Does Juvenile Play Programme the Equine Musculoskeletal System?
Abstract
Simple Summary
Abstract
1. Introduction
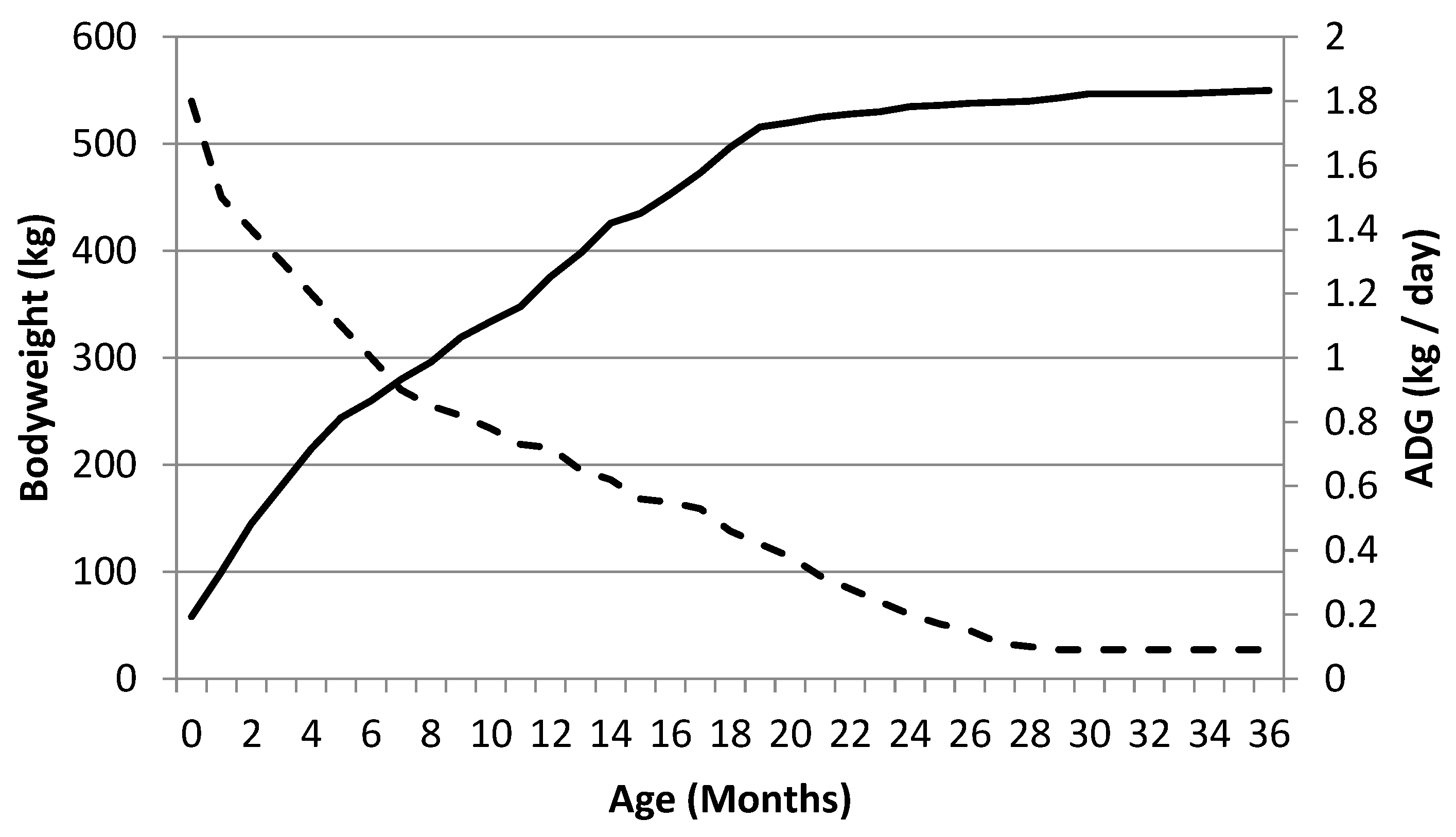
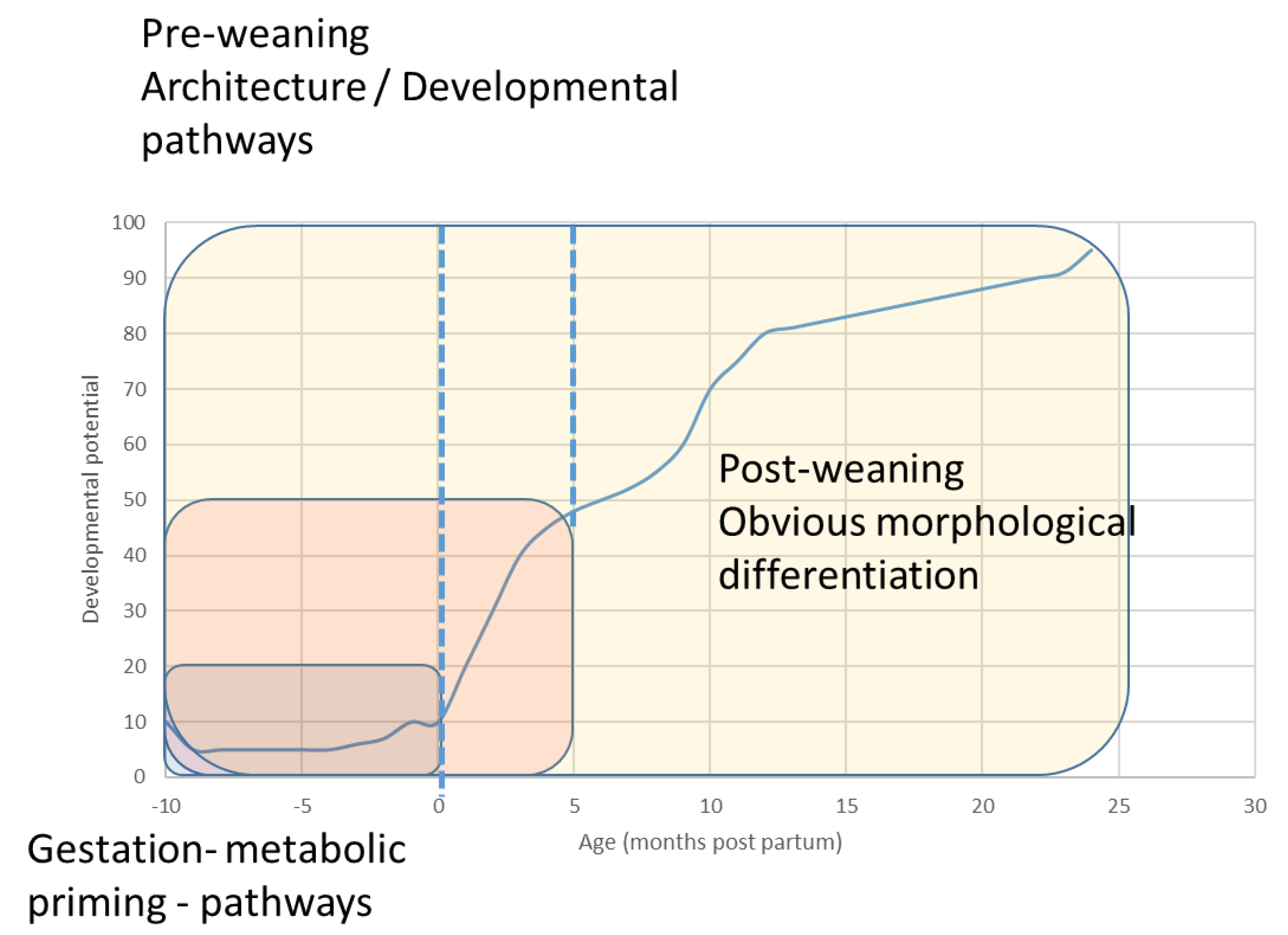
1.1. The Precocious Neonate
1.2. Innate Play in the Foal—What Does It Do?
2. Bone Adaption
Bone Cells, Mechanosensation and Transduction
3. Play
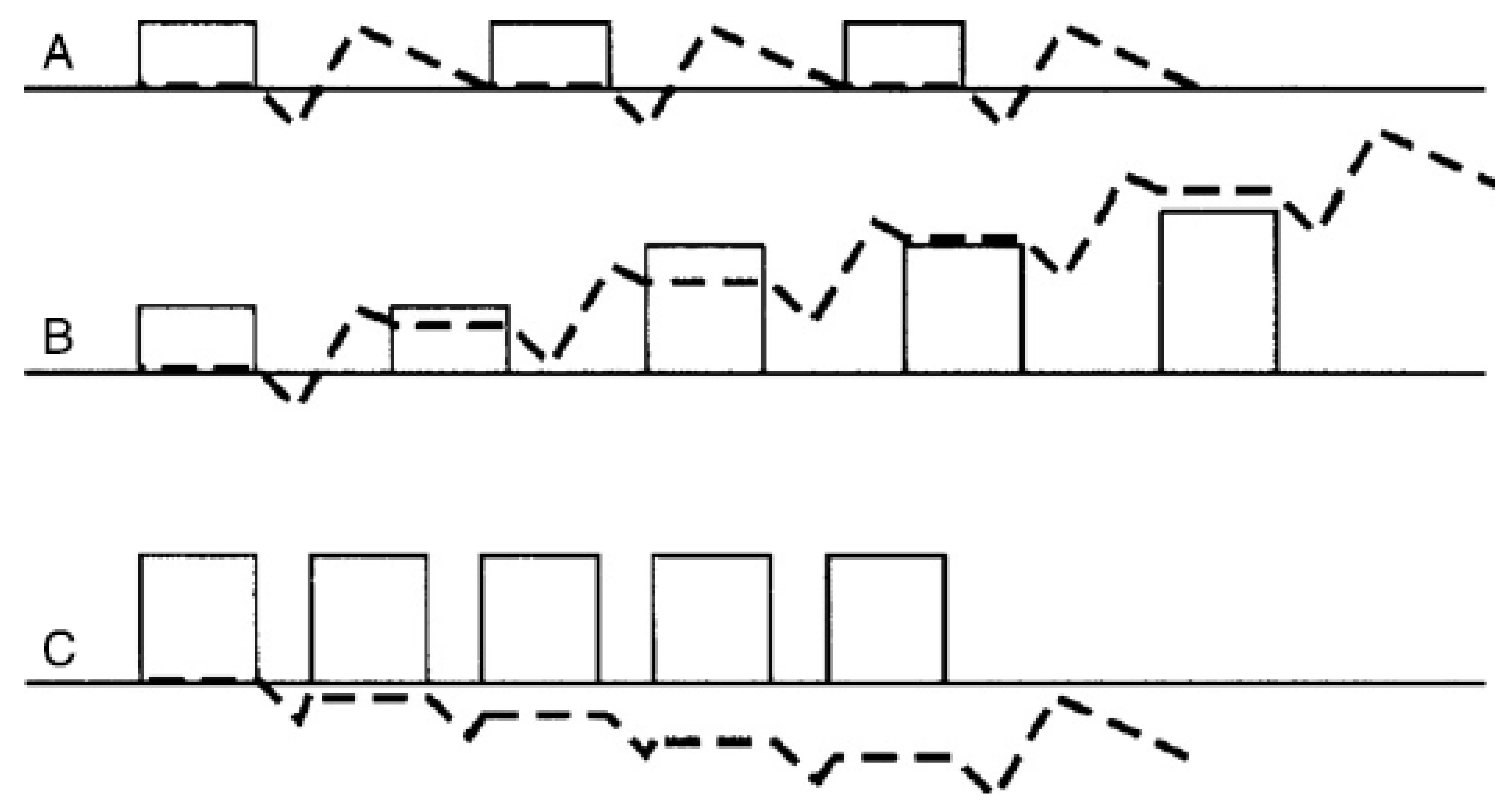
3.1. Play and the Mechanostat Theory
3.2. Play Should Be Osteo-Inductive
3.3. In Modern Management Systems, Play Drives Orientation of the Tissue for Future Load
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Barker, D.J.P. The origins of the developmental origins theory. J. Intern. Med. 2007, 261, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.W.; Firth, E.C.; McIlwraitth, C.W.; Barneveld, A.; Goodship, A.E.; Kawcak, C.E.; Smith, R.K.W.; van Weeren, P.R. Evaluation of a new strategy to modulate skeletal development in Thoroughbred performance horses by imposing track-based exercise during growth. Equine Vet. J. 2008, 40, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.W.; Bolwell, C.F.; Tanner, J.C.; Van Weeren, P.R. Early exercise in the horse. J. Vet. Behav. Clin. Appl. Res. 2012, 7, 375–379. [Google Scholar] [CrossRef]
- Cameron, E.Z.; Linklater, W.L.; Stafford, K.J.; Minot, E.O. Maternal investment results in better foal condition through increased play behaviour in horses. Anim. Behav. 2008, 76, 1511–1518. [Google Scholar] [CrossRef]
- Frost, H.M. Bone mass and the mechanostat—A proposal. Anat. Record. 1987, 219, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.T.; Lanyon, L.E. Regulation of bone-formation by applied dynamic loads. J. Bone Joint Surg. 1984, 66, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, C.; Birch, H.L.; Goodship, A.E.; Smith, R.K.W. 8—Skeletal physiology: Responses to exercise and training. In Equine Sports Medicine and Surgery, 2nd ed.; Hinchcliff, K., Kaneps, A.J., Geor, R.J., Eds.; WB Saunders: Philadelphia, PA, USA, 2014; pp. 145–165. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; Bakker, A.D.; Bacabac, R.G.; Vatsa, A.; Weinbaum, S. Mechanosensation and transduction in osteocytes. Bone 2013, 54, 182–190. [Google Scholar] [CrossRef]
- Franz-Odendaal, T.A.; Hall, B.K.; Witten, P.E. Buried Alive: How Osteoblasts Become Osteocytes. Dev. Dyn. 2006, 235, 176–190. [Google Scholar] [CrossRef]
- Han, Y.; Cowin, S.C.; Schaffler, M.B.; Weinbaum, S. Mechanotransduction and strain amplification in osteocyte cell processes. Proc. Natl. Acad. Sci. USA 2004, 101, 16689–16694. [Google Scholar] [CrossRef]
- Wang, B.; Lai, X.; Price, C.; Thompson, W.R.; Li, W.; Quabili, T.R.; Tseng, W.J.; Liu, X.S.; Zhang, H.; Pan, J.; et al. Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. J. Bone Min. Res. 2014, 29, 878–891. [Google Scholar] [CrossRef]
- Turner, C.H.; Forwood, M.R.; Otter, M.W. Mechanotransduction in bone: Do bone cells act as sensors of fluid flow? FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1994, 8, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Price, C.; Zhou, X.; Li, W.; Wang, L. Real-time measurement of solute transport within the lacunar-canalicular system of mechanically loaded bone: Direct evidence for load-induced fluid flow. J. Bone Min. Res. 2011, 26, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Henstock, J.R.; Rotherham, M.; Rose, J.B.; El Haj, A.J. Cyclic hydrostatic pressure stimulates enhanced bone development in the foetal chick femur in vitro. Bone 2013, 53, 468–477. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Cowin, S.C.; Schaffler, M.B.; Weinbaum, S. A model for strain amplification in the actin cytoskeleton of osteocytes due to fluid drag on pericellular matrix. J. Biomech. 2001, 34, 1375–1386. [Google Scholar] [CrossRef]
- Dittmer, K.E.; Firth, E.C. Mechanisms of bone response to injury. J. Vet. Diagn. Investig. 2017, 29, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Geoghegan, I.P.; Hoey, D.A.; McNamara, L.M. Integrins in Osteocyte Biology and Mechanotransduction. Curr. Osteoporos Rep. 2019, 17, 195–206. [Google Scholar] [CrossRef]
- Kringelbach, T.M.; Aslan, D.; Novak, I.; Ellegaard, M.; Syberg, S.; Andersen, C.K.B.; Kristiansen, K.A.; Vang, O.; Schwarz, P.; Jørgensen, N.R. Fine-tuned ATP signals are acute mediators in osteocyte mechanotransduction. Cell. Signal. 2015, 27, 2401–2409. [Google Scholar] [CrossRef] [PubMed]
- Genetos, D.C.; Kephart, C.J.; Zhang, Y.; Yellowley, C.E.; Donahue, H.J. Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J. Cell. Physiol. 2007, 212, 207–214. [Google Scholar] [CrossRef]
- Zaman, G.; Pitsillides, A.A.; Rawlinson, S.C.F.; Suswillo, R.F.L.; Mosley, J.R.; Cheng, M.Z.; Platts, L.A.M.; Hukkanen, M.; Polak, J.M.; Lanyon, L.E. Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J. Bone Miner. Res. 1999, 14, 1123–1131. [Google Scholar] [CrossRef]
- Jiang, J.X.; Cheng, B. Mechanical Stimulation of Gap Junctions in Bone Osteocytes is Mediated by Prostaglandin E2. Cell Commun. Adhes. 2001, 8, 283–288. [Google Scholar] [CrossRef]
- Santos, A.; Bakker, A.D.; Zandieh-Doulabi, B.; de Blieck-Hogervorst, J.M.; Klein-Nulend, J. Early activation of the beta-catenin pathway in osteocytes is mediated by nitric oxide, phosphatidyl inositol-3 kinase/Akt, and focal adhesion kinase. Biochem. Biophys. Res. Commun. 2010, 391, 364–369. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, M.A.; Burra, S.; Kar, R.; Lampe, P.D.; Jiang, J.X. Mitogen-activated Protein Kinase (MAPK) Activated by Prostaglandin E2 Phosphorylates Connexin 43 and Closes Osteocytic Hemichannels in Response to Continuous Flow Shear Stress. J. Biol. Chem. 2015, 290, 28321–28328. [Google Scholar] [CrossRef] [PubMed]
- Plotkin, L.I.; Mathov, I.; Aguirre, J.I.; Parfitt, A.M.; Manolagas, S.C.; Bellido, T. Mechanical stimulation prevents osteocyte apoptosis: Requirement of integrins, Src kinases and ERKs. Am. J. Physiol. Cell Physiol. 2005, 289, C633–C643. [Google Scholar] [CrossRef] [PubMed]
- Kitase, Y.; Barragan, L.; Qing, H.; Kondoh, S.; Jiang, J.X.; Johnson, M.L.; Bonewald, L.F. Mechanical induction of PGE2 in osteocytes blocks glucocorticoid-induced apoptosis through both the beta-catenin and PKA pathways. J. Bone Min. Res. 2010, 25, 2657–2668. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Temiyasathit, S.; Lee, P.; Kim, C.H.; Tummala, P.; Yao, W.; Kingery, W.; Malone, A.M.; Kwon, R.Y.; Jacobs, C.R. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone 2008, 42, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Tu, X.; Rhee, Y.; Condon, K.W.; Bivi, N.; Allen, M.R.; Dwyer, D.; Stolina, M.; Turner, C.H.; Robling, A.G.; Plotkin, L.I.; et al. Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 2012, 50, 209–217. [Google Scholar] [CrossRef]
- Lin, C.; Jiang, X.; Dai, Z.; Guo, X.; Weng, T.; Wang, J.; Li, Y.; Feng, G.; Gao, X.; He, L. Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J. Bone Min. Res. 2009, 24, 1651–1661. [Google Scholar] [CrossRef]
- Nishijima, Y.; Yamaguchi, M.; Kojima, T.; Aihara, N.; Nakajima, R.; Kasai, K. Levels of RANKL and OPG in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthod. Craniofacial Res. 2006, 9, 63–70. [Google Scholar] [CrossRef]
- Tyrovola, J.B. The “mechanostat Theory” of Frost and the OPG/RANKL/RANK System. J. Cell. Biochem. 2015, 116, 2724–2729. [Google Scholar] [CrossRef]
- Zhang, C.; Bakker, A.D.; Klein-Nulend, J.; Bravenboer, N. Studies on Osteocytes in Their 3D Native Matrix Versus 2D in Vitro Models. Curr. Osteoporos Rep. 2019, 17, 207–216. [Google Scholar] [CrossRef]
- Hughes, J.M.; Petit, M.A. Biological underpinnings of Frost’s mechanostat thresholds: The important role of osteocytes. J. Musculoskelet. Neuronal Interaction. 2010, 10, 128–135. [Google Scholar]
- Pontzer, H.; Raichlen, D.A.; Gordon, A.D.; Schroepfer-Walker, K.K.; Hare, B.; O’Neill, M.C.; Muldoon, K.M.; Dunsworth, H.M.; Wood, B.M.; Isler, K.; et al. Primate energy expenditure and life history. Proc. Natl. Acad. Sci. USA 2014, 111, 1433–1437. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.H.; Gillooly, J.F.; Allen, A.P.; Savage, V.M.; West, G.B. Toward a metabolic theory of ecology. Ecology 2004, 85, 1771–1789. [Google Scholar] [CrossRef]
- Berghanel, A.; Schulke, O.; Ostner, J. Locomotor play drives motor skill acquisition at the expense of growth: A life history trade-off. Sci. Adv. 2015, 1. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, L.L.; Clutton-Brock, T.H.; Brotherton, P.N.M.; Cameron, E.Z.; Cherry, M.I. Experimental provisioning increases play in free-ranging meerkats. Anim. Behav. 2002, 64, 113–121. [Google Scholar] [CrossRef]
- Byers, J.A.; Walker, C. Refining the motor training hypothesis for the evolution of play. Am. Nat. 1995, 146, 25–40. [Google Scholar] [CrossRef]
- McDonnell, S.M.; Poulin, A. Equid play ethogram. Appl. Anim. Behav. Sci. 2002, 78, 263–290. [Google Scholar] [CrossRef]
- Hampson, B.A.; Pollit, C.C. GPS analysis of activity of domestic mares and newborn foals. In Proceedings of the 6th International Conference on Equine Locomotion, Cabourg, France, 16–19 June 2008; p. 17. [Google Scholar]
- Hampson, B.A.; Morton, J.M.; Mills, P.C.; Trotter, M.G.; Lamb, D.W.; Pollitt, C.C. Monitoring distances travelled by horses using GPS tracking collars. Aust. Vet. J. 2010, 88, 176–181. [Google Scholar] [CrossRef]
- Brama, P.A.; Karssenberg, D.; Barneveld, A.; Van Weeren, P.R. Contact areas and pressure distribution on the proximal articular surface of the proximal phalanx under sagittal plane loading. Equine Vet. J. 2001, 33, 26–32. [Google Scholar] [CrossRef]
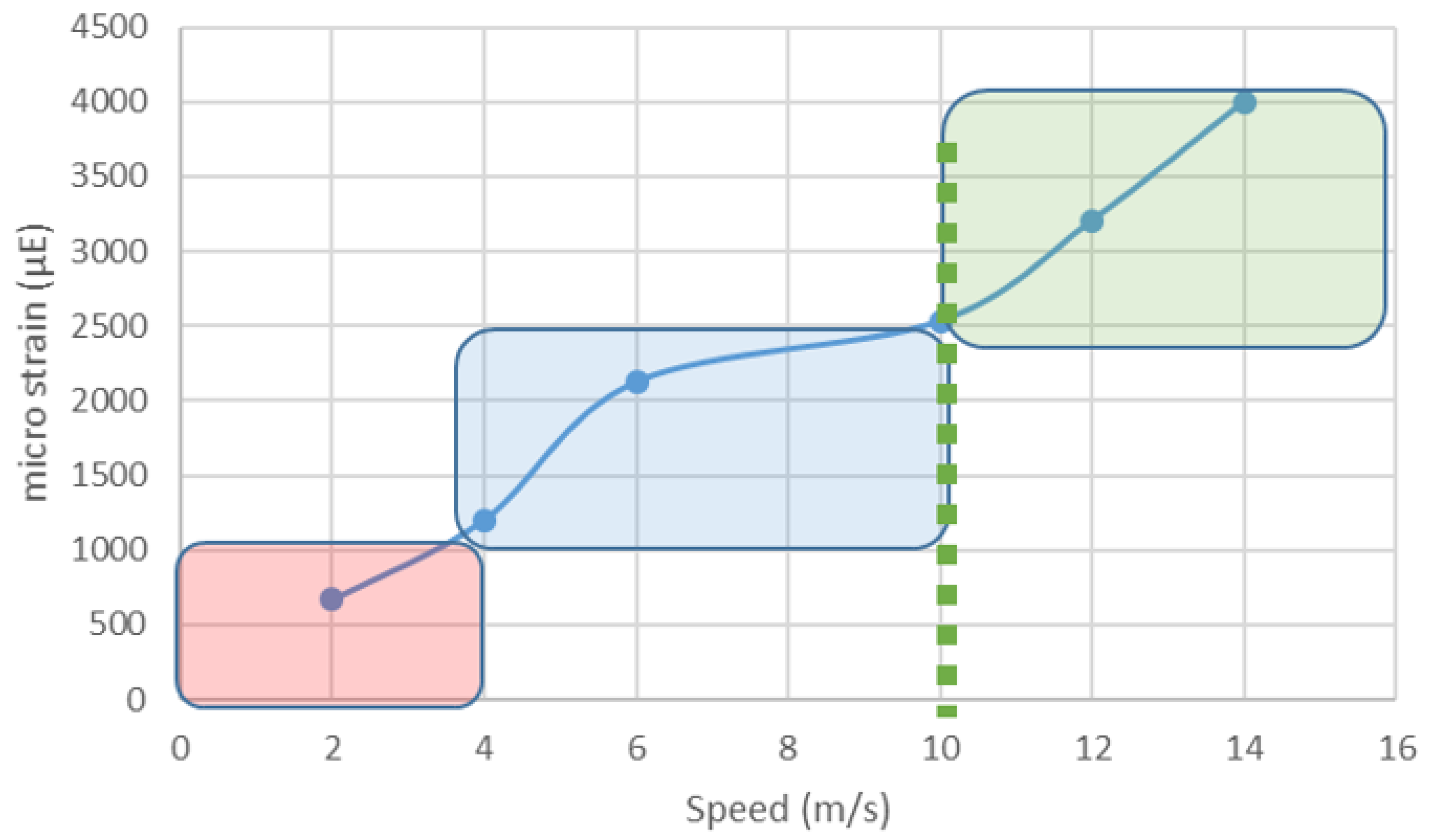
- Davies, H.M.S.; McCarthy, R.N.; Jeffcott, L.B. Surface strain on the dorsal metacarpus of Thoroughbreds at different speeds and gaits. Acta Anatatomica 1993, 146, 148–153. [Google Scholar] [CrossRef]
- Davies, H.M.S. The timing and distribution of strains around the surface of the midshaft of the third metacarpal bone during treadmill exercise in one Thoroughbred racehorse. Aust. Vet. J. 2005, 83, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Kurvers, C.M.H.C.; van Weeren, P.R.; Rogers, C.W.; van Dierendonck, M.C. Quantification of spontaneous locomotion activity in foals kept in pastures under various management conditions. Am. J. Vet. Res. 2006, 67, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Boy, V.; Duncan, P. Time-budgets of Carmargue horses. 1. Developmental changes in the time budgets of foals. Behaviour 1979, 71, 187–202. [Google Scholar] [CrossRef]
- Brama, P.A.; Tekoppele, J.M.; Bank, R.A.; van Weeren, P.R.; Barneveld, A. Influence of different exercise levels and age on the biochemical characteristics of immature equine articular cartilage. Equine Vet. J. Suppl. 1999, 31, 55–61. [Google Scholar] [CrossRef]
- Brama, P.A.; TeKoppele, J.M.; Bank, R.A.; Barneveld, A.; van Weeren, P.R. Development of biochemical heterogeneity of articular cartilage: Influences of age and exercise. Equine Vet. J. 2002, 34, 265–269. [Google Scholar] [CrossRef] [PubMed]
- van Weeren, P.R.; Firth, E.C.; Brommer, H.; Hyttinen, M.M.; Helminen, H.J.; Rogers, C.W.; De Groot, J.; Brama, P.A.J. Early exercise advances the maturation of glycosaminoglycans and collagen in the extracellular matrix of articular cartilage in the horse. Equine Vet. J. 2008, 40, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Dykgraaf, S.; Firth, E.C.; Rogers, C.W.; Kawcak, C.E. Effects of exercise on chondrocyte viability and subchondral bone sclerosis in the distal third metacarpal and metatarsal bones of young horses. Vet. J. 2008, 178, 53–61. [Google Scholar] [CrossRef]
- Barneveld, A.; van Weeren, P.R. Conclusions regarding the influence of exercise on the development of the equine musculoskeletal system with special reference to osteochondrosis. Equine Vet. J. Suppl. 1999, 31, 112–119. [Google Scholar] [CrossRef]
- Firth, E.C.; Rogers, C.W.; van Weeren, P.R.; Barneveld, A.; McIlwraith, C.W.; Kawcak, C.E.; Goodship, A.E.; Smith, R.K.W. Mild exercise early in life produces changes in bone size and strength but not density in proximal phalangeal, third metacarpal and third carpal bones of foals. Vet. J. 2011, 190, 383–389. [Google Scholar] [CrossRef]
- Firth, E.C.; Rogers, C.W.; van Weeren, P.R.; Barneveld, A.; McIlwraith, C.W.; Kawcak, C.E.; Goodship, A.E.; Smith, R.K.W. The effect of previous conditioning exercise on diaphyseal and metaphyseal bone responses to imposition and withdrawal of training in young Thoroughbred horses. Vet. J. 2012, 192, 34–40. [Google Scholar] [CrossRef]
- Chalmers, J.; Ray, R.D. The growth of transplanted foetal bones in different immunological environments. J. Bone Jt. Surg. Br. Vol. 1962, 44, 149–164. [Google Scholar] [CrossRef]
- Frost, H.M. A 2003 update of bone physiology and Wolff’s Law for clinicians. Angle Orthod. 2004, 74, 3–15. [Google Scholar] [PubMed]
- Robling, A.G. Is Bone’s Response to Mechanical Signals Dominated by Muscle Forces? Med. Sci. Sports Exerc. 2009, 41, 2044–2049. [Google Scholar] [CrossRef] [PubMed]
- Schoenua, E. From mechanostat theory to development of the “Functional Muscle-Bone-Unit”. J. Musculoskelet Neuronal Interact. 2005, 5, 232–238. [Google Scholar]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.J.; Walrand, S.; Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef] [PubMed]
- Back, W.; Smit, L.D.; Schamhardt, H.C.; Barneveld, A. The Influence of Different Exercise Regimens on the Development of Locomotion in the Foal. Equine Vet. J. 1999, 31, 106–111. [Google Scholar] [CrossRef]
- Clayton, H.M.; Hobbs, S.J. Ground Reaction Forces: The Sine Qua Non of Legged Locomotion. J. Equine Vet. Sci. 2019, 76, 25–35. [Google Scholar] [CrossRef]
- Harrison, S.M.; Whitton, R.C.; Kawcak, C.E.; Stover, S.M.; Pandy, M.G. Relationship between muscle forces, joint loading and utilization of elastic strain energy in equine locomotion. J. Exp. Biol. 2010, 213, 3998–4009. [Google Scholar] [CrossRef]
- Chateau, H.; Camus, M.; Holden-Douilly, L.; Falala, S.; Ravary, B.; Vergari, C.; Lepley, J.; Denoix, J.M.; Pourcelot, P.; Crevier-Denoix, N. Kinetics of the forelimb in horses circling on different ground surfaces at the trot. Vet. J. 2013, 198, E20–E26. [Google Scholar] [CrossRef]
- Rogers, C.W.; Rivero, J.L.L.; van Breda, E.A.L.; Sloet van Oldruitenborgh Oosterbaan, M. ICEEP7–Workshop—Workload and Conditioning: Describing workload and scientific information on conditioning horses. Equine Comp. Exerc. Physiol. 2007, 4, 1–6. [Google Scholar] [CrossRef]
- Crowelldavis, S.L.; Houpt, K.A.; Kane, L. Play development in Welsh pony (Equus-Caballus) foals. Appl. Anim. Behav. Sci. 1987, 18, 119–131. [Google Scholar] [CrossRef]
- Firth, E.C. The response of bone, articular cartilage and tendon to exercise in the horse. J. Anat. 2006, 208, 513–526. [Google Scholar] [CrossRef] [PubMed]
- Brama, P.A.J.; Tekoppele, J.M.; Bank, R.A.; Barneveld, A.; van Weeren, P.R. Functional adaptation of equine articular cartilage: The formation of regional biochemical characteristics up to age one year. Equine Vet. J. 2000, 32, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Rogers, C.W.; Firth, E.C.; McIlwraith, C.W.; Barneveld, A.; Goodship, A.E.; Kawcak, C.E.; Smith, R.K.W.; van Weeren, P.R. Evaluation of a new strategy to modulate skeletal development in racehorses by imposing track-based exercise during growth: The effects on 2-and 3-year-old racing careers. Equine Vet. J. 2008, 40, 119–127. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rogers, C.W.; Dittmer, K.E. Does Juvenile Play Programme the Equine Musculoskeletal System? Animals 2019, 9, 646. https://doi.org/10.3390/ani9090646
Rogers CW, Dittmer KE. Does Juvenile Play Programme the Equine Musculoskeletal System? Animals. 2019; 9(9):646. https://doi.org/10.3390/ani9090646
Chicago/Turabian StyleRogers, Chris W., and Keren E. Dittmer. 2019. "Does Juvenile Play Programme the Equine Musculoskeletal System?" Animals 9, no. 9: 646. https://doi.org/10.3390/ani9090646
APA StyleRogers, C. W., & Dittmer, K. E. (2019). Does Juvenile Play Programme the Equine Musculoskeletal System? Animals, 9(9), 646. https://doi.org/10.3390/ani9090646




