The Opuntia effect and the Reactivation of Ovarian Function and Blood Metabolite Concentrations of Anestrous Goats Exposed to Active Males
Abstract
Simple Summary
Abstract
1. Introduction
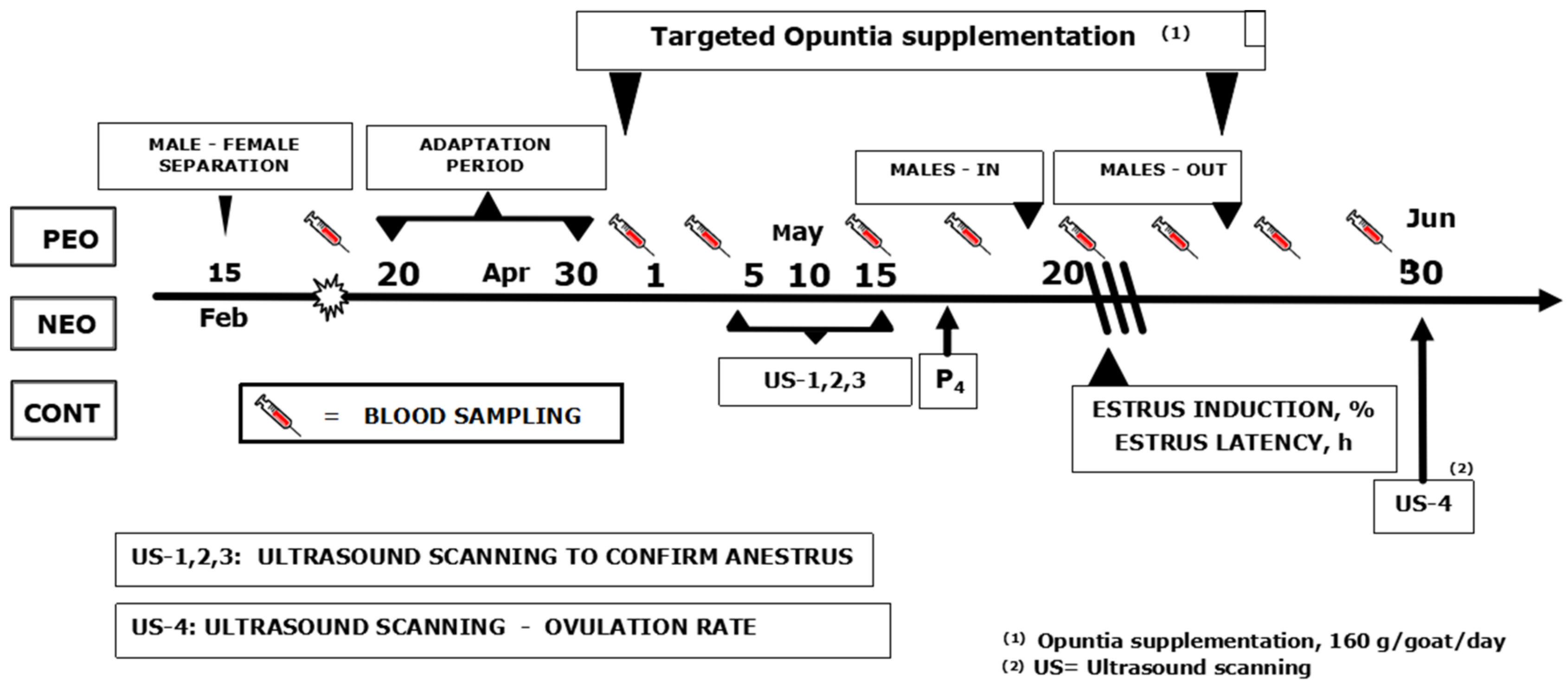
2. Material and Methods
2.1. General
2.2. Location, Environmental and Rangeland Conditions, Animal Care
2.3. Animals and Experimental Treatments
2.4. Experimental Supplements and Supplementation Schedule
2.5. Male-to-Female Interaction: The Quest for the Male Effect
2.6. Ultrasonographic Evaluation of the Ovary Function and Structures
2.7. Intermittent Blood Sampling, Serum Progesterone and Blood Analytes Quantification
2.8. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gonzalez-Bulnes, A.; Meza-Herrera, C.A.; Rekik, M.; Ben Salem, H.; Kridli, R.T. Limiting factors and strategies for improving reproductive outputs of small ruminants reared in semi-arid environments. In Semi-Arid Environments: Agriculture, Water Supply and Vegetation; Degenovine, K.M., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2011; Chapter 2; pp. 41–60. ISBN 978-1-61761-541-2. [Google Scholar]
- Escareño, L.; Wurzinger, M.; Iñiguez, L.; Soelkner, J.; Salinas, H.; Meza-Herrera, C.A. Dairy goat production systems in dry areas: Status-quo, perspectives and challenges. Trop. Anim. Health Prod. 2013, 45, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Arechiga, C.F.; Aguilera, J.I.; Rincón, R.M.; Mendez de Lara, S.; Bañuelos, V.R.; Meza-Herrera, C.A. Role and perspectives of goat production in a global world. Trop. Subtrop. Agroecosyst. 2008, 9, 1–14. [Google Scholar]
- Pérez-Razo, M.A.; Sánchez, F.; Torres-Hernández, G.; Becerril-Pérez, C.; Gallegos-Sánchez, J.; González-Cosío, F.; Meza-Herrera, C.A. Risk factors associated with dairy goats stayability. Livest. Prod. Sci. 2004, 89, 139–146. [Google Scholar] [CrossRef]
- Sakly, C.; Rekik, M.; Ben-Salem, I.; Lassoued, N.; Gonzalez-Bulnes, A.; Ben-Salem, H. Reproductive response of fat-tailed Barbarine ewes subjected to short-term nutritional treatments including spineless cactus (Opuntia ficus-indica f. inermis) cladodes. J. Anim. Physiol. Anim. Nutr. 2014, 98, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Arellano-Rodriguez, G.; Meza-Herrera, C.A.; Rodríguez-Martinez, R.; Dionisio-Tapia, R.; Hallford, D.M.; Mellado, M.; Gonzalez-Bulnes, A. Short-term intake of B-carotene supplemented diets enhances ovarian function and progesterone synthesis in goats. J. Anim. Physiol. Anim. Nutr. 2009, 93, 710–715. [Google Scholar] [CrossRef] [PubMed]
- Ben-Salem, H.; Smith, T. Feeding strategies to increase small ruminant production in dry environments. Small Rumin. Res. 2008, 77, 174–194. [Google Scholar] [CrossRef]
- De Santiago-Miramontes, M.A.; Luna-Orozco, j.r.; Meza-Herrera, C.A.; Rivas-Muñoz, R.; Carrillo, E.; Veliz-Deras, G.; Mellado, M. The effect of flushing and stimulus of estrogenized does on reproductive performance of anovulatory-range goats. Trop. Anim. Health Prod. 2011, 43, 1595–1600. [Google Scholar] [CrossRef]
- Meza-Herrera, C.A.; Torres-Moreno, M.; Lopez-Medrano, J.I.; Gonzalez-Bulnes, A.; Veliz, F.G.; Mellado, M.; Wurzinger, M.; Soto-Sanchez, M.J.; Calderon-Leyva, M.G. Glutamate supply positively affects serum release of triiodothyronine and insulin across time without increases of glucose during the onset of puberty in the female goat. Anim. Reprod. Sci. 2011, 125, 74–80. [Google Scholar] [CrossRef]
- Meza-Herrera, C.A.; Tena-Sempere, M. Interface between nutrition and reproduction. In Animal Reproduction in Livestock—Encyclopedia of Life Support Systems; Astiz, S., Gonzalez, A., Eds.; Eolss Publishers: Oxford, UK, 2012. [Google Scholar]
- El-Mostafa, K.; El Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; El Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef]
- Ben-Salem, H.; Abidi, S. Recent advances on the potential use of Opuntia spp. in livestock feeding. Acta Horticult. 2009, 811, 317–324. [Google Scholar] [CrossRef]
- Akanni, G.B.; du Preez, J.C.; Steyn, L.; Kilian, S.G. Protein enrichment of an Opuntia [ficus-indica cladode hydrolysate by cultivation of Candida utilis and Kluyveromyces marxianus. J. Sci. Food Agric. 2015, 95, 1094–1102. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.A.; Rodríguez, C.; Reis, E.M.; Nozaki, J. Production of fungal protein by solid substrate fermentation of cactus Cereus peruvianus and Opuntia ficus indica. Quim. Nova 2001, 24, 307–310. [Google Scholar] [CrossRef]
- Díaz-Plascencia, D.; Rodríguez-Muela, C.; Mancillas-Flores, P.; Ruíz-Holguín, N.; Mena-Mungía, S.; Salvador-Torres, F.; Duran-Melendez, L. In vitro fermentation of forage Opuntia inoculated with Kluyveromyces lactis obtined from apple residues. Rev. Electr. Vet. 2012, 13, 1–11. [Google Scholar]
- Araujo, L.M.; Medeiros, A.N.; Neto, A.P. Protein enrichment of cactus pear (Opuntia ficus-indica Mill) using Saccharomyces cerevisiae in solid-state fermentation. Brazil. Arch. Biol. Technol. 2005, 48, 161–168. [Google Scholar] [CrossRef]
- Alvarado-Espino, A.S.; Meza-Herrera, C.A.; Carrillo, E.; González-Álvarez, V.H.; Guillen-Muñoz, J.M.; Angel-García, O.; Mellado, M.; Veliz-Deras, F.G. Reproductive outcomes of Alpine goats primed with progesterone and treated with human chorionic gonadotropin during the anestrus-to-estrus transition season. Anim. Reprod. Sci. 2016, 167, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.B.; Rodger, J.; Blache, D. Nutritional and environmental effects on reproduction in small ruminants. Reprod. Fertil. Dev. 2004, 16, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Flores-Najera, M.J.; Meza-Herrera, C.A.; Echavarria, F.G.; Villagomez, E.; Iñiguez, L.; Salinas, H.; Gonzalez-Bulnes, A. Influence of nutritional and socio-sexual cues upon reproductive efficiency of goats exposed to the male effect under extensive conditions. Anim. Prod. Sci. 2010, 50, 897–901. [Google Scholar] [CrossRef]
- Luna-Orozco, J.R.; Guillen-Muñoz, J.M.; De Santiago-Miramontes, M.A.; García, J.E.; Rodríguez-Martínez, R.; Meza-Herrera, C.A.; Veliz, F.G. Influence of sexually inactive bucks subjected to long photoperiod or testosterone on the induction of estrus in anovulatory goats. Trop. Anim. Health Prod. 2012, 44, 71–75. [Google Scholar] [CrossRef] [PubMed]
- FASS. Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, 3rd ed.; Federation Animal Science Society: Champaing, IL, USA, 2010; p. 177. [Google Scholar]
- NAM-National Academy of Medicine. Guide for the Care and Use of Laboratory Animals. Co-Produced by the National Academy of Medicine–Mexico and the Association for Assessment and Accreditation of Laboratory Animal Care International, 1st ed.; Harlan: Mexico City, Mexico, 2010. [Google Scholar]
- Mellado, M.; Rodriguez, A.; Lozano, E.A.; Dueñez, J.; Aguilar, C.N.; Arevalo, J.R. The food habits of goats on rangelands with different amounts of fourwing saltbush (Atriplex canecens) cover. J. Arid Environ. 2012, 84, 91–96. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids and New World Camelids; National Academy Press: Washington, DC, USA, 2007. [Google Scholar]
- Dickie, A.; Paterson, M.M.; Anderson, C.; Boyd, J.S. Determination of corpora lutea numbers in Booroola-Texel ewes using transrectal ultrasound. Theriogenology 1999, 51, 1209–1224. [Google Scholar] [CrossRef]
- Littell, R.C.; Henry, P.R.; Ammerman, C.B. Statistical analysis of repeated measures using SAS procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef] [PubMed]
- Rekik, M.; Ben-Salem, H.; Lassoued, N.; Chalouati, H.; Ben-Salem, I. Supplementation of Barbarine ewes with spineless cactus (Opuntia ficus-indica f. inermis) cladodes during late gestation-early suckling: Effects on mammary secretions, blood metabolites, lamb growth and postpartum ovarian activity. Small Rumin. Res. 2010, 90, 53–57. [Google Scholar] [CrossRef]
- Sakly, C.; Rekik, M.; Ben Salem, I.; Lassoued, N.; Mtaallah, B.; Kraiem, K.; Gonzalez-Bulnes, A. Reproductive response of Barbarine ewes to supplementation with alternative feed prior to and during mating under semi-arid extensive conditions. Eur. Assoc. Anim. Prod. 2012, 131, 235–239. [Google Scholar]
- Rekik, M.; Gonzalez-Bulnes, A.; Lassoued, N.; Ben-Salem, H.; Tounsi, A.; Ben-Salem, I. The cactus effect: an alternative to the lupin effect for increasing ovulation rate in sheep reared in semi-arid regions? J. Anim. Physiol. Anim. Nutr. 2012, 96, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Ordaz-Ochoa, G.; Juárez-Caratachea, A.; Pérez-Sánchez, R.E.; Román-Bravo, R.M.; Ortiz-Rodríguez, R. Effect of spineless cactus intake (Opuntia ficus-indica) on blood glucose levels in lactating sows and its impact on feed intake, body weight loss, and weaning-estrus interval. Trop. Anim. Health Prod. 2017, 49, 1025–1033. [Google Scholar] [CrossRef][Green Version]
- Tejada, L.M.; Meza-Herrera, C.A.; Rivas-Muñoz, R.; Rodriguez-Martinez, R.; Carrillo, E.; Mellado, M.; Véliz-Deras, F.G. Appetitive and consummatory sexual behaviors of rams treated with exogenous testosterone and exposed to anestrus Dorper ewes: Efficacy of the male effect. Arch. Sexual Behav. 2017, 46, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Fabre, N.C.; Chanvallon, A.; François, D.; Fassier, T.; Menassol, J.; Scaramuzzi, R.J. Condition on the Pituitary and Ovarian Responses of Anoestrous Ewes to the “Ram Effect”. J. Veterinar. Sci. Technol. 2011, S2, 001. [Google Scholar]
- Meza-Herrera, C.A. Puberty, kisspeptin and glutamate: A ceaseless golden braid. In Advances in Medicine and Biology; Berhardt, L.V., Ed.; Nova Science Publishers Inc.: Hauppauge, NY, USA, 2012; Volume 52, Chapter 3; pp. 97–124. ISBN 978-1-62081-314-0. [Google Scholar]
- Keller, M.; Levy, F. The main but not the accessory olfactory system is involved in the processing of socially relevant chemosignals in ungulates. Front. Neuroanat. 2012, 6, art39. [Google Scholar] [CrossRef]
- Murata, K.; Tamogami, S.; Itou, M.; Ohkubo, H.; Okamura, H.; Takeuchi, Y.; Mori, Y. Identification of an olfactory signal molecule that activates the central regulator of reproduction in goats. Curr. Biol. 2014, 24, 681–686. [Google Scholar] [CrossRef]
- De Bond, J.A.; Li, Q.; Millar, R.P.; Clarke, I.J.; Smith, J.T. Kisspeptin signaling is required for the luteinizing hormone response in anestrous ewes following the introduction of males. PLoS ONE 2013, 8, e57972. [Google Scholar] [CrossRef]
- Sakamoto, K.; Wakabayashi, Y.; Yamamura, T.; Tanaka, T.; Takeuchi, Y.; Mori, Y.; Okamura, H. A population of Kisspeptin/Neurokinin B neurons in the arcuate nucleus may be the central target of the male effect phenomenon in goats. PLoS ONE 2013, 8, e81017. [Google Scholar] [CrossRef] [PubMed]
- Dupont, J.; Scaramuzzi, R.J.; Reverchon, M. The effect of nutrition and metabolic status on the development of follicles, oocytes and embryos in ruminants. Animal 2014, 8, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Lucy, M.C.; Butler, S.T.; Garverick, H.A. Endocrine and metabolic mechanisms linking postpartum glucose with early embryonic and foetal development in dairy cows. Animal 2014, 8 (Suppl. 1), 82–90. [Google Scholar] [CrossRef]
- Kaneko, J.J.; Harvery, W.; Bruss, M.L. Clinical Biochemistry of Domestic Animals, 6th ed.; Academic Press-Elsevier: San Diego, CA, USA; London, UK; Boston, MA, USA; New York, NY, USA; Tokyo, Japan; Toronto, ON, Canada, 2008. [Google Scholar]
- Ye, G.; Zhu, Y.; Liu, J.; Chen, X.; Huang, K. Preparation of glycerol-enriched yeast culture and its effect on blood metabolites and ruminal fermentation in goats. PLoS ONE 2014, 9, e94410. [Google Scholar] [CrossRef][Green Version]
- Meza-Herrera, C.A.; Cano-Villegas, O.; Flores-Hernandez, A.; Veliz-Deras, F.G.; Calderon-Leyva, G.; Guillen-Muñoz, G.M.; de la Peña, C.G.; Rosales-Nieto, C.A.; Macias-Cruz, U.; Avendaño-Reyes, L. Reproductive outcomes of anestrous goats supplemented with spineless Opuntia megacantha Salm-Dyck protein-enriched cladodes and exposed to the male effect. Trop. Anim. Health Prod. 2017, 49, 1511–1516. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzzi, R.J.; Baird, D.T.; Campbell, B.K.; Driancourt, M.A.; Dupont, J.; Fortune, J.E.; Gilchrist, R.B.; Martin, G.B.; McNatty, K.P.; McNeilly, A.S.; et al. Regulation of folliculogenesis and the determination of ovulation rate in ruminants. Reprod. Fertil. Dev. 2011, 23, 444–467. [Google Scholar] [CrossRef]
- Samadi, F.; Phillips, N.J.; Blache, D.; Martin, G.B.; D’Occhio, M.J. Interrelationships of nutrition, metabolic hormones and resumption of ovulation in multiparous suckled beef cows on subtropical pastures. Anim. Reprod. Sci. 2013, 137, 137–144. [Google Scholar] [CrossRef]
| Components | NEO, Fresh | NEO, Dry | PEO, Fresh | PEO, Dry |
|---|---|---|---|---|
| DM, % | 12.9 | 92.1 | 12.5 | 92.0 |
| CP, % | 6.4 | 4.9 | 29.8 | 20.5 |
| NDF, % | 21.3 | 14.7 | 18.3 | 17.5 |
| ADF, % | 19.7 | 11.9 | 16.6 | 17.9 |
| NFC, % | 43.8 | 53.3 | 24.4 | 33.9 |
| TND, % | 53.1 | 61.0 | 57.2 | 56.4 |
| NEm, Mcal/kg DM | 1.9 | 2.3 | 2.3 | 2.2 |
| Ash, % | 27.9 | 24.7 | 25.5 | 26.7 |
| Variables | NEO | PEO | CON | S.E. (2) |
|---|---|---|---|---|
| LW-initial, kg | 43.9 a | 44.5 a | 45.1 a | 1.6 |
| BCS-initial, units | 2.6 a | 2.6 a | 2.6 a | 0.10 |
| LW-d30, kg | 43.9 a | 43.8 a | 45.4 a | 1.7 |
| BCS-final, units | 2.5 a | 2.5 a | 2.6 a | 0.20 |
| Glucose, mg/dL | 102.0 a | 98.1 a | 96.6 a | 8.7 |
| Cholesterol, mg/dL | 158.1 a | 165.1 a | 156.1 a | 8.2 |
| Total protein, g/dL | 6.2 a | 5.9 a | 5.9 a | 0.4 |
| Urea, mg/dL | 49.1 a | 46.5 a | 46.9 a | 2.4 |
| Estrus induction, % | 57 b | 100 a | 43b | 12 |
| Estrus latency, h | 60 a | 62 a | 32 b | 4.1 |
| Ovulation rate, units | 0.71 b | 1.33 a | 0.43 b | 0.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meza-Herrera, C.A.; Romero-Rodríguez, C.A.; Nevárez-Dominguez, A.; Flores-Hernández, A.; Cano-Villegas, O.; Macías-Cruz, U.; Mellado, M.; Calderón-Leyva, G.; Carrillo-Moreno, D.; Véliz-Deras, F.G. The Opuntia effect and the Reactivation of Ovarian Function and Blood Metabolite Concentrations of Anestrous Goats Exposed to Active Males. Animals 2019, 9, 550. https://doi.org/10.3390/ani9080550
Meza-Herrera CA, Romero-Rodríguez CA, Nevárez-Dominguez A, Flores-Hernández A, Cano-Villegas O, Macías-Cruz U, Mellado M, Calderón-Leyva G, Carrillo-Moreno D, Véliz-Deras FG. The Opuntia effect and the Reactivation of Ovarian Function and Blood Metabolite Concentrations of Anestrous Goats Exposed to Active Males. Animals. 2019; 9(8):550. https://doi.org/10.3390/ani9080550
Chicago/Turabian StyleMeza-Herrera, Cesar A., Carlos A. Romero-Rodríguez, Adrian Nevárez-Dominguez, Arnoldo Flores-Hernández, Omag Cano-Villegas, Ulises Macías-Cruz, Miguel Mellado, Guadalupe Calderón-Leyva, Dalia Carrillo-Moreno, and Francisco G. Véliz-Deras. 2019. "The Opuntia effect and the Reactivation of Ovarian Function and Blood Metabolite Concentrations of Anestrous Goats Exposed to Active Males" Animals 9, no. 8: 550. https://doi.org/10.3390/ani9080550
APA StyleMeza-Herrera, C. A., Romero-Rodríguez, C. A., Nevárez-Dominguez, A., Flores-Hernández, A., Cano-Villegas, O., Macías-Cruz, U., Mellado, M., Calderón-Leyva, G., Carrillo-Moreno, D., & Véliz-Deras, F. G. (2019). The Opuntia effect and the Reactivation of Ovarian Function and Blood Metabolite Concentrations of Anestrous Goats Exposed to Active Males. Animals, 9(8), 550. https://doi.org/10.3390/ani9080550






