognitive bias and paw preference in the domestic dog, Canis familiaris. J. Comp. Psychol. 2017, 131, 317–325. [Google Scholar] [CrossRef]
- Murphy, J.; Sutherland, A.; Arkins, S. Idiosyncratic motor laterality in the horse. Appl. Anim. Behav. Sci. 2005, 91, 297–310. [Google Scholar] [CrossRef]
- Wells, D.L. Lateralised behaviour in the domestic dog, Canis familiaris. Behav. Proc. 2003, 61, 27–35. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Thomson, P.C. Differences in motor laterality in breeds of performance horse. Appl. Anim. Behav. Sci. 2006, 99, 183–190. [Google Scholar] [CrossRef]
- McGreevy, P.D.; Brueckner, A.; Thomson, P.C.; Branson, N.J. Motor laterality in 4 breeds of dogs. J. Vet. Behav. 2010, 5, 318–323. [Google Scholar] [CrossRef]
- Hart, B.L.; Hart, L.A. Your Ideal Cat: Insights into Breed and Gender Differences in Cat Behavior; Purdue University Press: West Lafayette, IN, USA, 2013. [Google Scholar]
- Mendl, M.; Harcourt, R. Individuality in the domestic cat: Origins, development and stability. In The Domestic Cat: The Biology of its Behaviour; Turner, D.C., Bateson, P., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 48–64. [Google Scholar]
- Wilhelmy, J.; Serpell, J.; Brown, D.; Siracusa, C. Behavioral associations with breed, coat type, and eye color in single-breed cats. J. Vet. Behav. Clin. Appl. Res. 2016, 13, 80–87. [Google Scholar] [CrossRef]
- New, J.C., Jr.; Salman, M.; King, M.; Scarlett, J.M.; Kass, P.H.; Hutchison, J.M. Characteristics of shelter-relinquished animals and their owners compared with animals and their owners in US pet-owning households. J. Appl. Anim. Welf. Sci. 2000, 3, 179–201. [Google Scholar] [CrossRef]
- Powell, L.; Chia, D.; McGreevy, P.; Podberscek, A.L.; Edwards, K.M.; Neilly, B.; Guastella, A.J.; Lee, V.; Stamatakis, E. Expectations for dog ownership: Perceived physical, mental and psychosocial health consequences among prospective adopters. PLoS ONE 2018, 13, e0200276. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.J. Laterality in the Domestic Cat, Felis silvestris. Unpublished. Ph.D. Thesis, Queen’s University Belfast, Northern Ireland, UK, 2017. Unpublished. [Google Scholar]
- Pike, A.V.L.; Maitland, D.P. Paw preferences in cats (Felis silvestris catus) living in a household environment. Behav. Proc. 1997, 39, 241–247. [Google Scholar] [CrossRef]
- Tan, U.; Yaprak, M.; Kutlu, N. Paw preference in cats: Distribution and sex differences. Int. J. Neurosci. 1990, 50, 195–208. [Google Scholar] [CrossRef]
- Deckel, A.W. Laterality of aggressive responses in Anolis. J. Exp. Zool. 1995, 272, 194–200. [Google Scholar] [CrossRef]
- Siniscalchi, M.; Sasso, R.; Pepe, A.M.; Vallortigara, G.; Quaranta, A. Dogs turn left to emotional stimuli. Behav. Brain Res. 2010, 208, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Quaranta, A.; Siniscalchi, M.; Vallortigara, G. Asymmetric tail-wagging responses by dogs to different emotive stimuli. Curr. Biol. 2007, 17, R199–R201. [Google Scholar] [CrossRef] [PubMed]
- Siniscalchi, M.; Lusito, R.; Vallortigara, G.; Quaranta, A. Seeing left- or right-asymmetric tail wagging produces different emotional responses in dogs. Curr. Biol. 2013, 22, 2279–2282. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.J. Cerebral asymmetry, emotion, and affective style. In Brain Asymmetry; Davidson, R.J., Hugdahl, K., Eds.; MIT Press: Cambridge, MA, UAS, 1995; pp. 361–387. [Google Scholar]
- Yousry, T.A.; Schmid, U.D.; Jassoy, A.G.; Schmidt, D.; Eisner, W.E.; Reulen, H.J.; Reiser, M.F.; Lissner, J. Topography of the cortical motor hand area—Prospective-study with functional MR-imaging and direct motor mapping at surgery. Radiology 1995, 195, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Bennett, A. Handedness and approach-avoidance behaviour in chimpanzees. J. Exp. Psychol. 1994, 20, 413–418. [Google Scholar]
- Rogers, L.J. Relevance of brain and behavioural lateralization to animal welfare. Appl. Anim. Behav. Sci. 2010, 127, 1–11. [Google Scholar] [CrossRef]
- Branson, N.J.; Rogers, L.J. Relationship between paw preference strength and noise phobia in Canis familiaris. J. Comp. Psychol. 2006, 120, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Wells, D.L.; Hepper, P.G.; Milligan, A.D.S.; Barnard, S. Lack of association between paw preference and behaviour problems in the domestic dog, Canis familiaris. Appl. Anim. Behav. Sci. 2019, 210, 81–87. [Google Scholar] [CrossRef]
- Dharmaretnam, M.; Rogers, L.J. Hemispheric specialization and dual processing in strongly versus weakly lateralized chicks. Behav. Brain Res. 2005, 162, 62–70. [Google Scholar] [CrossRef]
- Quaranta, A.; Siniscalchi, M.; Frate, A.; Vallortigara, G. Paw preference in dogs: Relations between lateralised behaviour and immunity. Behav. Brain Res. 2004, 153, 521–525. [Google Scholar] [CrossRef]
- Batt, L.S.; Batt, M.S.; Baguley, J.A.; McGreevy, P.D. Factors associated with success in guide dog training. J. Vet. Behav. 2008, 3, 143–151. [Google Scholar] [CrossRef]
- Schneider, L.A.; Delfabbro, P.H.; Burns, N.R. Temperament and lateralization in the domestic dog (Canis familaris). J. Vet. Behav. 2013, 8, 124–134. [Google Scholar] [CrossRef]
- Bianki, V.L.; Filippova, E.B. Sex Differences in Lateralization in the Animal Brain; Harwood Academic Publishers: Sydney, Australia, 2001. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wells, D.L.; McDowell, L.J. Laterality as a Tool for Assessing Breed Differences in Emotional Reactivity in the Domestic Cat, Felis silvestris catus. Animals 2019, 9, 647. https://doi.org/10.3390/ani9090647
Wells DL, McDowell LJ. Laterality as a Tool for Assessing Breed Differences in Emotional Reactivity in the Domestic Cat, Felis silvestris catus. Animals. 2019; 9(9):647. https://doi.org/10.3390/ani9090647
Chicago/Turabian StyleWells, Deborah L., and Louise J. McDowell. 2019. "Laterality as a Tool for Assessing Breed Differences in Emotional Reactivity in the Domestic Cat, Felis silvestris catus" Animals 9, no. 9: 647. https://doi.org/10.3390/ani9090647
APA StyleWells, D. L., & McDowell, L. J. (2019). Laterality as a Tool for Assessing Breed Differences in Emotional Reactivity in the Domestic Cat, Felis silvestris catus. Animals, 9(9), 647. https://doi.org/10.3390/ani9090647




