Effects of Dietary Xylanase and Arabinofuranosidase Combination on the Growth Performance, Lipid Peroxidation, Blood Constituents, and Immune Response of Broilers Fed Low-Energy Diets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Nutrient Digestibility
2.3. Biochemical Analysis
2.4. Serum Antibody Titers
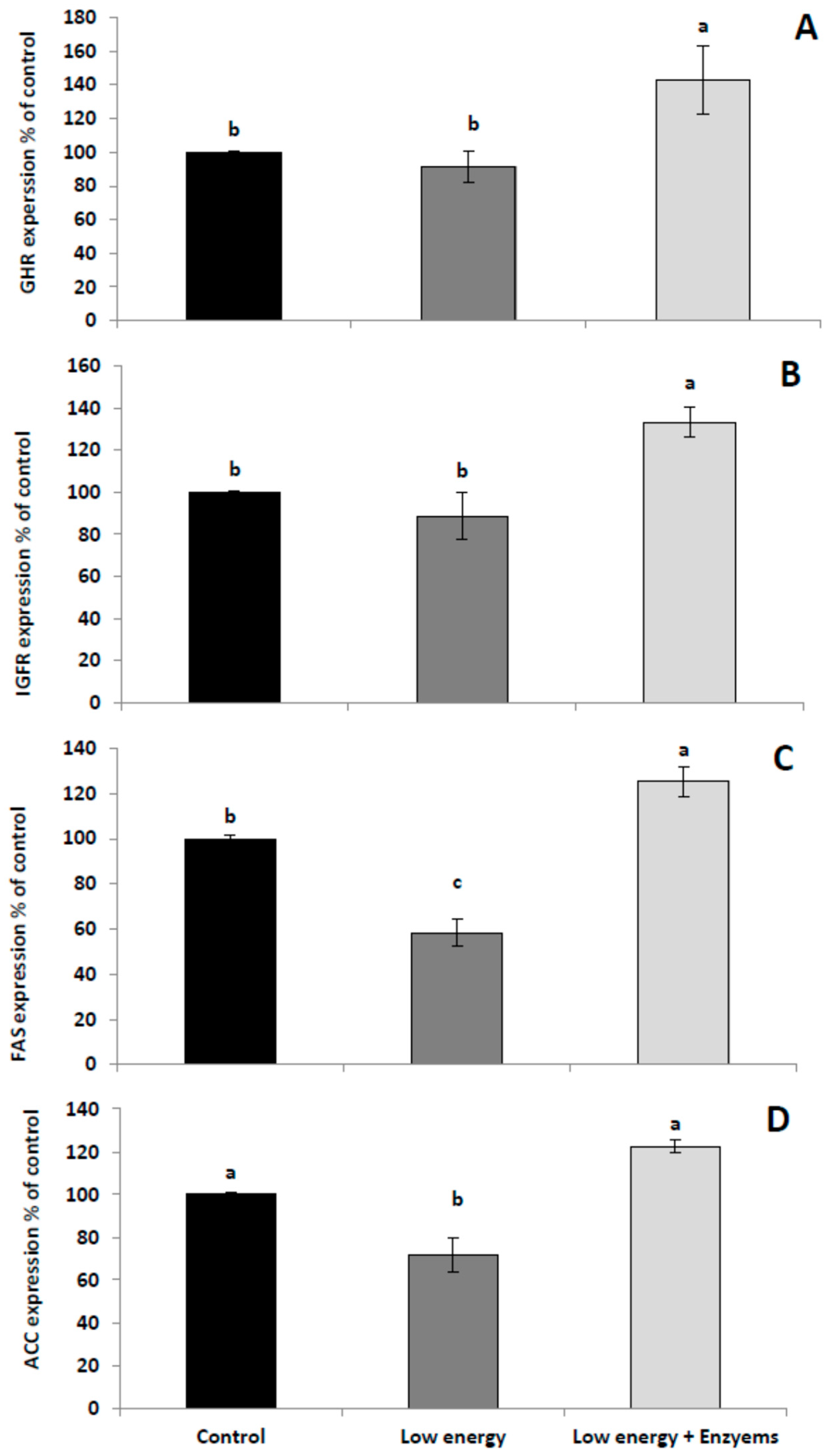
2.5. RNA Analysis
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Donohue, M.; Cunningham, D.L. Effects of grain and oilseed prices on the costs of US poultry production. J. Appl. Poult. Res. 2009, 18, 325–337. [Google Scholar] [CrossRef]
- Horvatovic, M.P.; Glamocic, D.; Zikic, D.; Hadnadjev, T.D. Performance and some intestinal functions of broilersfed diets with different inclusion levels of sunflower meal and supplemented or not with enzymes. Braz. J. Poult. Sci. 2015, 17, 25–30. [Google Scholar] [CrossRef]
- Saleh, A.A.; El-Far, A.H.; Abdel-Latif, M.A.; Emam, M.A.; Ghanem, R.; Abd El-Hamid, H.S. Exogenous dietary enzyme formulations improve growth performance of broiler chickens fed a low-energy diet targeting the intestinal nutrienttransporter genes. PLoS ONE 2018, 13, e0198085. [Google Scholar] [CrossRef] [PubMed]
- Almirall, M.; Francesch, M.; Perez-Vendrell, A.M.; Brufau, J.; Esteve-Garcia, E. The differences in intestinalviscosity produced by barley and beta-glucanase alter digesta enzyme activities and ileal nutrient digestibilities more in broiler chicks than in cocks. J. Nutr. 1995, 125, 947–955. [Google Scholar] [PubMed]
- Abdel-Latif, M.A.; El-Far, A.H.; Elbestawy, A.R.; Ghanem, R.; Mousa, S.A.; Abd El-Hamid, H.S. Exogenous dietary lysozyme improves the growth performance and gut microbiota in broiler chickens targeting the antioxidant and non-specific immunity mRNA expression. PLoS ONE 2017, 12, e0185153. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.R. The Effect of Enzymes on Digestion. J. Appl. Poult. Res. 1996, 5, 370–378. [Google Scholar] [CrossRef]
- Dibner, J.J.; Richards, J.D. The Digestive System: Challenges and Opportunities. J. Appl. Poult. Res. 2004, 13, 86–93. [Google Scholar] [CrossRef]
- Saleh, A.A.; Gálik, B.; Arpášová, H.; Capcarová, M.; Kalafová, A.; Šimko, M.; Juráček, M.; Rolinec, M.; Bíro, D.; Abudabos, A.M. Synergistic effect of feeding Aspergillus Awamori and Lactic acid bacteria on performance, egg traits, egg yolk cholesterol and fatty acid profile in laying hens. Ital. J. Anim. Sci. 2017, 16, 132–139. [Google Scholar] [CrossRef]
- Cho, J.H.; Kim, I.H. Effects of beta mannanase and xylanase supplementation in low energy density diets on performances, nutrient digestibility, blood profiles and meat quality in finishing pigs. Vet. Adv. 2013, 8, 622–630. [Google Scholar]
- Knudsen, K.E.B. Fiber and nonstarch polysaccharide content and variation in common crops used in broiler diets. Poult. Sci. 2014, 93, 2380–2393. [Google Scholar] [CrossRef]
- Cozannet, P.; Kidd, M.T.; Montanhini Neto, R.; Geraert, P.A. Next-generation non-starch polysaccharide-degrading, multi-carbohydrase complex rich in xylanase and arabinofuranosidase to enhance broiler feed digestibility. Poult. Sci. 2017, 96, 2743–2750. [Google Scholar] [CrossRef] [PubMed]
- De la Mare, M.; Guais, O.; Bonnin, E.; Weber, J.; Francois, J.M. Molecular and biochemical characterization of three GH62 α-I-383 arabinofuranosidases from the soil deuteromycete Penicillium funiculosum. Enzym. Microb. Technol. 2013, 53, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Ravn, J.L.; Glitsø, V.; Pettersson, D.; Ducatelle, R.; van Immerseel, F.; Pedersen, N.R. Combined endo-β-1,4-xylanase and α-l-arabinofuranosidase increases butyrate concentration during broiler cecal fermentation of maize glucurono-arabinoxylan. Anim. Feed Sci. Technol. 2018, 236, 159–169. [Google Scholar] [CrossRef]
- AOAC. International Official Methods of Analysis, 18th ed.; Current through Revision 2 (Online); AOAC International: Gaithersburg, MD, USA, 1994. [Google Scholar]
- Saleh, A.A. Effects of fish oil on the production performances, polyunsaturated fatty acids and cholesterol levels of yolk in hens. Emir. J. Food Agric. 2013, 25, 605–661. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- OIE. A Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 5th ed.; Part 2 Section 21; OIE: Tokyo, Japan, 2009; Chapter 2; pp. 7–12. [Google Scholar]
- Abramoff, M.D.; Magelhaes, P.J.; Ram, S.J. Image processing with Image. J. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Nortey, T.N.; Patience, J.F.; Sands, J.S.; Trottier, N.L.; Zijlstra, R.T. Effects of xylanase supplementation on the apparent digestibility and digestible calculated nutritional content of energy, amino acids, phosphorus, and calcium in wheat and wheat by-products from dry milling fed to grower pigs. J. Anim. Sci. 2008, 86, 3450–3464. [Google Scholar] [CrossRef] [PubMed]
- Slominski, B.A.; Gdala, J.; Boros, D.; Campbell, L.D.; Guenter, W.; Jones, O. Variability in chemical and nutritive composition of Canadian wheat and the potential for its minimization by enzyme use. In Proceedings of the XXI World Poultry Congress, Montreal, QC, Canada, 20–24 August 2000. [Google Scholar]
- Cowieson, A.J.; Ravindran, V. Effects of exogenous enzymes in maize-based diets varying in nutrient density for young broilers: Growth performance and digestibility of energy, mineral and amino acids. Br. Poult. Sci. 2008, 49, 37–44. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Chung, T.K.; Moughan, P.J. The effect of a commercial enzyme preparation on apparent metabolizable energy, the true ileal amino acid digestibility, and endogenous ileal lysine losses in broiler chickens. Poult. Sci. 2007, 86, 665–672. [Google Scholar] [CrossRef]
- Cowieson, A.J.; Ravindran, V. Sensitivity of broiler starter to three doses of an enzyme cocktail in maize based diets. Br. Poult. Sci. 2008, 49, 340–346. [Google Scholar] [CrossRef]
- Meng, X.; Slominski, B.A. Nutritive value of corn, soybean meal, canola meal, and peas for broiler chickens as affected by a multicarbohydrase preparation of cell wall degrading enzymes. Poult. Sci. 2005, 84, 1242–1251. [Google Scholar] [CrossRef] [PubMed]
- Francesch, M.; Perez-Vendrell, A.M.; Esteve-Garcia, E.; Brufau, J. Effects of cultivar, pelleting and enzyme addition on nutritive value of barley in poultry diets. Br. Poult. Sci. 1994, 35, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Yi, G.F.; Song, G.L.; Hou, Y.Q.; Huang, J.W.; Vasquez-Anon, M.; Knight, C.D. Impact of feeding 2-hydroxy-4-(methyltio)butanoic acid and dl-methionine supplemented maize-soybean-rapeseed meal diets on growth performance and carcass quality of broilers. Br. Poult. Sci. 2007, 48, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Farran, M.T.; Barbour, G.W.; Usayran, N.N.; Darwish, A.H.; Machlab, H.H.; Hruby, M.; Ashkarian, V.M. Performance and carcass quality of broiler chickens fed a corn-soybean meal diet containing graded levels without or with enzyme. J. Poult. Sci. 2010, 47, 34–40. [Google Scholar] [CrossRef]
- Garipoglu, A.V.; Saricicek, B.Z.; Kilic, U. Effects of the Commercial Enzyme Supplementation to the Rations on Broiler Performance. Asian J. Anim. Vet. Adv. 2006, 1, 42–48. [Google Scholar]
- Kocher, A.; Choct, M.; Ross, G.; Broz, J.; Chung, T.K. Effects of enzyme combinations on apperent metabolisable energy of corn-soybean meal-based diets in broilers. J. Appl. Poult. Res. 2003, 12, 275–283. [Google Scholar] [CrossRef]
- Garcia, E.; Brufau, J.; Perez-Vandrell, A.; Miguel, A.; Duven, K. Bioefficiacy of enzyme preparations containing beta-glucanase and xylanase activities in broiler diets based on barley or wheat in combination whit flavomycin. Poult. Sci. 1997, 76, 1728–1737. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.I.; Marzorati, M.; Grootaert, C.; Baran, M.; Van Craeyveld, V.; Courtin, C.M.; Broekaert, W.F.; Delcour, J.A.; Verstraete, W.; Van De Wiele, T. Arabinoxylan-oligosaccharides (AXOS) affect the protein/carbohydrate fermentation balance and microbial population dynamics of the simulator of human intestinal microbial ecosystem. Microb. Biotechnol. 2009, 2, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Damen, B.; Verspreet, J.; Pollet, A.; Broekaert, W.F.; Delcour, J.A.; Courtin, C.M. Prebiotic effects and intestinal fermentation of cereal arabinoxylans and arabinoxylan oligosaccharides in rats depend strongly on their structural properties and joint presence. Mol. Nutr. Food Res. 2011, 55, 1862–1874. [Google Scholar] [CrossRef]
- Saleh, A.A.; Hayashi, K.; Ijiri, D.; Ohtsuka, A. Beneficial effects of Aspergillus awamori in broiler nutrition. World’s Poult. Sci. J. 2014, 70, 857–864. [Google Scholar] [CrossRef]
- Eeckhaut, V.; Van Immerseel, F.; Dewulf, J.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R.; Courtin, C.M.; Delcour, J.A.; Broekaert, W.F. Arabinoxylooligosaccharides from wheat bran inhibit Salmonella colonization in broiler chickens. Poult. Sci. 2008, 87, 2329–2334. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.A. Effect of dietary mixture of Aspergillus probiotic and selenium nano-particles on growth, nutrient digestibilities, selected blood parameters and muscle fatty acid profile in broiler chickens. Anim. Sci. Pap. Rep. 2014, 32, 65–79. [Google Scholar]
- Lei, Z.; Shao, Y.; Yin, X.; Yin, D.; Guo, Y.; Yuan, J. Combination of xylanase and debranching enzymes specific to wheat arabinoxylan improve the growth performance and gut health of broilers. J. Agric. Food Chem. 2016, 64, 4932–4942. [Google Scholar] [CrossRef] [PubMed]
- Pettey, L.A.; Carter, S.D.; Senne, B.W.; Shriver, J.A. Effect of β-mannanase addition to corn-soybean meal diets on growth performance, carcass traits and nutrient digestibility of weanling and growing-finishing pigs. J. Anim. Sci. 2002, 80, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Butt, M.S.; Hussain, R.; Ahmed, A.; Riaz, M. Effect of Oral Application of Xylanase on Some Hematological and Serum Biochemical Parameters in Broilers. Pak. Vet. J. 2013, 33, 388–390. [Google Scholar]
- Head, B.; Bionaz, M.; Cherian, G. Flaxseed and carbohydrase enzyme SupplementatioAlters hepatic n-3 polyunsaturated fatty AcidMolecular species and expression of genes associated with lipid metabolism in broiler chickens. Vet. Sci. 2019, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Liu, D.; Zhao, X.; Li, C.; Guo, Y. Xylanase supplementation of a wheat-based diet improved nutrientdigestion and mRNA expression of intestinal nutrient transporters in broiler chickens infected with Clostridiumperfringens. Poult. Sci. 2014, 93, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Manafi, M.; Nazarizadeh, H. Effects of Xylanase Supplementation and Citric Acid on Performance, Ileal Nutrients Digestibility, and Gene Expression of Intestinal Nutrient Transporters in Broilers Challenged with Clostridium perfringens. J. Poult. Sci. 2017, 54, 149–156. [Google Scholar] [CrossRef]
- Tong, L. Acetyl-coenzyme A carboxylase: Crucial metabolic enzyme and attractive target for drug discovery. Cell. Mol. Life Sci. 2005, 62, 1784–1803. [Google Scholar] [CrossRef]
- Prentice, A.M.; Jebb, S.A. Fast foods, energy density and obesity: A possible mechanistic link. Obes. Rev. 2003, 4, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.A.; Ebeid, T.A. Feeding sodium selenite and nano-selenium stimulates growth and oxidation resistance in broilers. S. Afr. J. Anim. Sci. 2019, 49, 176–184. [Google Scholar] [CrossRef]
- Rosebrough, R.W.; Russell, B.A.; Richards, M.P. Further studies on short-term adaptations in the expression of lipogenic genes in broilers. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 159, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.A.; El-Magd, M.A. Beneficial effects of dietary silver nanoparticles and silver nitrateon broiler nutrition. Environ. Sci. Pollut. Res. 2018, 25, 27031–27038. [Google Scholar] [CrossRef] [PubMed]
| Ingredient | Starter (1–10 days) | Grower (11–25 days) | Finisher (26–35 days) | |||
|---|---|---|---|---|---|---|
| Control | Low Energy | Control | Low Energy | Control | Low Energy | |
| Yellow corn | 507 | 543 | 548 | 584 | 578 | 613 |
| Soybean meal, 46% | 370 | 352 | 317 | 300 | 280 | 266 |
| Corn gluten meal, 60% | 38 | 39 | 50 | 50 | 50 | 47 |
| Soya oil | 37 | 17 | 41 | 22 | 51 | 32 |
| Calcium carbonate | 14.0 | 14.8 | 13.8 | 14.0 | 12.6 | 13.4 |
| Dicalcium phosphate | 20.0 | 20.0 | 17.5 | 17.5 | 16.0 | 16.0 |
| Salt | 2.3 | 2.3 | 2.4 | 2.4 | 2.3 | 2.3 |
| Sodium sulfate | 1.8 | 1.8 | 1.6 | 1.6 | 1.6 | 1.6 |
| Dl Methionine, 99% | 2.7 | 2.4 | 2.0 | 1.8 | 1.9 | 1.8 |
| l-Lysine HCl, 98% | 2.5 | 2.4 | 2.3 | 2.3 | 2.2 | 2.2 |
| l-Threonine | 1.1 | 1.0 | 0.7 | 0.7 | 0.6 | 0.6 |
| Choline chloride, 60% | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 |
| Premix * | 2 | 2 | 2 | 2 | 2 | 2 |
| Anticoccidia | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Anticlostridia | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 |
| Antimycotoxin biology | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 | 0.25 |
| Silica | 1 | 1 | 1 | 1 | 1 | 1 |
| Chemical Analysis on DM basis | ||||||
| AME kcal | 3000 | 2910 | 3100 | 3010 | 3200 | 3110 |
| Crude protein, % | 23.0 | 22.4 | 21.5 | 20.9 | 20.0 | 19.4 |
| Fat, % | 6.3 | 4.5 | 6.9 | 5.1 | 7.9 | 6.2 |
| Digestible LYS, % | 1.28 | 1.24 | 1.15 | 1.11 | 1.06 | 1.02 |
| Digestible M and C, % | 0.95 | 0.92 | 0.87 | 0.84 | 0.83 | 0.80 |
| Digestible THR, % | 0.86 | 0.83 | 0.77 | 0.74 | 0.71 | 0.68 |
| Digestible ARG, % | 1.37 | 1.33 | 1.25 | 1.20 | 1.14 | 1.11 |
| Digestible ILE, % | 0.90 | 0.87 | 0.85 | 0.82 | 0.77 | 0.74 |
| Digestible LEU, % | 1.87 | 1.83 | 1.84 | 1.78 | 1.74 | 1.68 |
| Digestible VAL, % | 0.96 | 0.93 | 0.91 | 0.87 | 0.84 | 0.81 |
| Calcium, % | 0.96 | 0.96 | 0.87 | 0.84 | 0.81 | 0.76 |
| Available P, % | 0.48 | 0.48 | 0.44 | 0.42 | 0.41 | 0.38 |
| Sodium, % | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
| Chloride, % | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 | 0.23 |
| Control | Low Energy | Low Energy and Enzymes | p-Value | |
|---|---|---|---|---|
| Initial body weight, g | 40.9 ± 0.5 | 40.6 ± 0.7 | 41.0 ± 0.5 | 0.29 |
| Final body weight, g/35 d | 2382 ± 52 a | 2296 ± 57 b | 2358 ± 56 ab | 0.03 |
| Body weight gain, g/35 d | 2341 ± 21.9 a | 2255 ± 27.2 b | 2317.5 ± 16.4 ab | 0.04 |
| Feed intake, g/35 d | 3485 ± 34.5 | 3500 ± 38.9 | 3410 ± 32.7 | 0.18 |
| FCR, g/g | 1.49 ± 0.02 b | 1.55 ± 0.01 a | 1.47 ± 0.02 b | 0.02 |
| Crude protein digestibility, % | 72 ± 3.6 a | 64 ± 5.4 b | 71 ± 4.8 a | 0.05 |
| Crude fat digestibility, % | 44 ± 2.4 b | 41 ± 3.5 b | 52 ± 4.3 a | 0.05 |
| Control | Low Energy | Low Energy and Enzymes | p-Value | |
|---|---|---|---|---|
| Carcass | 64 ± 0.63 | 65 ± 0.90 | 63 ± 0.63 | 0.33 |
| Breast muscle weight | 23 ± 0.49 ab | 22 ± 0.63 b | 25 ± 1.11 a | 0.07 |
| Thigh muscle weight | 18 ± 0.37 | 18 ± 0.34 | 17 ± 0.29 | 0.75 |
| Liver weight | 1.85 ± 0.07 | 1.72 ± 0.08 | 1.82 ± 0.17 | 0.70 |
| Gizzard weight | 1.28 ± 0.08 | 1.28 ± 0.08 | 1.19 ± 0.06 | 0.61 |
| Heart weight | 0.37 ± 0.02 | 0.39 ± 0.01 | 0.43 ± 0.03 | 0.27 |
| Spleen weight | 0.14 ± 0.02 | 0.11 ± 0.08 | 0.12 ± 0.02 | 0.46 |
| Abdominal fat weight | 1.18 ± 0.12 a | 0.81 ± 0.09 b | 0.89 ± 0.02 b | 0.02 |
| Bursa of Fabricius weight | 0.13 ± 0.02 b | 0.14 ± 0.02 b | 0.20 ± 0.02 a | 0.04 |
| Control | Low Energy | Low Energy and Enzymes | p-Value | |
|---|---|---|---|---|
| GPT, I/U | 19 ± 2.33 | 18 ± 2.61 | 15 ± 1.05 | 0.38 |
| GOT, I/U | 399 ± 18.10 | 361 ± 24.70 | 374 ± 40.60 | 0.64 |
| Total protein, mg/dL | 2.83 ± 0.10 | 2.98 ± 0.09 | 3.21 ± 0.12 | 0.06 |
| Albumin, mg/dL | 1.58 ± 0.05 | 1.59 ± 0.09 | 1.56 ± 0.08 | 0.97 |
| Globulin, mg/dL | 1.26 ± 0.09 b | 1.43 ± 0.04 b | 1.7 ± 0.11 a | 0.01 |
| Total cholesterol, mg/dL | 150 ± 5.3 a | 135 ± 3.4 b | 133 ± 2.3 b | 0.02 |
| Triglycerides, mg/dL | 21 ± 2.18 | 25 ± 2.47 | 18 ± 3.02 | 0.20 |
| HDL-cholesterol, mg/dL | 56.98 ± 3.01 b | 67.47 ± 3.82 ab | 71.83 ± 4.44 a | 0.04 |
| LDL-cholesterol, mg/dL | 79.28 ± 4.2 | 87.10 ± 11.9 | 75.81 ± 5.5 | 0.60 |
| Glucose, mg/dL | 193 ± 3.7 | 193 ± 4.8 | 199 ± 4.4 | 0.49 |
| Creatinine, mg/dL | 0.47 ± 0.04 | 0.50 ± 0.02 | 0.51 ± 0.02 | 0.49 |
| ND, titer | 2.75 ± 0.47 b | 3.75 ± 0.47 ab | 5.25 ± 0.62 a | 0.03 |
| H9N1, titer | 0.25 ± 0.03 | 1.0 ± 0.04 | 1.25 ± 0.03 | 0.11 |
| Control | Low Energy | Low Energy and Enzymes | p-Value | |
|---|---|---|---|---|
| TBARS, nanomole/g | 7.08 ± 0.71 a | 5.03 ± 0.46 b | 5.35 ± 0.34 b | 0.05 |
| Lysine, g/100 g protein | 5.89 ± 0.43 | 5.91 ± 0.49 | 5.93 ± 0.50 | 0.99 |
| Methionine, g/100 g protein | 1.14 ± 0.04 | 1.20 ± 0.08 | 1.20 ± 0.01 | 0.56 |
| Total lipids, g/100 g muscle | 3.13 ± 0.07 b | 3.66 ± 0.48 ab | 4.98 ± 0.54 a | 0.03 |
| Oleic acid, mg/100 g fat | 0.124 ± 0.008 | 0.152 ± 0.012 | 0.155 ± 0.005 | 0.96 |
| Linoleic acid, mg/100 g fat | 0.226 ± 0.009 | 0.777 ± 0.065 | 0.424 ± 0.025 | 0.63 |
| Linolenic acid, mg/100 g fat | 0.095 ± 0.003 | 0.083 ± 0.008 | 0.157 ± 0.007 | 0.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleh, A.A.; Kirrella, A.A.; Abdo, S.E.; Mousa, M.M.; Badwi, N.A.; Ebeid, T.A.; Nada, A.L.; Mohamed, M.A. Effects of Dietary Xylanase and Arabinofuranosidase Combination on the Growth Performance, Lipid Peroxidation, Blood Constituents, and Immune Response of Broilers Fed Low-Energy Diets. Animals 2019, 9, 467. https://doi.org/10.3390/ani9070467
Saleh AA, Kirrella AA, Abdo SE, Mousa MM, Badwi NA, Ebeid TA, Nada AL, Mohamed MA. Effects of Dietary Xylanase and Arabinofuranosidase Combination on the Growth Performance, Lipid Peroxidation, Blood Constituents, and Immune Response of Broilers Fed Low-Energy Diets. Animals. 2019; 9(7):467. https://doi.org/10.3390/ani9070467
Chicago/Turabian StyleSaleh, Ahmed A., Abeer A. Kirrella, Safaa E. Abdo, Mahmoud M. Mousa, Nemat A. Badwi, Tarek A. Ebeid, Ahmed L. Nada, and Mahmoud A. Mohamed. 2019. "Effects of Dietary Xylanase and Arabinofuranosidase Combination on the Growth Performance, Lipid Peroxidation, Blood Constituents, and Immune Response of Broilers Fed Low-Energy Diets" Animals 9, no. 7: 467. https://doi.org/10.3390/ani9070467
APA StyleSaleh, A. A., Kirrella, A. A., Abdo, S. E., Mousa, M. M., Badwi, N. A., Ebeid, T. A., Nada, A. L., & Mohamed, M. A. (2019). Effects of Dietary Xylanase and Arabinofuranosidase Combination on the Growth Performance, Lipid Peroxidation, Blood Constituents, and Immune Response of Broilers Fed Low-Energy Diets. Animals, 9(7), 467. https://doi.org/10.3390/ani9070467






