Maternal Metabolic Demands Caused by Pregnancy and Lactation: Association with Productivity and Offspring Phenotype in High-Yielding Dairy Ewes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Assessment of Maternal Milk Yield
- Total milk yield over the lactation period (TMY). Based on the own data and percentile distributions, ewes with a TMY of <220 L were defined as low-yielding (L-TMY); those with 220 –371 L as average-yielding (A-TMY); and those with >371 L as high-yielding (H-TMY).
- Yield per day in milk (YDIM) from conception to drying off (YDIMd). Ewes with a YDIMd of <0.77 L/d were defined as low-yielding (L-YDIMd); those with 0.77–1.12 L/d as average-yielding (A-YDIMd); and those with >1.12 L/d as high-yielding (H-YDIMd).
- YDIM during the month of conception (YDIMc). Ewes with a YDIMc of <0.91 L/d were defined as low-yielding (L-YDIMc); those with 0.91–1.35 L/d as average-yielding (A-YDIMc); and those with >1.35 L/d as high-yielding (H-YDIMc).
2.2. Assessment of Productivity
2.3. Statistical Analyses
3. Results
3.1. Productivity
3.2. Maternal Body Weight and Condition Correlated with Milk Yield in Mature Ewes
3.3. Maternal Metabolite Levels Correlated with Milk Yield in Mature Ewes
3.4. Lamb BW, Body Size, and Plasma Metabolites Correlated with Maternal Milk Yield in Mature Ewes
3.5. Maternal Features during Gestation on Milk Yield during the Subsequent Lactation
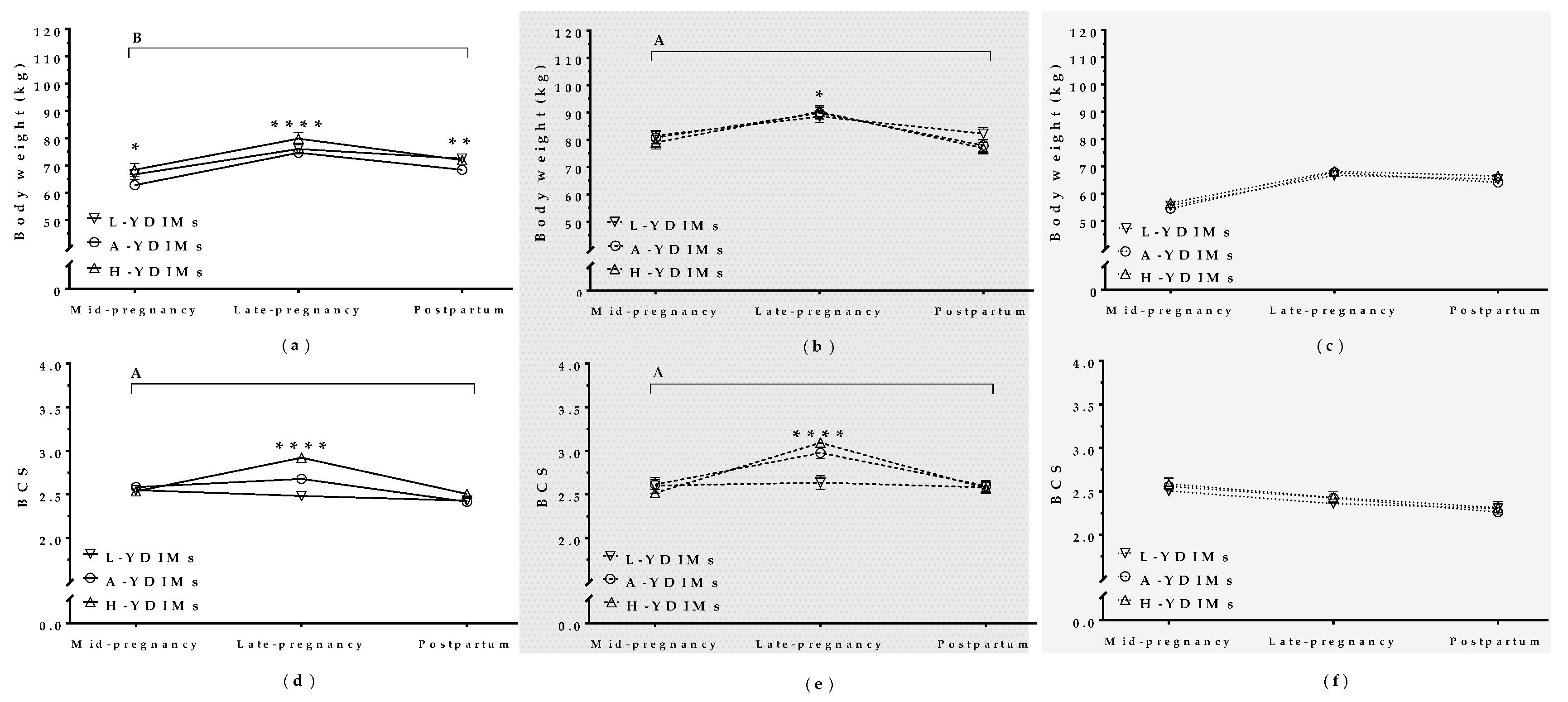
3.5.1. Maternal Weight and BCS during Concurrent Gestation and Lactation Affected Milk Yield in the Subsequent Lactation
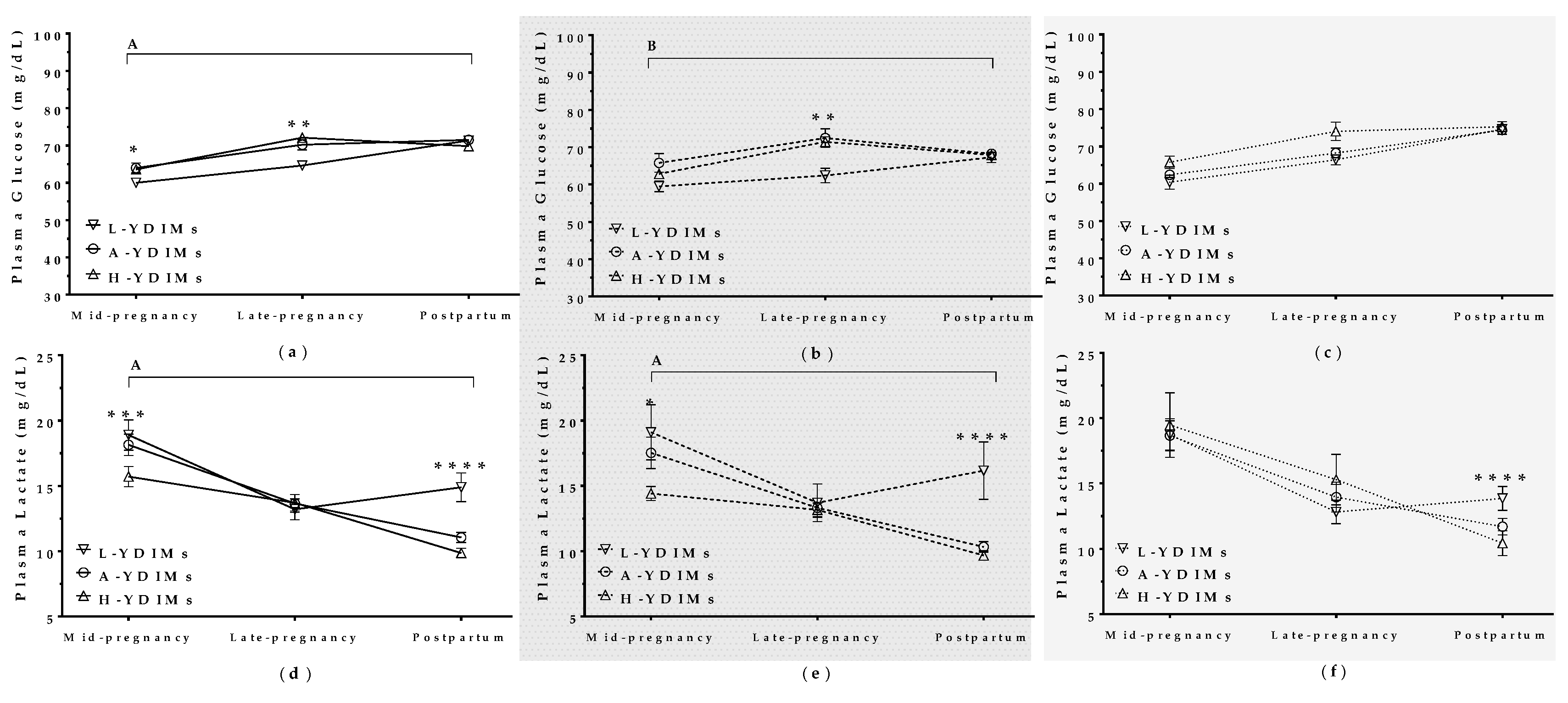
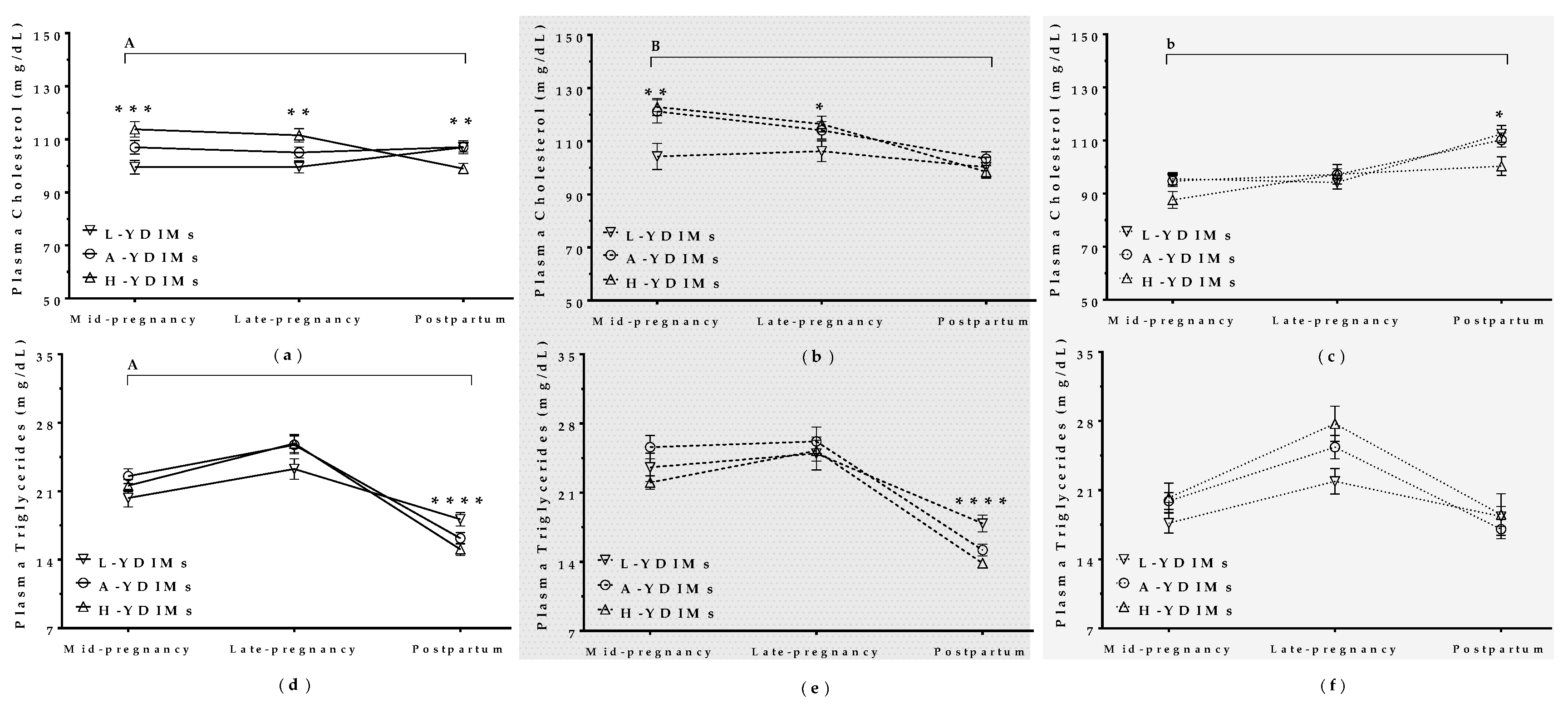
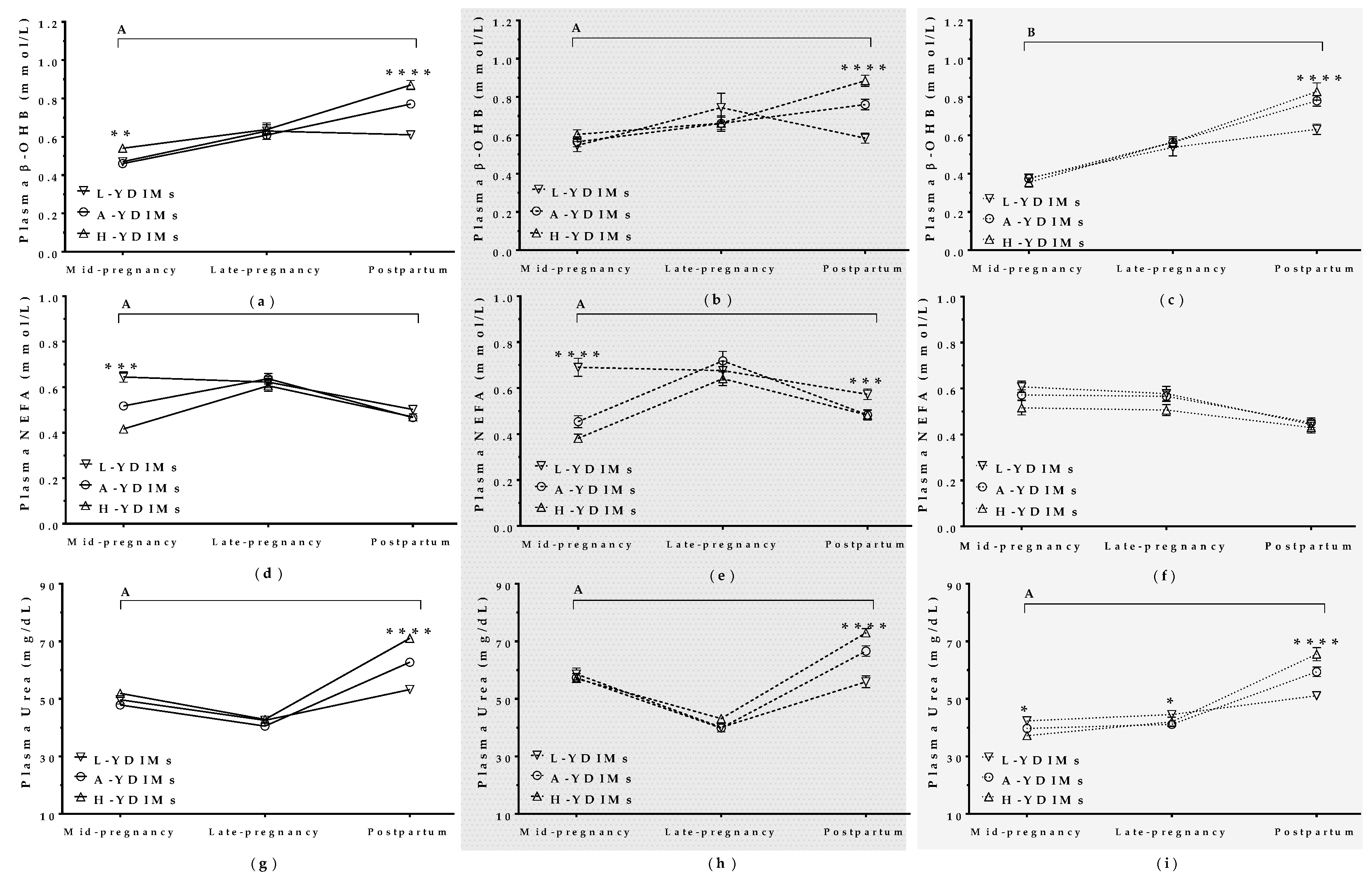
3.5.2. Maternal Metabolite Levels during Pregnancy Correlated with Productivity in the Subsequent Lactation
Glucose
Lactate
Cholesterol
Triglycerides
β-hydroxybutyrate (β-OHB)
Non-Esterified Fatty Acids (NEFA)
Urea
3.5.3. Ewe Age, Pregnancy Rank, and Concurrent Lactation with Pregnancy Affected Milk Yield in the Subsequent Lactation
3.6. Lamb Phenotypes Correlated with Maternal Yield during the Subsequent Lactation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bell, A.W. Regulation of organic nutrient metabolism during transition from late pregnancy to early lactation. J. Anim. Sci. 1995, 73, 2804–2819. [Google Scholar] [CrossRef]
- Davis, A.J.; Fleet, I.R.; Goode, J.A.; Hamon, M.H.; Maule Walker, F.M.; Peaker, M. Changes in mammary function at the onset of lactation in the goat: Correlation with hormonal changes. J. Physiol. 1979, 288, 33–44. [Google Scholar]
- Bell, A.W.; Bauman, D.E. Adaptations of glucose metabolism during pregnancy and lactation. J. Mammary Gland Biol. Neoplasia 1997, 2, 265–278. [Google Scholar] [CrossRef]
- Joy, M.; Ripoll-Bosch, R.; Sanz, A.; Molino, F.; Blasco, I.; Álvarez-Rodríguez, J. Effects of concentrate supplementation on forage intake, metabolic profile and milk fatty acid composition of unselected ewes raising lambs. Anim. Feed Sci. Technol. 2014, 187, 19–29. [Google Scholar] [CrossRef]
- Bauman, D.E.; Bruce Currie, W. Partitioning of nutrients during pregnancy and lactation: A review of mechanisms involving homeostasis and homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A.; Faulconnier, Y.; Bonnet, M.; Rouel, J.; Bocquier, F. Adipose tissue metabolism and its role in adaptations to undernutrition in ruminants. Proc. Nutr. Soc. 2000, 59, 127–134. [Google Scholar] [CrossRef]
- Opsomer, G.; Van Eetvelde, M.; Kamal, M.; Van Soom, A. Epidemiological evidence for metabolic programming in dairy cattle. Reprod. Fertil. Dev. 2017, 29, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, R.M.; Belo, A.T.; Santos, C.C.; Vazques, M.I.; Portugal, A.V. The effect of body condition score on blood metabolites and hormonal profiles in ewes. Small Rumin. Res. 2007, 68, 233–241. [Google Scholar] [CrossRef]
- Drackley, J.K.; Richard, M.J.; Beitz, D.C.; Young, J.W. Metabolic changes in dairy cows with ketonemia in response to feed restriction and dietary 1,3-butanediol. J. Dairy Sci. 1992, 75, 1622–1634. [Google Scholar] [CrossRef]
- Miller, D.R.; Blache, D.; Jackson, R.B.; Downie, E.F.; Roche, J.R. Metabolic maturity at birth and neonate lamb survival: Association among maternal factors, litter size, lamb birth weight, and plasma metabolic and endocrine factors on survival and behavior. J. Anim. Sci. 2010, 88, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Dunlap, K.A.; Brown, J.D.; Keith, A.B.; Satterfield, M.C. Factors controlling nutrient availability to the developing fetus in ruminants. J. Anim. Sci. Biotechnol. 2015, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Luther, J.; Aitken, R.; Milne, J.; Matsuzaki, M.; Reynolds, L.; Redmer, D.; Wallace, J. Maternal and fetal growth, body composition, endocrinology, and metabolic status in undernourished adolescent sheep. Biol. Reprod. 2007, 77, 343–350. [Google Scholar] [CrossRef]
- Kamal, M.M.; Van Eetvelde, M.; Depreester, E.; Hostens, M.; Vandaele, L.; Opsomer, G. Age at calving in heifers and level of milk production during gestation in cows are associated with the birth size of Holstein calves. J. Dairy Sci. 2014, 97, 5448–5458. [Google Scholar] [CrossRef] [PubMed]
- González-Recio, O.; Ugarte, E.; Bach, A. Trans-Generational effect of maternal lactation during pregnancy: A Holstein cow model. PLoS ONE 2012, 7, e51816. [Google Scholar] [CrossRef]
- Swali, A.; Wathes, D.C. Influence of the dam and sire on size at birth and subsequent growth, milk production and fertility in dairy heifers. Theriogenology 2006, 66, 1173–1184. [Google Scholar] [CrossRef]
- Karagiannis, I.; Panousis, N.; Kiossis, E.; Tsakmakidis, I.; Lafi, S.; Arsenos, G.; Boscos, C.; Brozos, C. Associations of pre-lambing body condition score and serum β-hydroxybutyric acid and non-esterified fatty acids concentrations with periparturient health of Chios dairy ewes. Small Rumin. Res. 2014, 120, 164–173. [Google Scholar] [CrossRef]
- Harmeyer, J.; Schlumbohm, C. Pregnancy impairs ketone body disposal in late gestating ewes: Implications for onset of pregnancy toxaemia. Res. Vet. Sci. 2006, 81, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, F.; Elvira, L.; Gonzalez Martin, J.V.; Astiz, S. Influence of dry period length on reproductive performance and productivity of Lacaune dairy sheep under an intensive management system. J. Dairy Res. 2012, 79, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Pollott, G.E.; Gootwine, E. Reproductive performance and milk production of Assaf sheep in an intensive management system. J. Dairy Sci. 2004, 87, 3690–3703. [Google Scholar] [CrossRef]
- Cranston, L.M.; Kenyon, P.R.; Corner Thomas, R.A.; Morris, S.T. The potential interaction between ewe body condition score and nutrition during very late pregnancy and lactation on the performance of twin-bearing ewes and their lambs. Asian Australas. J. Anim. Sci. 2017, 30, 1270–1277. [Google Scholar] [CrossRef]
- Abecia, J.A.; Palacios, C. Ewes giving birth to female lambs produce more milk than ewes giving birth to male lambs. Ital. J. Anim. Sci. 2018, 17, 736–739. [Google Scholar] [CrossRef]
- Pesántez Pacheco, J.; Heras Molina, A.; Torres Rovira, L.; Sanz Fernández, M.; García Contreras, C.; Vázquez Gómez, M.; Feyjoo, P.; Cáceres, E.; Frías Mateo, M.; Hernández, F.; et al. Influence of maternal factors (Weight, Body Condition, Parity, and Pregnancy Rank) on plasma metabolites of dairy ewes and their lambs. Animals 2019, 9, 122. [Google Scholar] [CrossRef] [PubMed]
- Rook, A.F. The role of carbohydrate metabolism in the regulation of milk production. Proc. Nutr. Soc. 1979, 38, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Woollett, L.A. The origins and roles of cholesterol and fatty acids in the fetus. Curr. Opin. Lipidol. 2001, 12, 305–312. [Google Scholar] [CrossRef]
- Palinski, W. Maternal–fetal cholesterol transport in the placenta. Circ. Res. 2009, 104, 569–571. [Google Scholar] [CrossRef]
- Kaneene, J.B.; Miller, R.A.; Herdt, T.H.; Gardiner, J.C. The association of serum nonesterified fatty acids and cholesterol, management and feeding practices with peripartum disease in dairy cows. Prev. Vet. Med. 1997, 31, 59–72. [Google Scholar] [CrossRef]
- Novoselec, J.; Speranda, M.; Klir, Z.; Mioc, B.; Steiner, Z.; Antunovic, Z. Blood biochemical indicators and concentration of thyroid hormones in heavily pregnant and lactating ewes depending on selenium supplementation. Acta Vet. Brno 2017, 86, 353–363. [Google Scholar] [CrossRef]
- González-García, E.; Tesniere, A.; Camous, S.; Bocquier, F.; Barillet, F.; Hassoun, P. The effects of parity, litter size, physiological state, and milking frequency on the metabolic profile of Lacaune dairy ewes. Domest. Anim. Endocrinol. 2015, 50, 32–44. [Google Scholar] [CrossRef]
- Meikle, A.; Kulcsar, M.; Chilliard, Y.; Febel, H.; Delavaud, C.; Cavestany, D.; Chilibroste, P. Effects of parity and body condition at parturition on endocrine and reproductive parameters of the cow. Reproduction 2004, 127, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Douglas, G.N.; Overton, T.R.; Bateman, H.G.; Dann, H.M.; Drackley, J.K. Prepartal plane of nutrition, regardless of dietary energy source, affects periparturient metabolism and dry matter intake in Holstein cows. J. Dairy Sci. 2006, 89, 2141–2157. [Google Scholar] [CrossRef]
- Butler, S.T.; Pelton, S.H.; Butler, W.R. Energy balance, metabolic status, and the first postpartum ovarian follicle wave in cows administered propylene glycol. J. Dairy Sci. 2006, 89, 2938–2951. [Google Scholar] [CrossRef]
- Katoh, N. Relevance of apolipoproteins in the development of fatty liver and fatty liver-related peripartum diseases in dairy cows. J. Vet. Med. Sci. 2002, 64, 293–307. [Google Scholar] [CrossRef]
- Zobel, G.; Leslie, K.; Weary, D.M.; von Keyserlingk, M.A.G. Ketonemia in dairy goats: Effect of dry period length and effect on lying behavior. J. Dairy Sci. 2015, 98, 6128–6138. [Google Scholar] [CrossRef]
- Holtenius, P.; Holtenius, K. New aspects of ketone bodies in energy metabolism of dairy cows: A review. J. Vet. Med. Ser. A 1996, 43, 579–587. [Google Scholar] [CrossRef]
- Moallem, U.; Rozov, A.; Gootwine, E.; Honig, H. Plasma concentrations of key metabolites and insulin in late-pregnant ewes carrying 1 to 5 fetuses. J. Anim. Sci. 2015, 90, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Nudda, A.; Battacone, G.; Bee, G.; Boe, R.; Castanares, N.; Lovicu, M.; Pulina, G. Effect of linseed supplementation of the gestation and lactation diets of dairy ewes on the growth performance and the intramuscular fatty acid composition of their lambs. Animal 2015, 9, 800–809. [Google Scholar] [CrossRef]
- Rumball, C.W.H.; Bloomfield, F.H.; Oliver, M.H.; Harding, J.E. Different periods of periconceptional undernutrition have different effects on growth, metabolic and endocrine status in fetal sheep. Pediatr. Res. 2009, 66, 605–613. [Google Scholar] [CrossRef]
- Elvira, L.; Hernandez, F.; Cuesta, P.; Cano, S.; Gonzalez Martin, J.V.; Astiz, S. Factors affecting the lactation curves of intensively managed sheep based on a clustering approach. J. Dairy Res. 2013, 80, 439–447. [Google Scholar] [CrossRef]
- Eastham, N.T.; Coates, A.; Cripps, P.; Richardson, H.; Smith, R.; Oikonomou, G. Associations between age at first calving and subsequent lactation performance in UK Holstein and Holstein-Friesian dairy cows. PLoS ONE 2018, 13, e01197764. [Google Scholar] [CrossRef] [PubMed]
- Zobel, G.; Weary, D.M.; Leslie, K.E.; Von Keyserlingk, M.A. Invited review: Cessation of lactation: Effects on animal welfare. J. Dairy Sci. 2015, 98, 8263–8277. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, R.; Abdullahpour, R.; Sefidmazgi, A.S. Effect of calf birth beight on milk production of holstein dairy cattle in desert climate. J. Anim. Behav. Biometeorol. 2016, 4, 65–70. [Google Scholar] [CrossRef]
- Ayadi, M.; Matar, A.M.; Aljumaah, R.S.; Alshaikh, M.A.; Abouheif, M. Factors affecting milk yield, composition and udder health of najdi ewes. Int. J. Anim. Vet. Adv. 2014, 6, 28–33. [Google Scholar] [CrossRef]
- Hinde, K.; Carpenter Abigail, J.; Clay John, S.; Bradford Barry, J. Holsteins favor heifers, not bulls: Biased milk production programmed during pregnancy as a function of fetal sex. PLoS ONE 2014, 9, e86169. [Google Scholar] [CrossRef] [PubMed]
- Selvaggi, M.; D’Alessandro, A.G.; Dario, C. Environmental and genetic factors affecting milk yield and quality in three Italian sheep breeds. J. Dairy Res. 2017, 84, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Pesantez-Pacheco, J.L.; Torres-Rovira, L.; Hernandez, F.; Sanz-Fernandez, M.V.; Villalobos, N.P.; Heras-Molina, A.; Garcia-Contreras, C.; Vazquez-Gomez, M.; Martinez-Ros, P.; Gonzalez-Martin, J.V.; et al. Efficiency and demographics of a high-yield dairy ewe farm with two managing systems involving five or 10 lambings per year. Animal 2018, 12, 2181–2190. [Google Scholar] [CrossRef]
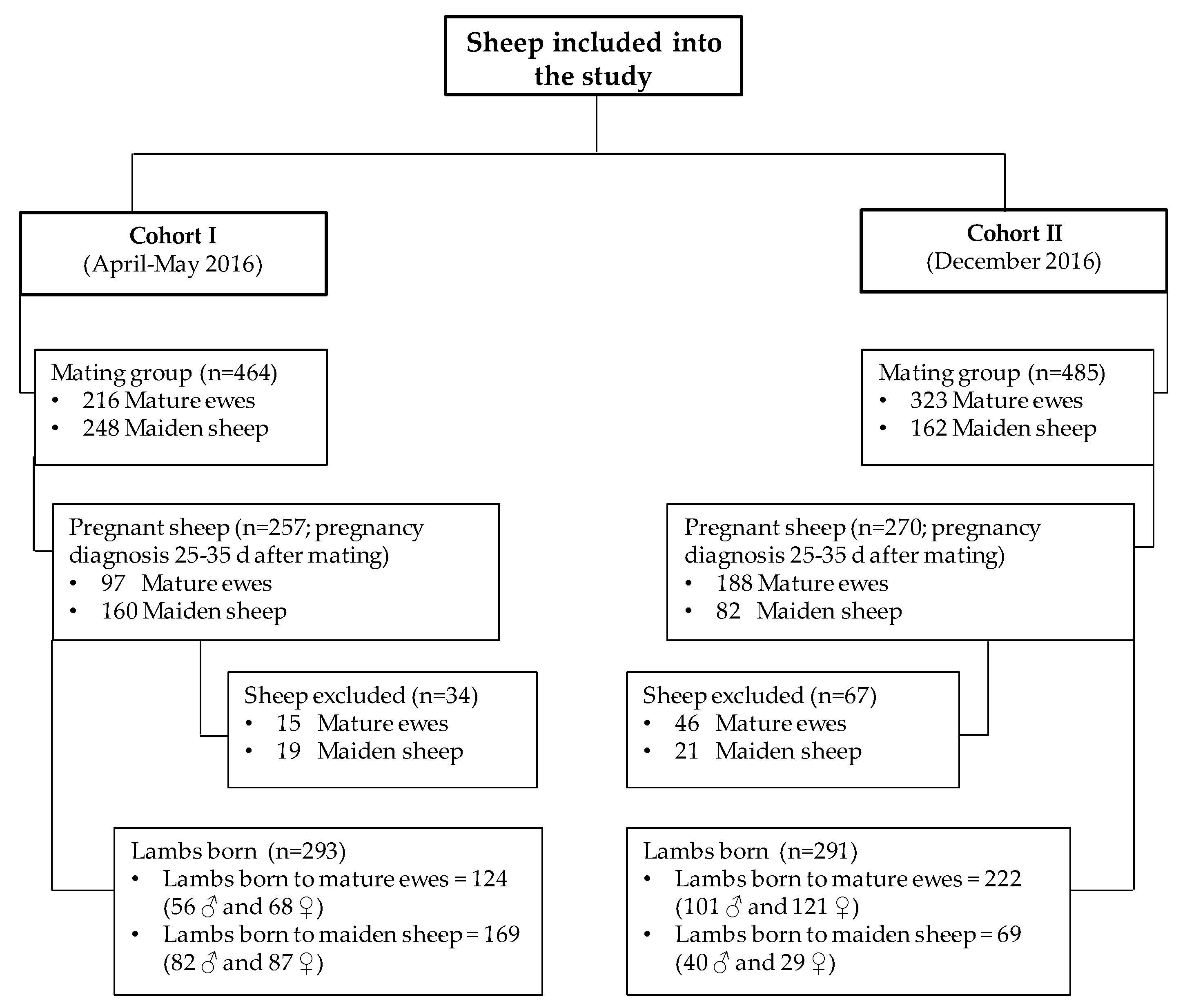
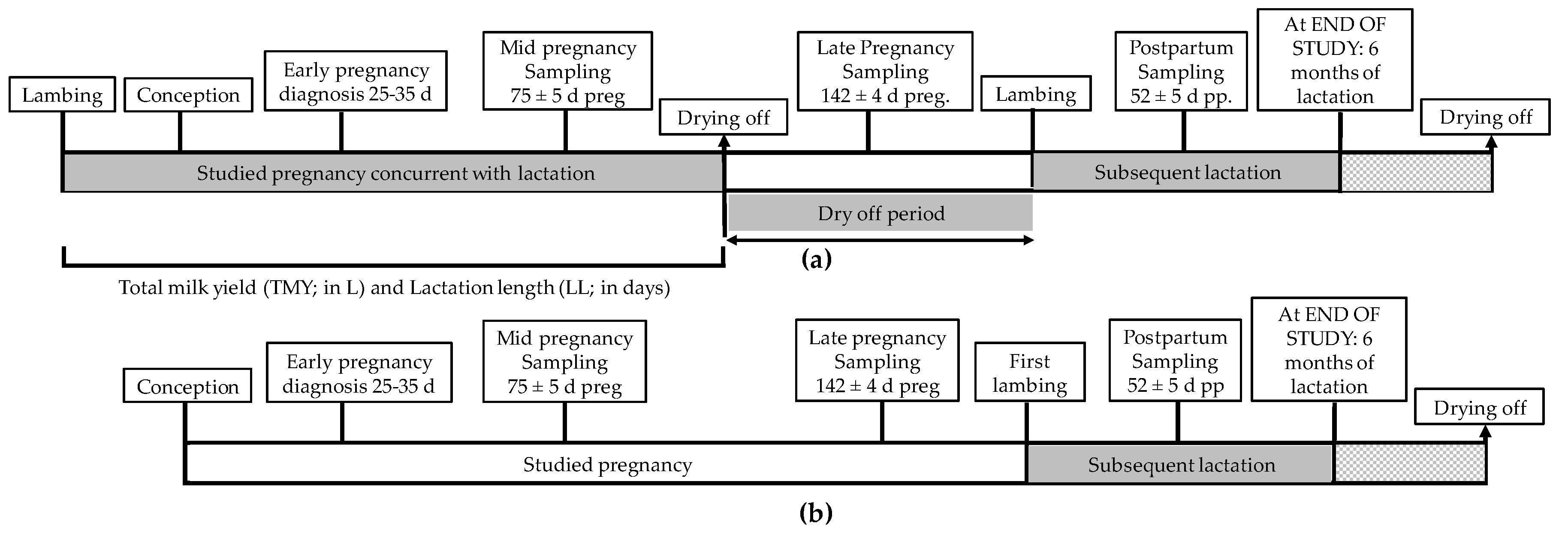
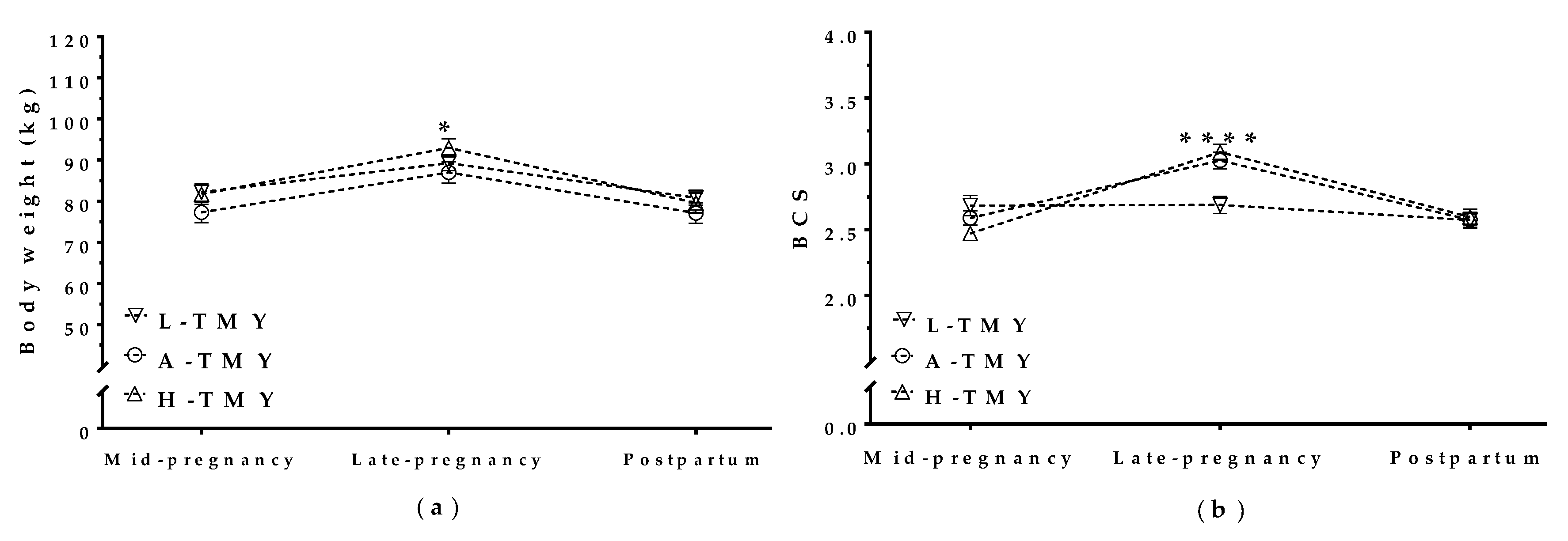
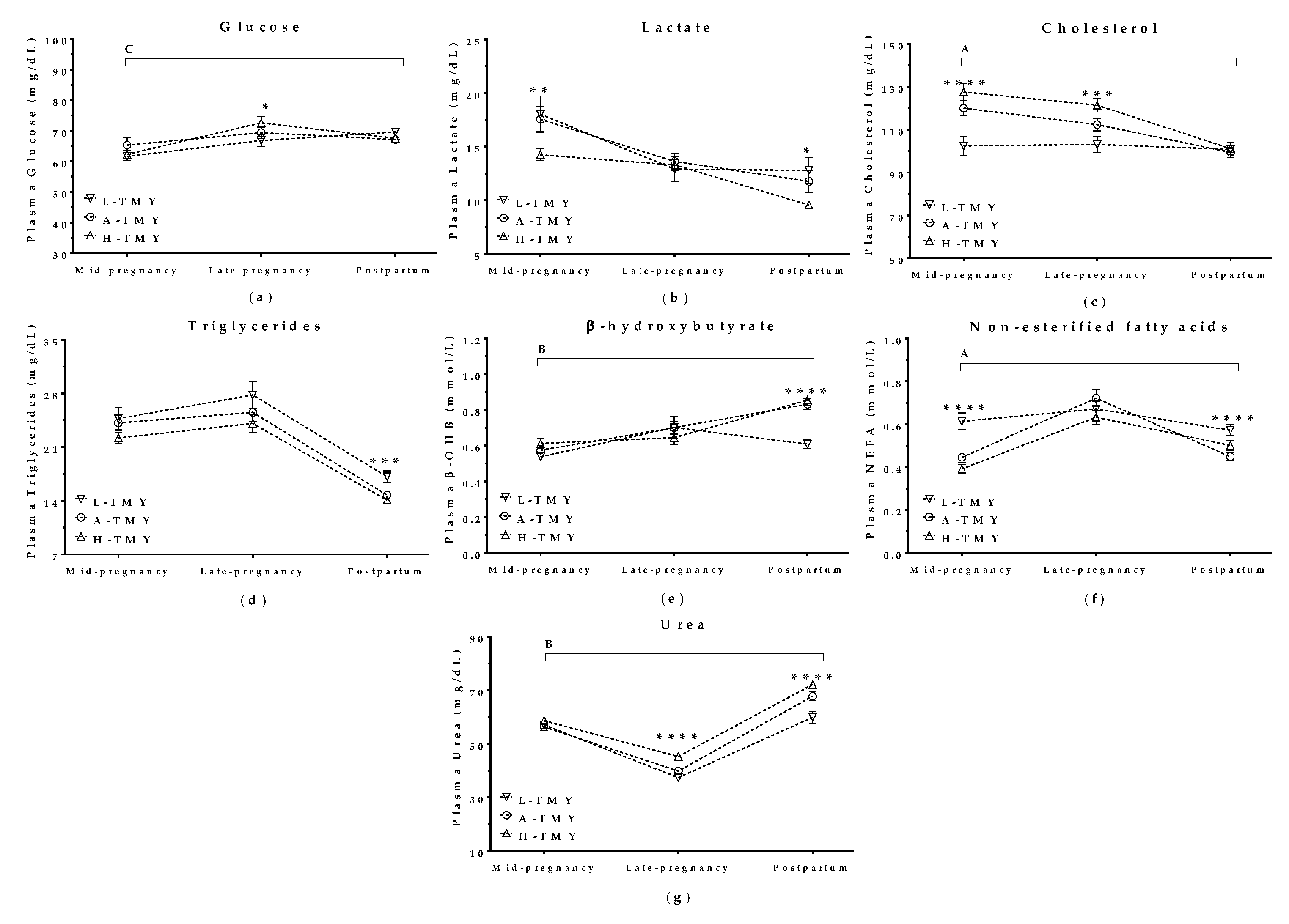
| Lactation | Parameter | Mature Ewes (n = 224) | Maiden Sheep (n = 179) | All Sheep (n = 403) |
|---|---|---|---|---|
| Concurrent gestation dlactation | Lactation length, LL (days) | 199.9 ± 56.7 | N/A | N/A |
| Total milk yield, TMY (L) | 331.0 ± 140.9 | N/A | N/A | |
| YDIMt (L/d) | 1.65 ± 0.43 | N/A | N/A | |
| YDIMb (L/d) | 2.01 ± 0.65 | N/A | N/A | |
| YDIMc (L/d) | 1.19 ± 0.43 | N/A | N/A | |
| YDIM in second month of pregnancy (L/d) | 1.03 ± 0.42 | N/A | N/A | |
| YDIM in third month of pregnancy (L/d) | 0.93 ± 0.44 | N/A | N/A | |
| YDIMd (L/d) | 1.02 ± 0.40 | N/A | N/A | |
| Dry period length, DPL (days) | 80.3 ± 48.8 | N/A | N/A | |
| Subsequent | Lactation length, LLs (days) | 200.1 ± 55.5 c | 217.0 ± 69.9 d | 207.8 ± 63.0 |
| Total milk yield, TMYs (L) | 373.6 ± 171.6 | 348.5 ± 144.9 | 362.1 ± 160.2 | |
| YDIMs (L/d) | 2.13 ± 0.72 a | 1.85 ± 0.58 b | 2.00 ± 0.67 | |
| YDIMts (L/d) | 1.81 ± 0.61 a | 1.60 ± 0.51 b | 1.72 ± 0.57 | |
| Dry period length, DLPs (days) | 57.6 ± 20.0 c | 52.8 ± 14.1 d | 55.3 ± 7.43 |
| Productivity Category | Values (Mean ± S.D.) (n) | |||
|---|---|---|---|---|
| TMY, L * | YDIMd, L/d * | YDIMc, L/d * | YDIMs, L/d ** | |
| Low yielding | 161.0 ± 41.6 (56) | 0.55 ± 0.15 (54) | 0.65 ± 0.18 (56) | 1.17 ± 0.31 (102) |
| Average yielding | 301.5 ± 35.0 (89) | 0.93 ± 0.10 (90) | 1.13 ± 0.13 (89) | 1.90 ± 0.18 (156) |
| High yielding | 485.7 ± 91.2 (79) | 1.42 ± 0.29 (80) | 1.66 ± 0.25 (79) | 2.70 ± 0.43 (145) |
| Total | 331.0 ± 140.86 (224) | 1.02 ± 0.39 (224) | 1.19 ± 0.43 (224) | 1.99 ± 0.67 (403) |
| Parameters | Total Milk Yield | Yield Per Day in Milk from Conception to Drying off | Yield Per Day in Milk during the Month of Conception | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L-TMY | A-TMY | H-TMY | L-YDIMd | A-YDIMd | H-YDIMd | L-YDIMc | A-YDIMc | H-YDIMc | |
| Phenotypic Parameters | (n = 78) | (n = 118) | (n = 121) | (n = 82) | (n = 119) | (n = 116) | (n = 81) | (n = 122) | (n = 114) |
| Birth weight, kg | 4.41 ± 0.89 j | 4.09 ± 0.87 k | 4.29 ± 0.74 j,k | 4.08 ± 0.86 | 4.31 ± 0.79 | 4.31 ± 0.84 | 4.11 ± 0.89 j | 4.20 ± 0.78 j,k | 4.40 ± 0.84 k |
| Body weight, 17 days *, kg | 7.40 ± 1.68 a | 8.88 ± 2.04 b | 9.63 ± 1.80 c | 8.68 ± 2.22 f,g | 8.46 ± 2.09 f | 9.23 ± 1.82 g | 8.65 ± 2.27 | 8.87 ± 1.95 | 8.83 ± 2.01 |
| Average daily weight gain, kg | 0.228 ± 0.1 k | 0.246 ± 0.07 j,k | 0.253 ± 0.06 j | 0.243 ± 0.07 | 0.240 ± 0.09 | 0.250 ± 0.07 | 0.238 ± 0.08 | 0.253 ± 0.08 | 0.239 ± 0.07 |
| Birth trunk length, cm | 30.10 ± 2.26 | 29.68 ± 2.02 | 30.05 ± 1.90 | 29.19 ± 2.35 | 30.30 ± 1.81 | 30.06 ± 1.91 | 29.35 ± 2.32 j | 30.04 ± 1.93 j,k | 30.21 ± 1.87 k |
| Trunk length 17 days *, cm | 36.93 ± 3.42 a | 39.36 ± 3.43 b | 40.44 ± 3.24 c | 38.63 ± 3.92 f | 38.97 ± 3.55 f,g | 39.76 ± 3.40 g | 38.54 ± 3.86 | 39.73 ± 3.31 | 39.03 ± 3.69 |
| Birth BMI, kg/m2 | 48.45 ± 7.71 | 46.05 ± 6.76 | 47.39 ± 6.40 | 47.60 ± 8.05 | 46.65 ± 6.20 | 47.34 ± 6.76 | 47.23 ± 6.85 | 46.37 ± 6.96 | 47.92 ± 6.88 |
| BMI, 17 days *, kg/m2 | 54.28 ± 10.52 f | 56.91 ± 9.32 f,g | 58.72 ± 9.04 g | 57.81 ± 10.86 j,k | 55.10 ± 8.87 j | 58.25 ± 9.28 k | 57.74 ± 10.75 | 55.88 ± 9.27 | 57.55 ± 9.19 |
| Thoracic girth, 17 days *, cm | 43.31 ± 3.56 a | 45.76 ± 3.80 b | 47.42 ± 3.51 c | 45.62 ± 4.18 f,g | 45.04 ± 3.95 f | 46.69 ± 3.65 g | 45.59 ± 4.30 | 45.69 ± 3.76 | 46.08 ± 3.93 |
| Abdominal girth, 17 days *, cm | 42.92 ± 4.03 a | 46.18 ± 4.43 b | 47.43 ± 4.51 b | 45.72 ± 4.66 j,k | 45.19 ± 4.62 j | 46.64 ± 4.72 k | 45.39 ± 4.78 | 46.04 ± 4.33 | 46.02 ± 5.02 |
| Metabolite Levels at 17 Days of age | |||||||||
| Glucose, mg/dL | 120.9 ± 20.0 | 120.1 ± 25.9 | 123.4 ± 26.6 | 117.3 ± 20.5 | 123.9 ± 27.0 | 122.2 ± 25.1 | 117.6 ± 25.1 j | 124.2 ± 25.8 k | 121.6 ± 23.3 k |
| Lactate, mg/dL | 19.7 ± 4.61 a | 19.7 ± 5.88 a | 17.4 ± 4.88 b | 19.4 ± 5.45 h | 19.5 ± 5.44 h | 17.7 ± 4.92 i | 19.7 ± 5.41 | 18.5 ± 5.41 | 18.5 ± 5.11 |
| Cholesterol, mg/dL | 91.4 ± 19.15 | 94.5 ± 16.43 | 95.6 ± 18.79 | 93.3 ± 17.18 | 94.0 ± 18.24 | 94.9 ± 18.54 | 94.6 ± 16.97 | 96.1 ± 19.21 | 91.7 ± 17.37 |
| Triglycerides, mg/dL | 51.3 ± 29.14 | 57.5 ± 32.30 | 54.2 ± 36.46 | 55.6 ± 42.69 | 52.9 ± 27.41 | 56.0 ± 31.02 | 57.5 ± 41.97 | 52.5 ± 26.88 | 55.1 ± 32.24 |
| β-OHB, mmol/L | 0.116 ± 0.08 d | 0.129 ± 0.06 d,e | 0.145 ± 0.07 e | 0.121 ± 0.07 k | 0.136 ± 0.07 j | 0.136 ± 0.05 j | 0.125 ± 0.07 | 0.143 ± 0.07 | 0.126 ± 0.06 |
| NEFA, mmol/L | 0.537 ± 0.15 | 0.546 ± 0.20 | 0.541 ± 0.16 | 0.517 ± 0.16 | 0.530 ± 0.15 | 0.571 ± 0.19 | 0.523 ± 0.17 | 0.547 ± 0.16 | 0.550 ± 0.19 |
| Urea, mg/dL | 31.1 ± 7.92 d | 33.9 ± 11.78 d | 35.7 ± 8.78 e | 33.7 ± 11.58 | 33.1 ± 10.25 | 34.9 ± 8.30 | 33.2 ± 12.06 | 34.1 ± 9.57 | 34.3 ± 8.69 |
| Parameters | Age | Pregnancy Rank | ||||||
|---|---|---|---|---|---|---|---|---|
| Mature Ewes (n = 218) | Maiden Sheep (n = 179) | Single (n = 186) | Multiple (n = 211) | |||||
| LLs, days | 200.1 ± 55.5 e | 217.0 ± 69.9 f | 208.6 ± 62.5 | 207.1 ± 64.7 | ||||
| TMYs, L | 373.6 ± 171.6 | 348.5 ± 144.9 | 355.5 ± 154.7 | 369.0 ± 167.4 | ||||
| YDIMs, L/d | 2.13 ± 0.72 a | 1.85 ± 0.58 b | 1.93 ± 0.63 g | 2.07 ± 0.71 h | ||||
| YDIMts, L/d | 1.81 ± 0.61 a | 1.60 ± 0.51 b | 1.68 ± 0.55 | 1.76 ± 0.60 | ||||
| DLPs, days | 57.6 ± 20.0 g | 52.8 ± 14.1 h | 54.1 ± 12.4 | 56.3 ± 21.2 | ||||
| Single Pregnancy (n = 86) | Multiple Pregnancy (n = 132) | Single Pregnancy (n = 100) | Multiple Pregnancy (n = 79) | Mature Ewes (n = 86) | Maiden Sheep (n = 100) | Mature Ewes (n = 132) | Maiden Sheep (n = 79) | |
| LLs, days | 196.1 ± 49.2 | 202.3 ± 59.6 | 219.4 ± 70.5 | 215.1 ± 72.0 | 196.1 ± 49.2 g | 219.4 ± 70.5 h | 202.3 ± 59.6 | 215.1 ± 72.0 |
| TMYs, L | 355.0 ± 158.6 | 386.9 ± 180.7 | 355.9 ± 152.1 | 339.1 ± 138.5 | 355.0 ± 158.6 | 355.9 ± 152.1 | 386.9 ± 180.7 g | 339.1 ± 138.5 h |
| YDIMs, L/d | 2.00 ± 0.66 g | 2.22 ± 0.75 h | 1.87 ± 0.59 | 1.82 ± 0.57 | 2.00 ± 0.66 | 1.87 ± 0.59 | 2.22 ± 0.75 c | 1.82 ± 0.57 d |
| YDIMts, L/d | 1.75 ± 0.56 | 1.86 ± 0.64 | 1.61 ± 0.53 | 1.59 ± 0.49 | 1.75 ± 0.56 | 1.61 ± 0.53 | 1.86 ± 0.64 c | 1.59 ± 0.49 d |
| DLPs, days | 56.4 ± 13.5 | 58.6 ± 23.5 | 52.3 ± 11.3 | 53.0 ± 17.0 | 56.4 ± 13.5 g | 52.3 ± 11.3 h | 58.6 ± 23.5 g | 53.0 ± 17.0 h |
| Phenotypic Parameters | L-YDIMs (n = 124) | A-YDIMs (n = 201) | H-YDIMs (n = 200) |
|---|---|---|---|
| Birth weight, kg | 3.81 ± 0.92 d | 3.92 ± 0.96 de | 4.10 ± 0.85 e |
| Body weight, 17 days *, kg | 7.40 ± 2.0 a | 7.79 ± 2.0 a | 8.62 ± 2.2 b |
| Average daily weight gain, kg | 0.228 ± 0.007 | 0.229 ± 0.005 | 0.238 ± 0.005 |
| Birth trunk length, cm | 28.93 ± 2.50 f | 29.01 ± 2.61 f,g | 29.67 ± 2.04 g |
| Trunk length 17 days *, cm | 35.80 ± 3.96 a | 37.32 ± 3.85 b | 38.79 ± 3.66 c |
| Birth BMI, kg/m2 | 44.92 ± 7.11 h | 45.91 ± 6.67 hi | 46.22 ± 6.68 i |
| BMI, 17 days *, kg/m2 | 57.31 ± 10.45 | 55.45 ± 10.08 | 56.73 ± 10.15 |
| Thoracic girth, 17 days *, cm | 43.35 ± 3.92 a | 43.93 ± 4.24 a | 45.38 ± 4.15 b |
| Abdominal girth, 17 days *, cm | 42.89 ± 4.47 a | 43.85 ± 8.45 a | 45.60 ± 4.92 b |
| Parameters | Male | Female | Male + Female | Female + Female | Male + Male |
|---|---|---|---|---|---|
| (n = 95) | (n = 91) | (n = 111) | (n = 58) | (n = 39) | |
| LLs, days | 206.7 ± 57.9 | 210.6 ± 67.3 | 211.7 ± 68.7 | 201.8 ± 66.2 | 201.7 ± 48.9 |
| TMYs, L | 367.6 ± 165.0 | 342.7 ± 143.0 | 362.6 ± 167.9 | 394.5 ± 190.1 | 349.2 ± 123.8 |
| YDIMs, L/d | 2.02 ± 0.68 a | 1.84 ± 0.56 b | 2.02 ± 0.69 | 2.22 ± 0.80 | 1.99 ± 0.60 |
| YDIMts, L/d | 1.73 ± 0.58 | 1.62 ± 0.51 | 1.68 ± 0.60 | 1.91 ± 0.60 | 1.75 ± 0.57 |
| DLPs, days | 54.5 ± 13.2 | 53.7 ± 11.7 | 58.9 ± 26.7 | 53.5 ± 9.8 | 54.4 ± 18.5 |
| Parameters | Mature Ewes | Maiden Sheep | Mature Ewes | Maiden Sheep | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male (40) | Female (46) | Male (55) | Female (45) | Male + Female (73) | Female + Female (36) | Male + Male (21) | Male + Female (38) | Female + Female (22) | Male + Male (18) | |
| LLs, days | 207.0 ± 54.5 | 186.6 ± 42.5 | 206.5 ± 60.7 a | 235.2 ± 78.7 b | 200.5 ± 63.3 | 207.7 ± 63.0 | 199.1 ± 38.4 | 233.2 ± 74.4 | 192.2 ± 71.7 | 204.7 ± 59.9 |
| TMYs, L | 386.5 ± 174.3 | 327.6 ± 139.8 | 353.9 ± 158.1 | 358.3 ± 146.2 | 367.0 ± 180.7 | 443.8 ± 192.0 | 358.8 ± 143.0 | 354.2 ± 141.9 | 313.8 ± 160.4 | 338.1 ± 99.7 |
| YDIMs, L/d | 2.10 ± 0.73 | 1.91 ± 0.59 | 1.96 ± 0.64 | 1.76 ± 0.52 | 2.13 ± 0.72 | 2.46 ± 0.77 | 2.11 ± 0.75 | 1.80 ± 0.58 | 1.82 ± 0.72 | 1.85 ± 0.31 |
| YDIMts, L/d | 1.82 ± 0.58 | 1.69 ± 0.55 | 1.67 ± 0.57 | 1.54 ± 0.46 | 1.75 ± 0.63 a | 2.10 ± 0.56 b | 1.82 ± 0.69 a,b | 1.54 ± 0.52 | 1.60 ± 0.53 | 1.67 ± 0.38 |
| DLPs, days | 58.6 ± 15.9 | 54.3 ± 10.2 | 51.5 ± 9.8 | 53.3 ± 12.8 | 62.3 ± 31.3 | 54.7 ± 9.65 | 55.2 ± 11.4 | 53.7 ± 16.7 | 51.5 ± 10.0 | 53.6 ± 23.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesántez-Pacheco, J.L.; Heras-Molina, A.; Torres-Rovira, L.; Sanz-Fernández, M.V.; García-Contreras, C.; Vázquez-Gómez, M.; Feyjoo, P.; Cáceres, E.; Frías-Mateo, M.; Hernández, F.; et al. Maternal Metabolic Demands Caused by Pregnancy and Lactation: Association with Productivity and Offspring Phenotype in High-Yielding Dairy Ewes. Animals 2019, 9, 295. https://doi.org/10.3390/ani9060295
Pesántez-Pacheco JL, Heras-Molina A, Torres-Rovira L, Sanz-Fernández MV, García-Contreras C, Vázquez-Gómez M, Feyjoo P, Cáceres E, Frías-Mateo M, Hernández F, et al. Maternal Metabolic Demands Caused by Pregnancy and Lactation: Association with Productivity and Offspring Phenotype in High-Yielding Dairy Ewes. Animals. 2019; 9(6):295. https://doi.org/10.3390/ani9060295
Chicago/Turabian StylePesántez-Pacheco, José Luis, Ana Heras-Molina, Laura Torres-Rovira, María Victoria Sanz-Fernández, Consolación García-Contreras, Marta Vázquez-Gómez, Pablo Feyjoo, Elisa Cáceres, Millán Frías-Mateo, Fernando Hernández, and et al. 2019. "Maternal Metabolic Demands Caused by Pregnancy and Lactation: Association with Productivity and Offspring Phenotype in High-Yielding Dairy Ewes" Animals 9, no. 6: 295. https://doi.org/10.3390/ani9060295
APA StylePesántez-Pacheco, J. L., Heras-Molina, A., Torres-Rovira, L., Sanz-Fernández, M. V., García-Contreras, C., Vázquez-Gómez, M., Feyjoo, P., Cáceres, E., Frías-Mateo, M., Hernández, F., Martínez-Ros, P., González-Martin, J. V., González-Bulnes, A., & Astiz, S. (2019). Maternal Metabolic Demands Caused by Pregnancy and Lactation: Association with Productivity and Offspring Phenotype in High-Yielding Dairy Ewes. Animals, 9(6), 295. https://doi.org/10.3390/ani9060295





