The Impact of Pre-Slaughter Stress on Beef Eating Quality
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Blood and Muscle Sampling
2.2. Consumer Sensory Testing
2.3. Statistical Analysis
3. Results
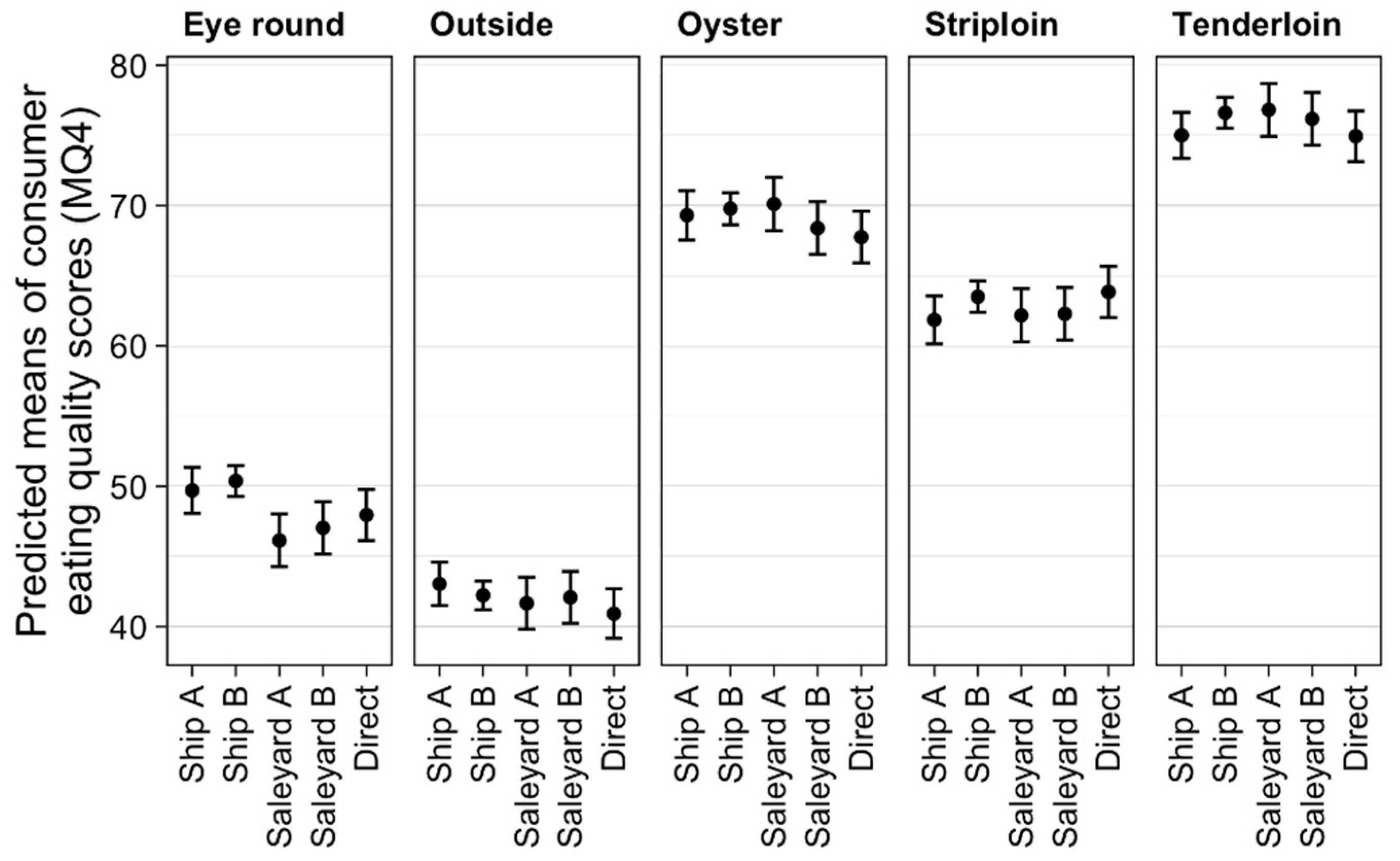
3.1. Effect of Treatments on Eating Quality
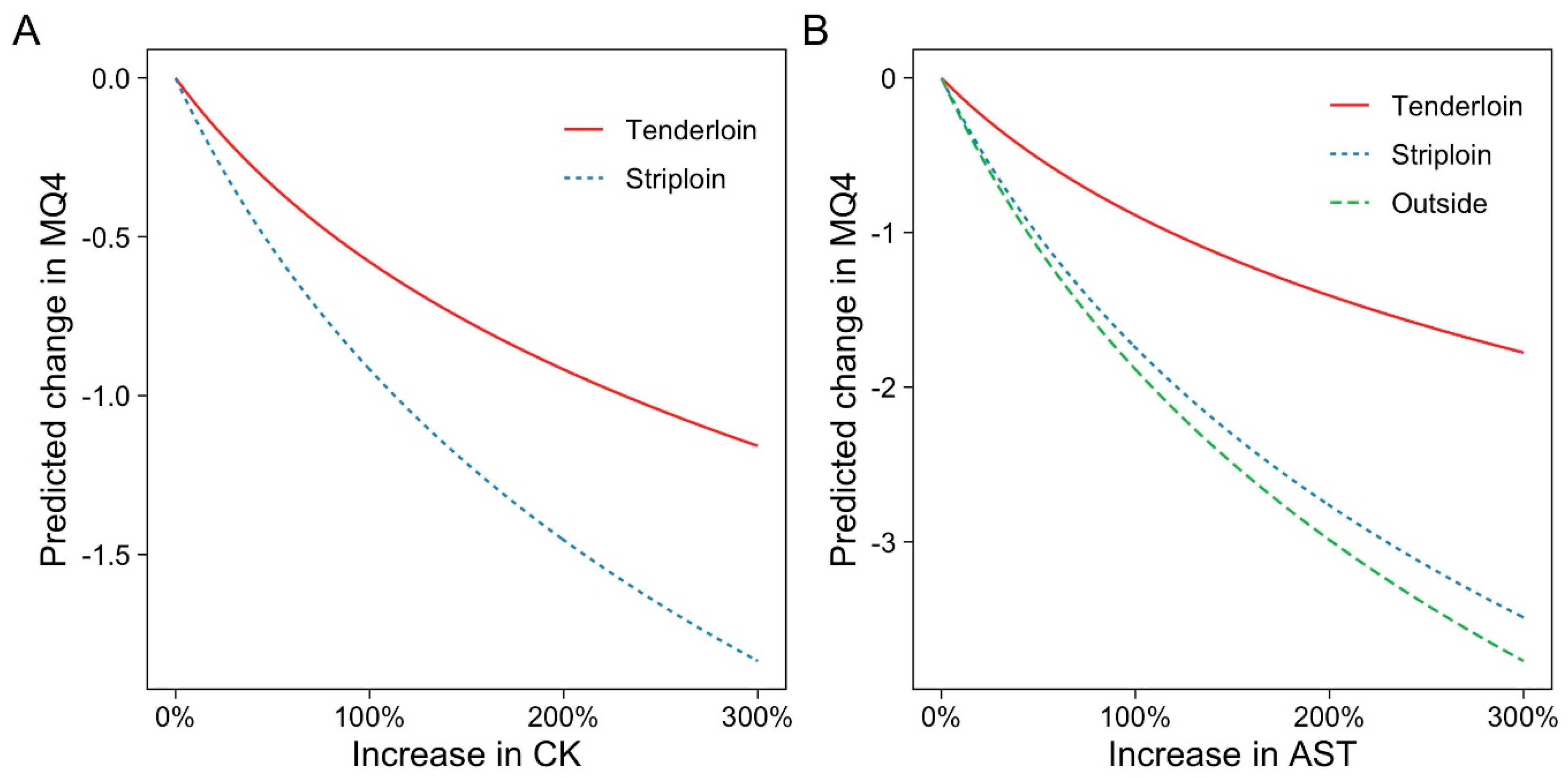
3.2. Associations between Blood Metabolites and Eating Quality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Watson, R.; Polkinghorne, R.; Thompson, J. Development of the meat standards australia (msa) prediction model for beef palatability. Aust. J. Exp. Agric. 2008, 48, 1368–1379. [Google Scholar] [CrossRef]
- Thompson, J. Managing meat tenderness. Meat Sci. 2002, 62, 295–308. [Google Scholar] [CrossRef]
- Polkinghorne, R.; Philpott, J.; Gee, A.; Doljanin, A.; Innes, J. Development of a commercial system to apply the Meat Standards Australia grading model to optimise the return on eating quality in a beef supply chain. Aust. J. Exp. Agric. 2008, 48, 1451–1458. [Google Scholar] [CrossRef]
- Lyford, C.; Thompson, J.; Polkinghorne, R.; Miller, M.; Nishimura, T.; Neath, K.; Allen, P.; Belasco, E.; Malcolm, B.; Wright, V. Is willingness to pay (WTP) for beef quality grades affected by consumer demographics and meat consumption preferences. Australas. Agribus. Rev. 2010, 18, 1–17. [Google Scholar]
- Griffith, G.R.; Thompson, J.M. The aggregate economic benefits to the Australian beef industry from the adoption of Meat Standards Australia: Updated to 2010/11. Australas. Agribus. Rev. 2012, 20, 1–28. [Google Scholar]
- Bonny, S.; Hocquette, J.F.; Pethick, D.; Legrand, I.; Wierzbicki, J.; Allen, P.; Farmer, L.; Polkinghorne, R.; Gardner, G. The variability of the eating quality of beef can be reduced by predicting consumer satisfaction. Animal 2018, 12, 2434–2442. [Google Scholar] [CrossRef]
- Ferguson, D.; Bruce, H.; Thompson, J.; Egan, A.; Perry, D.; Shorthose, W. Factors affecting beef palatability—Farmgate to chilled carcass. Aust. J. Exp. Agric. 2001, 41, 879–891. [Google Scholar] [CrossRef]
- Ferguson, D.; Warner, R. Have we underestimated the impact of pre-slaughter stress on meat quality in ruminants? Meat Sci. 2008, 80, 12–19. [Google Scholar] [CrossRef]
- Tarrant, P. Animal behaviour and environment in the dark-cutting condition in beef—A review. Ir. J. Food Sci. Technol. 1989, 12, 1–21. [Google Scholar]
- Warner, R.; Ferguson, D.; Cottrell, J.; Knee, B. Acute stress induced by the preslaughter use of electric prodders causes tougher beef meat. Aust. J. Exp. Agric. 2007, 47, 782–788. [Google Scholar] [CrossRef]
- Gruber, S.; Tatum, J.; Engle, T.; Chapman, P.; Belk, K.; Smith, G. Relationships of behavioral and physiological symptoms of preslaughter stress to beef longissimus muscle tenderness 1. J. Anim. Sci. 2010, 88, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, D.; Warner, R.; Walker, P.; Knee, B. Effect of cattle marketing method on beef quality and palatability. Aust. J. Exp. Agric. 2007, 47, 774–781. [Google Scholar] [CrossRef]
- Polkinghorne, R.; Philpott, J.; Thompson, J. Do extended transport times and rest periods impact on eating quality of beef carcasses? Meat Sci. 2018, 140, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Colditz, I.; Ferguson, D.; Greenwood, P.; Doogan, V.; Petherick, J.; Kilgour, R. Regrouping unfamiliar animals in the weeks prior to slaughter has few effects on physiology and meat quality in Bos taurus feedlot steers. Aust. J. Exp. Agric. 2007, 47, 763–769. [Google Scholar] [CrossRef]
- Loudon, K.; Tarr, G.; Pethick, D.; Lean, I.; Polkinghorne, R.; Mason, M.; Dunshea, F.; Gardner, G.; McGilchrist, P. The use of biochemical measurements to identify pre-slaughter stress in pasture finished beef cattle. Animals 2019, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.M.; Exton, J.H. A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes. Anal. Biochem. 1976, 71, 96–105. [Google Scholar] [CrossRef]
- Noll, F. L+ lactate Determination. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; VCH Verlagsgesellschaft: Weinheim, Germany, 1985; pp. 583–588. [Google Scholar]
- Watson, R.; Gee, A.; Polkinghorne, R.; Porter, M. Consumer assessment of eating quality–development of protocols for Meat Standards Australia (MSA) testing. Aust. J. Exp. Agric. 2008, 48, 1360–1367. [Google Scholar] [CrossRef]
- Gee, A.; Porter, M.; Polkinghorne, R. Protocols for the Thawing, Preparation and Serving of Beef for MSA Trials for 5 Different Cooking Methods. Meat and Livestock Australia: North Sydney. Verified 14 July 2008, 2005. Available online: www.mla.com.au/msa (accessed on 26 July 2019).
- Core, R.T. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www. R-project.org (accessed on 26 July 2019).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2014, 67, 1–48. [Google Scholar]
- Lenth, R. Emmeans: Estimated marginal means, aka least-squares means. Am. Stat. 2018, 34, 216–221. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysi; Springer: New York, NY, USA, 2016. [Google Scholar]
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical tests, P values, confidence intervals, and power: A guide to misinterpretations. Eur. J. Epidemiol. 2016, 31, 337–350. [Google Scholar] [CrossRef]
- Radostits, O.M.; Gay, C.C.; Hinchcliff, K.W.; Constable, P.D. Veterinary Medicine—A Textbook of the Diseases of Cattle, Horses, Sheep, Pigs and Goats, 9th ed.; WB Saunders Co: Sydney, Australia, 2000. [Google Scholar]
- Eckersall, P.; Young, F.; McComb, C.; Hogarth, C.; Safi, S.; Fitzpatrick, J.; Nolan, A.; Weber, A.; McDonald, T. Acute phase proteins in serum and milk from dairy cows with clinical mastitis. Vet. Rec. 2001, 148, 35–41. [Google Scholar] [CrossRef]
- Trevisi, E.; Amadori, M.; Cogrossi, S.; Razzuoli, E.; Bertoni, G. Metabolic stress and inflammatory response in high-yielding, periparturient dairy cows. Res. Vet. Sci. 2012, 93, 695–704. [Google Scholar] [CrossRef]
- Laven, R.; Lawrence, K.; Livesey, C. The assessment of blood copper status in cattle: A comparison of measurements of caeruloplasmin and elemental copper in serum and plasma. N. Z. Vet. J. 2007, 55, 171–176. [Google Scholar] [CrossRef]
- Gardner, G.; McIntyre, B.; Tudor, G.; Pethick, D. The impact of nutrition on bovine muscle glycogen metabolism following exercise. Aust. J. Agric. Res. 2001, 52, 461–470. [Google Scholar] [CrossRef]
- McVeigh, J.; Tarrant, P. Glycogen content and repletion rates in beef muscle, effect of feeding and fasting. J. Nutr. 1982, 112, 1306–1314. [Google Scholar] [CrossRef]
- Stewart, S.; McGilchrist, P.; Gardner, G.; Pethick, D. Lamb loin tenderness is not associated with plasma indicators of pre-slaughter stress. Meat Sci. 2018, 137, 147–152. [Google Scholar] [CrossRef]
- Rhee, M.S.; Wheeler, T.; Shackelford, S.; Koohmaraie, M. Variation in palatability and biochemical traits within and among eleven beef muscles. J. Anim. Sci. 2004, 82, 534–550. [Google Scholar] [CrossRef]
- Warriss, P.; Brown, S.; Knowles, T.; Kestin, S.; Edwards, J.; Dolan, S.; Phillips, A. Effects on cattle of transport by road for up to 15 hours. Vet. Rec. 1995, 136, 319–323. [Google Scholar] [CrossRef]
- Knowles, G.; Warriss, P.; Brown, S.; Edwards, J. Effects on cattle of transportation by road for up to 31 hours. Vet. Rec. 1999, 145, 575. [Google Scholar] [CrossRef]
- Tarrant, P.; Kenny, F.; Harrington, D.; Murphy, M. Long distance transportation of steers to slaughter: Effect of stocking density on physiology, behaviour and carcass quality. Livest. Prod. Sci. 1992, 30, 223–238. [Google Scholar] [CrossRef]
- Warriss, P.; Kestin, S.; Brown, S.; Wilkins, L. The time required for recovery from mixing stress in young bulls and the prevention of dark cutting beef. Meat Sci. 1984, 10, 53–68. [Google Scholar] [CrossRef]
- Bond, J.; Can, L.; Warner, R. The effect of exercise stress, adrenaline injection and electrical stimulation on changes in quality attributes and proteins in Semimembranosus muscle of lamb. Meat Sci. 2004, 68, 469–477. [Google Scholar] [CrossRef]
- Bond, J.; Warner, R. Ion. distribution and protein proteolysis affect water holding capacity of Longissimus thoracis et lumborum in meat of lamb subjected to antemortem exercise. Meat Sci. 2007, 75, 406–414. [Google Scholar] [CrossRef]
- Henckel, P.; Karlsson, A.; Jensen, M.T.; Oksbjerg, N.; Petersen, J.S. Metabolic conditions in porcine longissimus muscle immediately pre-slaughter and its influence on peri-and post mortem energy metabolism. Meat Sci. 2002, 62, 145–155. [Google Scholar] [CrossRef]
- Daroit, D.; Brandelli, A. Implications of skeletal muscle creatine kinase to meat quality. J. Anim. Feed Sci. 2008, 17, 285–294. [Google Scholar] [CrossRef]
- Lawrie, R. Meat Science; Pergamon Press: Oxford, UK, 1985. [Google Scholar]
- Wyss, M.; Smeitink, J.; Wevers, R.A.; Wallimann, T. Mitochondrial creatine kinase: A key enzyme of aerobic energy metabolism. Biochim. Et Biophys. Acta BBA Bioenerg. 1992, 1102, 119–166. [Google Scholar] [CrossRef]
- Scopes, R. The influence of post-mortem conditions on the solubilities of muscle proteins. Biochem. J. 1964, 91, 201. [Google Scholar] [CrossRef]
- O’Halloran, G.; Troy, D.; Buckley, D.; Reville, W. The role of endogenous proteases in the tenderisation of fast glycolysing muscle. Meat Sci. 1997, 47, 187–210. [Google Scholar] [CrossRef]
- Fischer, C.; Hamm, R.; Honikel, K. Changes in solubility and enzymic activity of muscle glycogen phosphorylase in PSE-muscles. Meat Sci. 1979, 3, 11–19. [Google Scholar] [CrossRef]
- Scopes, R.; Lawrie, R. Post-mortem lability of skeletal muscle proteins. Nature 1963, 197, 1202. [Google Scholar] [CrossRef]
- Polkinghorne, R.; Thompson, J.; Watson, R.; Gee, A.; Porter, M. Evolution of the Meat Standards Australia (MSA) beef grading system. Aust. J. Exp. Agric. 2008, 48, 1351–1359. [Google Scholar] [CrossRef]
- Watson, R.; Polkinghorne, R.; Gee, A.; Porter, M.; Thompson, J.; Ferguson, D.; Pethick, D.; McIntyre, B. Effect of hormonal growth promotants on palatability and carcass traits of various muscles from steer and heifer carcasses from a Bos indicus–Bos taurus composite cross. Aust. J. Exp. Agric. 2008, 48, 1415–1424. [Google Scholar] [CrossRef]
- Polkinghorne, R.; Philpott, J.; Perovic, J.; Lau, J.; Davies, L.; Mudannayake, W.; Watson, R.; Tarr, G.; Thompson, J. The effect of packaging on consumer eating quality of beef. Meat Sci. 2018, 142, 59–64. [Google Scholar] [CrossRef]
- Stewart, S.M.; McGilchrist, P.; Gardner, G.E.; Pethick, D.W. Association between Loin Ultimate pH and Plasma Indicators of Pre-Slaughter Stressors in Australian Lamb 1. Meat Muscle Biol. 2018, 2, 254–264. [Google Scholar] [CrossRef]
- Stewart, S.M. The Influence of Acute Stress and Feed Deprivation on the Meat Quality and Intermediary Metabolism of Australian Lamb; Murdoch University: Perth, Australia, 2017. [Google Scholar]
- Moberg, G.P. Biological Response to Stress: Key to Assessment of Animal Well-Being? In Animal Stress; Springer: New York, NY, USA, 1985; pp. 27–49. [Google Scholar]
- Warriss, P. The handling of cattle pre-slaughter and its effects on carcass and meat quality. Appl. Anim. Behav. Sci. 1990, 2, 171–186. [Google Scholar] [CrossRef]
- Mudroň, P.; Rehage, J.; Sallmann, H.; Höltershinken, M.; Scholz, H. Stress Response in Dairy Cows Related to Different Blood Glucose. Acta Vet. Brno 2005, 74, 37–42. [Google Scholar] [CrossRef]
- Keul, J. Muscle Metabolism during Long Lasting Exercise. In Metabolic Adaptation to Prolonged Physical Exercise; Springer: Birkhauser, Basel, 1975; pp. 31–42. [Google Scholar]
- van der Walt, J.; Hattingh, J.; Petty, D.; Grobler, M.; Ganhao, M. Effect of a 72 hour fast on physiological stress indicators in feedlot cattle. J. S. Afr. Vet. Assoc. 1993, 64, 39–42. [Google Scholar]
- D’souza, D.; Warner, R.; Leury, B.; Dunshea, F. The effect of dietary magnesium aspartate supplementation on pork quality. J. Anim. Sci. 1998, 76, 104–109. [Google Scholar] [CrossRef]
- Gardner, G.; Jacob, R.; Pethick, D. The effect of magnesium oxide supplementation on muscle glycogen metabolism before and after exercise and at slaughter in sheep. Aust. J. Agric. Res. 2001, 52, 723–729. [Google Scholar] [CrossRef]
- Rayssiguier, Y. Hypomagnesemia resulting from adrenaline infusion in ewes: Its relation to lipolysis. Horm. Metab. Res. 1977, 9, 309–314. [Google Scholar] [CrossRef]
- Terashima, Y.; Muzushima, Y.; Yamaoka, R.; Itoh, H. Effects of intravenous infusion of noradrenaline on plasma levels of Ca, inorganic P and Mg in sheep fed high or low calcium diet. Jpn. J. Zootech. Sci. (Jpn.) 1983, 54, 59. [Google Scholar]
- Yano, H.; Nagaoka, C.; Kawashimai, R. Effects of intravenous glucose or epinephrine injection on plasma concentrations of potassium, magnesium and insulin in sheep. J. Nutr. Sci. Vitaminol. 1984, 30, 535–540. [Google Scholar]
- Bertschat, F.; Golf, S.; Riediger, H.; Graef, V.; Ising, H. Protective effects of magnesium on release of proteins from muscle cells during a marathon run. Magnes. Bull. 1986, 8, 310–313. [Google Scholar]
- Golf, S.; Bender, S.; Grüttner, J. On the significance of magnesium in extreme physical stress. Cardiovasc. Drugs Ther. 1998, 12, 197–202. [Google Scholar] [CrossRef]


| Variable | Mean | SD | Min | Max | Published Normal Basal Concentrations |
|---|---|---|---|---|---|
| Glucose (mmol/L) | 6.83 | 1.22 | 3.69 | 14.51 | 1.9–3.8 a |
| Lactate (mmol/L) | 13.25 | 3.17 | 5.78 | 23.48 | 0.6–2.2 a |
| CK (IU/L) | 870.15 | 1056.29 | 113.30 | 9384.90 | 35–280 a |
| AST (IU/L) | 122.14 | 62.06 | 55.57 | 636.17 | 78–132 a |
| βHB (mmol/L) | 0.26 | 0.14 | 0.03 | 0.85 | 0.35–0.47 a |
| NEFA (mmol/L) | 0.46 | 0.26 | 0.08 | 1.52 | <0.4 a |
| Magnesium (mmol/L) | 0.79 | 0.10 | 0.48 | 1.10 | 0.74–1.10 a |
| Sodium (mmol/L) | 147.84 | 3.45 | 125.70 | 160.80 | 132–152 a |
| Chloride (mmol/L) | 97.96 | 3.33 | 91.40 | 119.50 | 95–110 a |
| Haptoglobin (mg/mL) | 0.31 | 0.40 | 0.01 | 3.24 | 0.0–0.2 b |
| Ceruloplasmin (IU/L) | 95.99 | 42.34 | 20.00 | 285.00 | 15–68 |
| Muscle Glycogen (g/100 g) | 1.08 | 0.24 | 0.31 | 1.78 |
| Variable | Eye Round | Outside | Oyster | Stiploin | Tenderloin |
|---|---|---|---|---|---|
| Glucose (mmol/L) | −0.114 (0.759) | −0.422 (0.272) | −0.427 (0.139) | −0.323 (0.356) | 0.119 (0.584) |
| Lactate (mmol/L) | −0.231 (0.087) | −0.349 (0.015) | −0.100 (0.350) | −0.090 (0.502) | −0.024 (0.767) |
| CK (log) | −0.461 (0.429) | −0.444 (0.476) | 0.217 (0.669) | −1.323 (0.041) | −0.835 (0.017) |
| AST (log) | −0.116 (0.925) | −2.715 (0.037) | 1.505 (0.123) | −2.511 (0.034) | −1.281 (0.088) |
| βHB (mmol/L) | 7.590 (0.010) | 3.839 (0.225) | 3.949 (0.096) | 1.793 (0.538) | 3.625 (0.049) |
| NEFA (mmol/L) | 4.379 (0.013) | 1.973 (0.286) | 2.306 (0.093) | 1.134 (0.495) | 0.355 (0.734) |
| Magnesium (mmol/L) | 5.826 (0.136) | 3.683 (0.378) | 3.249 (0.297) | 9.921 (0.008) | 0.426 (0.859) |
| Sodium (mmol/L) | −0.211 (0.066) | −0.101 (0.419) | −0.057 (0.539) | −0.011 (0.923) | −0.048 (0.501) |
| Chloride (mmol/L) | 0.007 (0.951) | 0.051 (0.703) | −0.019 (0.846) | −0.008 (0.951) | −0.035 (0.648) |
| Haptoglobin (log) | −0.036 (0.928) | 0.128 (0.757) | −0.376 (0.231) | −0.411 (0.294) | −0.212 (0.384) |
| Ceruloplasmin (log) | −0.573 (0.627) | 0.037 (0.975) | −0.263 (0.767) | −0.624 (0.623) | 0.526 (0.449) |
| Muscle Glycogen (g/100 g) | −0.306 (0.897) | 3.083 (0.213) | −0.317 (0.868) | 2.728 (0.247) | −1.094 (0.449) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loudon, K.M.W.; Tarr, G.; Lean, I.J.; Polkinghorne, R.; McGilchrist, P.; Dunshea, F.R.; Gardner, G.E.; Pethick, D.W. The Impact of Pre-Slaughter Stress on Beef Eating Quality. Animals 2019, 9, 612. https://doi.org/10.3390/ani9090612
Loudon KMW, Tarr G, Lean IJ, Polkinghorne R, McGilchrist P, Dunshea FR, Gardner GE, Pethick DW. The Impact of Pre-Slaughter Stress on Beef Eating Quality. Animals. 2019; 9(9):612. https://doi.org/10.3390/ani9090612
Chicago/Turabian StyleLoudon, Kate M.W., Garth Tarr, Ian J. Lean, Rod Polkinghorne, Peter McGilchrist, Frank R. Dunshea, Graham E. Gardner, and David W. Pethick. 2019. "The Impact of Pre-Slaughter Stress on Beef Eating Quality" Animals 9, no. 9: 612. https://doi.org/10.3390/ani9090612
APA StyleLoudon, K. M. W., Tarr, G., Lean, I. J., Polkinghorne, R., McGilchrist, P., Dunshea, F. R., Gardner, G. E., & Pethick, D. W. (2019). The Impact of Pre-Slaughter Stress on Beef Eating Quality. Animals, 9(9), 612. https://doi.org/10.3390/ani9090612






