Effect of Dietary Modulation of Selenium Form and Level on Performance, Tissue Retention, Quality of Frozen Stored Meat and Gene Expression of Antioxidant Status in Ross Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Selenium Sources
2.2. Birds and Experimental Procedures
2.3. Growth Parameter Measurement
2.4. Sampling and Analytical Procedures
2.4.1. Tissue Retention of Selenium
2.4.2. Selenium Content in Serum Constituents
2.4.3. Laboratory Analysis for Meat Quality
Meat pH and Drip and Cooking Loss in Meat Samples
Preparation of Samples for Total Antioxidant Capacity
2.5. RNA Extraction, Reverse Transcription, and Quantitative Real-Time PCR.
2.6. Statistical Analysis
3. Results and Discussion
3.1. Growth Performance
3.2. Selenium Retention in Serum, Muscle, and Liver
3.3. The Effect of Different Levels and Sources of Se on Selected Serum Parameters
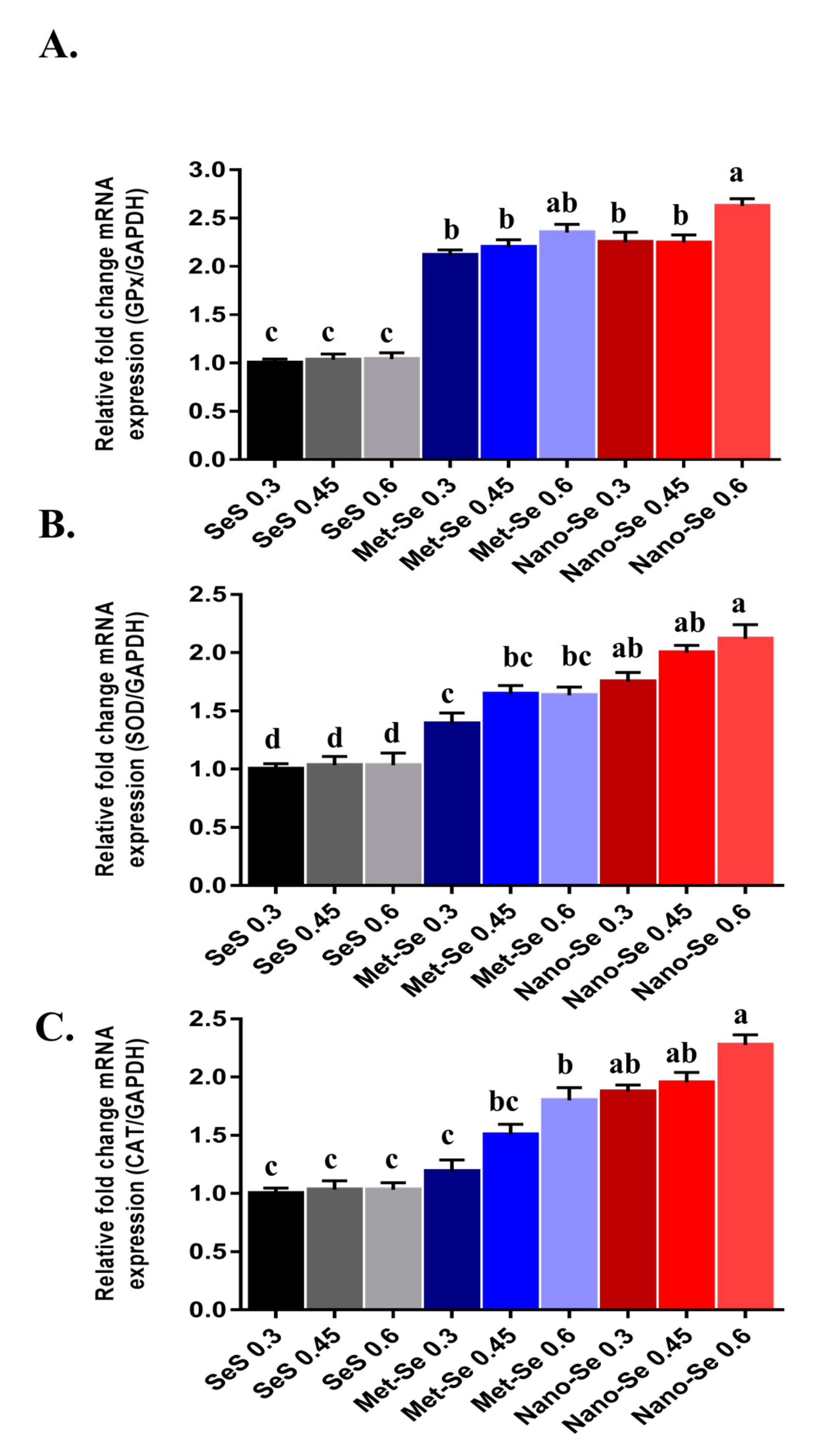
3.4. Antioxidant Potential of Different Sources and Levels of Se
3.5. Effect of Different Se Sources and Levels on Meat Quality
3.6. Post-Mortem pH of Meat, Cooking Loss and Drip Loss
3.7. Thiobarbituric acid Reactive Substances (TBRAS) Content of Meat as a Marker for Lipid Oxidation
3.8. Total Antioxidant Capacity of Meat
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kieliszek, M.; Błażejak, S. Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Surai, P.F. Selenium in Nutrition and Health; Nottingham University Press: Nottingham, UK, 2006; Volume 974. [Google Scholar]
- Skřivan, M.; Englmaierová, M.; Dlouhá, G.; Bubancová, I.; Skřivanová, V. High dietary concentrations of methionine reduce the selenium content, glutathione peroxidase activity and oxidative stability of chicken meat. Czech J. Anim. Sci. 2011, 56, 398–405. [Google Scholar] [CrossRef]
- Gao, X.; Xing, H.; Li, S.; Li, J.; Ying, T.; Xu, S. Selenium regulates gene expression of selenoprotein W in chicken gastrointestinal tract. Biol. Trace Elem. Res. 2012, 145, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Gromadzińska, J.; Reszka, E.; Bruzelius, K.; Wąsowicz, W.; Åkesson, B. Selenium and cancer: Biomarkers of selenium status and molecular action of selenium supplements. Eur. J. Nutr. 2008, 47, 29–50. [Google Scholar] [CrossRef] [PubMed]
- NRC. Nutrient Requirements of Poultry; National Academy Press: Washington, DC, USA, 1994. [Google Scholar]
- Heindl, J.; Ledvinka, Z.; Englmaierová, M.; Zita, L.; Tumová, E. The effect of dietary selenium sources and levels on performance, selenium content in muscle and glutathione peroxidase activity in broiler chickens. Czech J. Anim. Sci. 2010, 55, 572–578. [Google Scholar] [CrossRef]
- Wolffram, S.; Anliker, E.; Scharrer, E. Uptake of selenate and selenite by isolated intestinal brush border membrane vesicles from pig, sheep, and rat. Biol. Trace Elem. Res. 1986, 10, 293–306. [Google Scholar] [CrossRef]
- Briens, M.; Mercier, Y.; Rouffineau, F.; Vacchina, V.; Geraert, P.-A. Comparative study of a new organic selenium source v. seleno-yeast and mineral selenium sources on muscle selenium enrichment and selenium digestibility in broiler chickens. Br. J. Nutr. 2013, 110, 617–624. [Google Scholar] [CrossRef] [PubMed]
- Choct, M.; Naylor, A.; Reinke, N. Selenium supplementation affects broiler growth performance, meat yield and feather coverage. Br. Poult. Sci. 2004, 45, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Xu, T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: Comparison with se-methylselenocysteine in mice. Toxicol. Sci. 2008, 101, 22–31. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Yan, X.; Zhang, L. Comparison of short-term toxicity between Nano-Se and selenite in mice. Life Sci. 2005, 76, 1099–1109. [Google Scholar] [CrossRef]
- Cai, S.; Wu, C.; Gong, L.; Song, T.; Wu, H.; Zhang, L. Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poult. Sci. 2012, 91, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; McLELLAN, L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 1999, 31, 273–300. [Google Scholar] [CrossRef] [PubMed]
- Mahan, D.C.; Azain, M.; Crenshaw, T.D.; Cromwell, G.L.; Dove, C.R.; Kim, S.W.; Lindemann, M.D.; Miller, P.S.; Pettigrew, J.E.; Stein, H.H.; et al. Supplementation of organic and inorganic selenium to diets using grains grown in various regions of the United States with differing natural Se concentrations and fed to grower-finisher swine. J. Anim. Sci. 2014, 92, 4991–4997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, S.; Samaraweera, H.; Lee, E.J.; Ahn, D.U. Improving functional value of meat products. Meat Sci. 2010, 86, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.S.; Gao, X.Y.; Zhang, L.D.; Bao, Y.P. Biological effects of a nano red elemental selenium. Biofactors 2001, 15, 27–38. [Google Scholar] [CrossRef]
- Srivastava, N.; Mukhopadhyay, M. Green synthesis and structural characterization of selenium nanoparticles and assessment of their antimicrobial property. Bioprocess Biosyst. Eng. 2015, 38, 1723–1730. [Google Scholar] [CrossRef]
- Aviagen, W. Ross 308: Broiler Nutrition Specification; Aviagen Inc.: Huntsville, AL, USA,, 2007. [Google Scholar]
- Latimer, G.W. Official Methods of Analysis of AOAC International; AOAC International: Washington, DC, USA, 2012. [Google Scholar]
- Wahlen, R.; Evans, L.; Turner, J.; Hearn, R. The use of collision/reaction cell ICP-MS for the determination of elements in blood and serum samples. Spectroscopy 2005, 20, 84–89. [Google Scholar]
- Torres Filho, R.D.A.; Cazedey, H.P.; Fontes, P.R.; Ramos, A.D.S.; Ramos, E.M. Drip Loss Assessment by Different Analytical Methods and Their Relationships with Pork Quality Classification. J. Food Qual. 2017, 2017, 8. [Google Scholar] [CrossRef]
- Zeb, A.; Ullah, F. A Simple Spectrophotometric Method for the Determination of Thiobarbituric Acid Reactive Substances in Fried Fast Foods. J. Anal. Methods Chem. 2016, 2016, 5. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Manjunath, M.; Lavanya, G.; Sivajyothi, R.; Reddy, O.V.S. Antioxidant and radical scavenging activity of Actiniopteris radiata (Sw.) Link. Asian J. Exp. Sci. 2011, 25, 73–80. [Google Scholar]
- Lavanya, G.; Voravuthikunchai, S.P.; Towatana, N.H. Acetone Extract from Rhodomyrtus tomentosa: A Potent Natural Antioxidant. Evid.-Based Complement. Altern. Med. 2012, 212, 8. [Google Scholar] [CrossRef]
- Yarru, L.P.; Settivari, R.S.; Gowda, N.K.S.; Antoniou, E.; Ledoux, D.R.; Rottinghaus, G.E. Effects of turmeric (Curcuma longa) on the expression of hepatic genes associated with biotransformation, antioxidant, and immune systems in broiler chicks fed aflatoxin. Poultry Science 2009, 88, 2620–2627. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, Y.; Xiong, L.; Zhang, H.; Song, J.; Xia, M. Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim. Feed Sci. Technol. 2012, 177, 204–210. [Google Scholar] [CrossRef]
- Yoon, I.; Werner, T.M.; Butler, J.M. Effect of source and concentration of selenium on growth performance and selenium retention in broiler chickens. Poult. Sci. 2007, 86, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Couloigner, F.; Jlali, M.; Briens, M.; Rouffineau, F.; Geraert, P.-A.; Mercier, Y. Selenium deposition kinetics of different selenium sources in muscle and feathers of broilers. Poult. Sci. 2015, 94, 2708–2714. [Google Scholar] [CrossRef] [PubMed]
- Bakhshalinejad, R.; Akbari Moghaddam Kakhki, R.; Zoidis, E. Effects of different dietary sources and levels of selenium supplements on growth performance, antioxidant status and immune parameters in Ross 308 broiler chickens. Br. Poult. Sci. 2018, 59, 81–91. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, H.; Yuan, D.; Wang, Y.; Zhu, F. Comparison of different forms of dietary selenium supplementation on gene expression of cytoplasmic thioredoxin reductase, selenoprotein P, and selenoprotein W in broilers. Czech J. Anim. Sci. 2014, 59, 571–578. [Google Scholar] [CrossRef]
- Saleh, A.A. Effect of dietary mixture of Aspergillus probiotic and selenium nano-particles on growth, nutrient digestibilities, selected blood parameters and muscle fatty acid profile in broiler chickens. Anim. Sci. Pap. Rep. 2014, 32, 65–79. [Google Scholar]
- Liao, X.; Lu, L.; Li, S.; Liu, S.; Zhang, L.; Wang, G.; Li, A.; Luo, X. Effects of selenium source and level on growth performance, tissue selenium concentrations, antioxidation, and immune functions of heat-stressed broilers. Biol. Trace Elem. Res. 2012, 150, 158–165. [Google Scholar] [CrossRef]
- Wang, Y.-B.; Xu, B.-H. Effect of different selenium source (sodium selenite and selenium yeast) on broiler chickens. Anim. Feed Sci. Technol. 2008, 144, 306–314. [Google Scholar] [CrossRef]
- Wang, Y.J.; Linglin, F. Forms of Selenium Affect its Transport, Uptake and Glutathione Peroxidase Activity in the Caco-2 Cell Model. Biol. Trace Elem. Res. 2012, 149, 110–116. [Google Scholar] [CrossRef] [PubMed]
- AAFCO. Official Guidelines Suggested for Contaminants in Individual Mineral Feed Ingredients; Official Publication; Association of American Feed Control Officials Inc.: Olympia, WA, USA, 2011; p. 304. [Google Scholar]
- McConnell, K.P.; Cho, G.J. TRANSMUCOSAL MOVEMENT OF SELENIUM. Am. J. Physiol. 1965, 208, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.; Harwell, A.; van Saun, R.; Vorachek, W.; Stewart, W.; Galbraith, M.; Hooper, K.; Hunter, J.; Mosher, W.; Pirelli, G. Agronomic biofortification with selenium: Effects on whole blood selenium and humoral immunity in beef cattle. Anim. Feed Sci. Technol. 2011, 164, 184–190. [Google Scholar] [CrossRef]
- Liu, S.; Tan, H.; Wei, S.; Zhao, J.; Yang, L.; Li, S.; Zhong, C.; Yin, Y.; Chen, Y.; Peng, Y. Effect of selenium sources on growth performance and tissue selenium retention in yellow broiler chicks. J. Appl. Anim. Res. 2015, 43, 487–490. [Google Scholar] [CrossRef]
- Liao, C.-D.; Hung, W.-L.; Jan, K.-C.; Yeh, A.-I.; Ho, C.-T.; Hwang, L.S. Nano/sub-microsized lignan glycosides from sesame meal exhibit higher transport and absorption efficiency in Caco-2 cell monolayer. Food Chem. 2010, 119, 896–902. [Google Scholar] [CrossRef]
- Suzuki, K.T. Metabolomics of selenium: Se metabolites based on speciation studies. J. Health Sci. 2005, 51.2, 107–114. [Google Scholar] [CrossRef]
- Selim, N.A.; Radwan, N.L.; Youssef, S.F.; Eldin, T.S.; Elwafa, S.A. Effect of inclusion inorganic, organic or nano selenium forms in broiler diets on: 2-Physiological, immunological and toxicity statuses of broiler chicks. Int. J. Poult. Sci. 2015, 14, 144. [Google Scholar] [CrossRef]
- Eşrefoğlu, M. Cell injury and death: Oxidative stress and antioxidant defense system. Turk. Klin. J. Med Sci. 2009, 29, 1660–1676. [Google Scholar]
- Jiao, X.; Yang, K.; An, Y.; Teng, X.; Teng, X. Alleviation of lead-induced oxidative stress and immune damage by selenium in chicken bursa of Fabricius. Environ. Sci. Pollut. Res. 2017, 24, 7555–7564. [Google Scholar] [CrossRef]
- Zoidis, E.; Demiris, N.; Kominakis, A.; Pappas, A.C. Meta-analysis of selenium accumulation and expression of antioxidant enzymes in chicken tissues. Animal 2014, 8, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Yuan, D.; Wang, Y.-x.; Zhan, X.-a. The Protective Effects of Different Sources of Maternal Selenium on Oxidative Stressed Chick Embryo Liver. Biol. Trace Elem. Res. 2016, 172, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Zhan, X.; Wang, Y. Effects of selenium sources and levels on reproductive performance and selenium retention in broiler breeder, egg, developing embryo, and 1-day-old chick. Biol. Trace Elem. Res. 2011, 144, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.X.; Xue, W.; Yue, W.; Zhang, C.; Ren, Y.; Shi, L.; Wang, Q.; Yang, R.; Lei, F. Effect of sodium selenite, Se-yeast and nano-elemental selenium on growth performance, Se concentration and antioxidant status in growing male goats. Small Rumin. Res. 2011, 96, 49–52. [Google Scholar]
- Lubos, E.; Loscalzo, J.; Handy, D.E. Glutathione peroxidase-1 in health and disease: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 15, 1957–1997. [Google Scholar] [CrossRef] [PubMed]
- Hill, K.E.; Lyons, P.R.; Burk, R.F. Differential regulation of rat liver selenoprotein mRNAs in selenium deficiency. Biochem. Biophys. Res. Commun. 1992, 185, 260–263. [Google Scholar] [CrossRef]
- Pagmantidis, V.; Bermano, G.; Villette, S.; Broom, I.; Arthur, J.; Hesketh, J. Effects of Se-depletion on glutathione peroxidase and selenoprotein W gene expression in the colon. FEBS Lett. 2005, 579, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Jaeschke, H. Mechanisms of oxidant stress-induced acute tissue injury. Proc. Soc. Exp. Biol. Med. 1995, 209, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Tang, X.; Xue, Y.; Lin, G.; Xiong, Y.L. Dietary linseed oil supplemented with organic selenium improved the fatty acid nutritional profile, muscular selenium deposition, water retention, and tenderness of fresh pork. Meat Sci. 2017, 131, 99–106. [Google Scholar] [CrossRef]
- Lisiak, D.; Janiszewski, P.; Blicharski, T.; Borzuta, K.; Grześkowiak, E.; Lisiak, B.; Powałowski, K.; Samardakiewicz, Ł.; Batorska, M.; Skrzymowska, K. Effect of selenium supplementation in pig feed on slaughter value and physicochemical and sensory characteristics of meat. Ann. Anim. Sci. 2014, 14, 213–222. [Google Scholar] [CrossRef]
- Asghar, A.; Gray, J.I.; Miller, E.R.; Ku, P.K.; Booren, A.M.; Buckley, D.J. Influence of supranutritional vitamin E supplementation in the feed on swine growth performance and deposition in different tissues. J. Sci. Food Agric. 1991, 57, 19–29. [Google Scholar] [CrossRef]
- Huff-Lonergan, E.; Lonergan, S.M. Mechanisms of water-holding capacity of meat: The role of postmortem biochemical and structural changes. Meat Sci. 2005, 71, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Lambert, I.H.; Nielsen, J.H.; Andersen, H.J.; Ørtenblad, N. Cellular model for induction of drip loss in meat. J. Agric. Food Chem. 2001, 49, 4876–4883. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, T.F.B.; Rivera, D.F.R.; Mesquita, F.R.; Braga, H.; Ramos, E.M.; Bertechini, A.G. Effect of different sources and levels of selenium on performance, meat quality, and tissue characteristics of broilers. J. Appl. Poult. Res. 2014, 23, 15–22. [Google Scholar] [CrossRef]
- Chen, G.; Wu, J.; Li, C. Effect of different selenium sources on production performance and biochemical parameters of broilers. J. Anim. Physiol. Anim. Nutr. 2014, 98, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Baowei, W.; Guoqing, H.; Qiaoli, W.; Bin, Y. Effects of yeast selenium supplementation on the growth performance, meat quality, immunity, and antioxidant capacity of goose. J. Anim. Physiol. Anim. Nutr. 2011, 95, 440–448. [Google Scholar] [CrossRef]
- Perić, L.; Milošević, N.; Žikić, D.; Kanački, Z.; Džinić, N.; Nollet, L.; Spring, P. Effect of selenium sources on performance and meat characteristics of broiler chickens. J. Appl. Poult. Res. 2009, 18, 403–409. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y. Influence of dietary nano elemental selenium on growth performance, tissue selenium distribution, meat quality, and glutathione peroxidase activity in Guangxi Yellow chicken. Poult. Sci. 2011, 90, 680–686. [Google Scholar] [CrossRef]
- Goodla, L.; Manubolu, M.; Pathakoti, K.; Jayakumar, T.; Sheu, J.-R.; Fraker, M.; Tchounwou, P.B.; Poondamalli, P.R. Protective Effects of Ammannia baccifera Against CCl4-Induced Oxidative Stress in Rats. Int. J. Environ. Res. Public Health 2019, 16, 1440. [Google Scholar] [CrossRef]
- Juskiewicz, J.; Jankowski, J.; Zielinski, H.; Zdunczyk, Z.; Mikulski, D.; Antoszkiewicz, Z.; Kosmala, M.; Zdunczyk, P. The fatty acid profile and oxidative stability of meat from turkeys fed diets enriched with n-3 polyunsaturated fatty acids and dried fruit pomaces as a source of polyphenols. PLoS ONE 2017, 12, e0170074. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 13. [Google Scholar] [CrossRef]
- Da Silva, S.; Marangoni, C.; Brum, D.; Vendruscolo, R.; Silva, M.; de Moura, H.; Rampelotto, C.; Wagner, R.; de Menezes, C.; Barin, J. Effect of dietary olive leaves on the lipid and protein oxidation and bacterial safety of chicken hamburgers during frozen storage. Int. Food Res. J. 2018, 25, 383–391. [Google Scholar]
- Pappas, A.C.; Zoidis, E.; Papadomichelakis, G.; Fegeros, K. Supranutritional selenium level affects fatty acid composition and oxidative stability of chicken breast muscle tissue. J. Anim. Physiol. Anim. Nutr. 2012, 96, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Muela, E.; Sanudo, C.; Campo, M.M.; Medel, I.; Beltran, J.A. Effect of freezing method and frozen storage duration on lamb sensory quality. Meat Sci. 2012, 90, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Mikulski, D.; Jankowski, J.; Zduńczyk, Z.; Wróblewska, M.; Sartowska, K.; Majewska, T. The effect of selenium source on performance, carcass traits, oxidative status of the organism, and meat quality of turkeys. J. Anim. Feed Sci. 2009, 18, 518–530. [Google Scholar] [CrossRef]
- Zhao, L.Y.; Xu, S.Q.; Zhao, R.Q.; Peng, Z.Q.; Pan, X.J. Effects of selenium and methionine supplementation of breeder hen diets on selenium concentration and oxidative stability of lipids in the thigh muscles of progeny. J. Food. Sci. 2009, 74, C569–C574. [Google Scholar] [CrossRef] [PubMed]
- Calvo, L.; Toldrá, F.; Rodríguez, A.I.; López-Bote, C.; Rey, A.I. Effect of dietary selenium source (organic vs. mineral) and muscle pH on meat quality characteristics of pigs. Food Sci. Nutr. 2017, 5, 94–102. [Google Scholar] [CrossRef]
- Tsuruga, M.; Matsuoka, A.; Hachimori, A.; Sugawara, Y.; Shikama, K. The molecular mechanism of autoxidation for human oxyhemoglobin. Tilting of the distal histidine causes nonequivalent oxidation in the beta chain. J. Biol. Chem. 1998, 273, 8607–8615. [Google Scholar] [CrossRef] [PubMed]
- Juncher, D.; Rønn, B.; Mortensen, E.; Henckel, P.; Karlsson, A.; Skibsted, L.; Bertelsen, G. Effect of pre-slaughter physiological conditions on the oxidative stability of colour and lipid during chill storage of pork. Meat Sci. 2001, 58, 347–357. [Google Scholar] [CrossRef]
- Kusano, C.; Ferrari, B. Total antioxidant capacity: A biomarker in biomedical and nutritional studies. J. Cell. Mol. Biol. 2008, 7, 1–15. [Google Scholar]
- Serpen, A.; Gokmen, V.; Fogliano, V. Total antioxidant capacities of raw and cooked meats. Meat Sci. 2012, 90, 60–65. [Google Scholar] [CrossRef]
- Korzeniowska, M.; Króliczewska, B.; Kopeć, W. Effect of Dietary Selenium on Protein and Lipid Oxidation and the Antioxidative Potential of Selected Chicken Culinary Parts during Frozen Storage. J. Chem. 2018, 2018, 3492456. [Google Scholar] [CrossRef]
- Surai, P.F.; Dvorska, J.E. Effect of selenium and vitamin E content of the diet on lipid peroxidation in breast muscle tissue of broiler breeder hens during storage. In Proceedings of the 14th Annual Australian Poultry Science Symposium, Sydney, New South Wales, Australia, 11–13 Februray 2002; Available online: https://sydney.edu.au/vetscience/apss/proceedings/2002.shtml (accessed on 10 June 2019).
- Wang, Y.X.; Zhan, X.A.; Yuan, D.; Zhang, X.W.; Wu, R.J. Effects of selenomethionine and sodium selenite supplementation on meat quality, selenium distribution and antioxidant status in broilers. Czech J. Anim. Sci. 2011, 56, 305–313. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Yang, Z.; Zhang, Z.; Jiang, Y.; Gao, F.; Zhou, G. Effects of different selenium sources on growth performance, antioxidant capacity and meat quality of local Chinese Subei chickens. Biol. Trace Elem. Res. 2018, 181, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Tornberg, E. Effects of heat on meat proteins—Implications on structure and quality of meat products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Badii, F.; Howell, N.K. Effect of antioxidants, citrate, and cryoprotectants on protein denaturation and texture of frozen cod (Gadus morhua). J. Agric. Food Chem. 2002, 50, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Rowe, L.J.; Maddock, K.R.; Lonergan, S.M.; Huff-Lonergan, E. Influence of early postmortem protein oxidation on beef quality. J. Anim. Sci. 2004, 82, 785–793. [Google Scholar] [CrossRef] [PubMed]
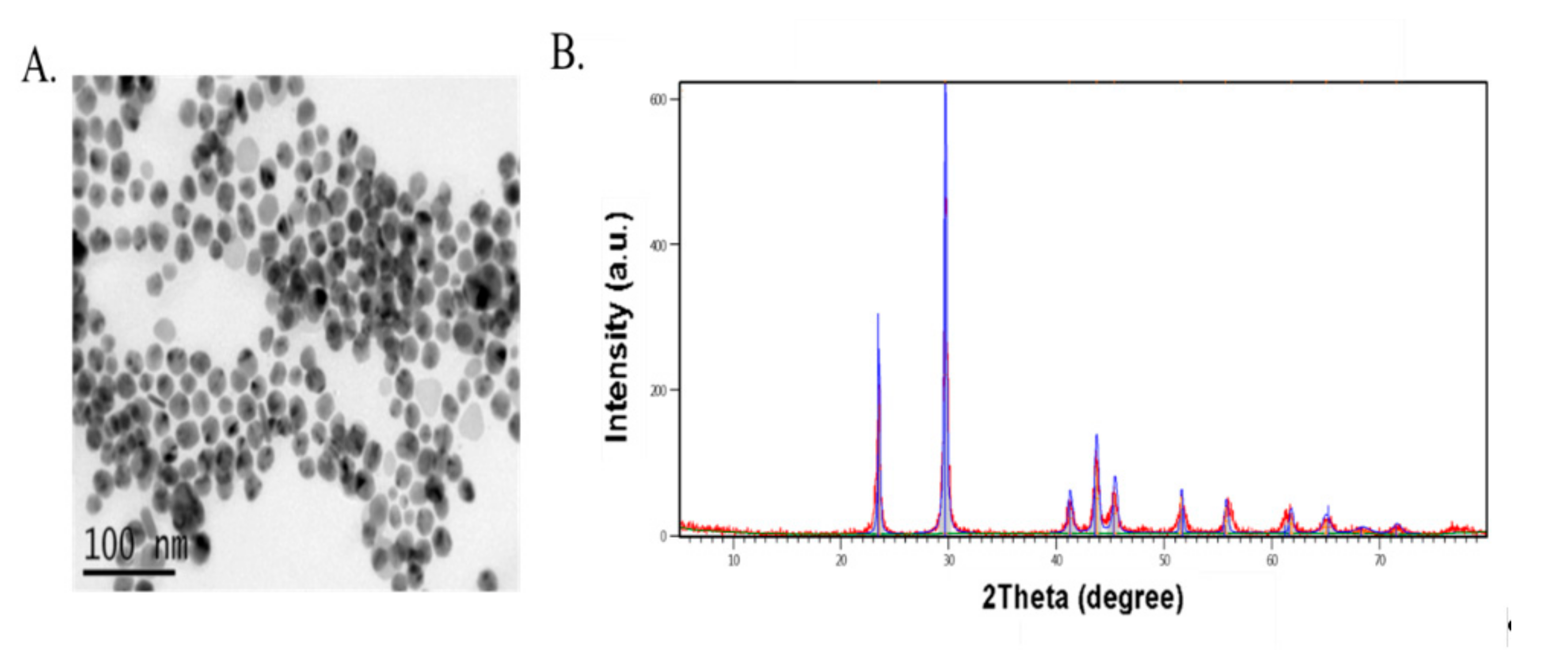
| Ingredients | Starter | Grower | Finisher |
|---|---|---|---|
| Corn, % | 56 | 61.7 | 62.5 |
| Soybean meal, % | 34.86 | 28.1 | 25 |
| Corn gluten, % | 3.5 | 3.3 | 3.5 |
| wheat bran, % | 0 | 1 | 1.9 |
| Soy oil, % | 1.8 | 2.2 | 3.66 |
| Calcium carbonate, % | 1 | 1 | 1 |
| Calcium diphasic phosphate, % | 1.8 | 1.7 | 1.5 |
| NaCl, % | 0.3 | 0.3 | 0.3 |
| Premix *, % | 0.3 | 0.3 | 0.3 |
| Methionine, % | 0.18 | 0.14 | 0.11 |
| Lysine, % | 0.16 | 0.16 | 0.13 |
| anti-mycotoxin, % | 0.1 | 0.1 | 0.1 |
| Total | 100 | 100 | 100 |
| Nutrient Levels b | |||
| Crude protein, % | 23.2952 | 20.527 | 19.3087 |
| ME (kcal/kg) | 3042.271 | 3105.028 | 3200.17 |
| Calcium, % | 0.9656 | 0.92681 | 0.86886 |
| Available P, % | 0.467822 | 0.43785 | 0.3962 |
| Methionine, % | 0.569576 | 0.49246 | 0.456018 |
| Lysine, % | 1.380138 | 1.18469 | 1.092276 |
| Ether extract, % | 4.28232 | 4.8086 | 2.6345 |
| Crude fiber, % | 2.64082 | 2.6282 | 6.2493 |
| Se mg/kg | 0.06986 | 0.0696 | 0.07615 |
| SeS | Met-Se | Nano-Se | SEM | p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 | 0.45 | 0.6 | 0.3 | 0.45 | 0.6 | 0.3 | 0.45 | 0.6 | Se Source | Se Level | Source × Level | ||
| BW | 2184 c | 2262 b | 2155 c | 2266 b | 2304 b | 2391 a | 2253 b | 2263 b | 2372 a | 4.82 | <0.001 | <0.001 | <0.001 |
| BWG | 2139 c | 2217 b | 2110 c | 2219 b | 2260 b | 2346 a | 2211 b | 2214 b | 2327 a | 4.18 | <0.001 | <0.001 | <0.001 |
| FI | 3932 | 3877 | 3881 | 3823 | 3879 | 3859 | 3811 | 3826 | 3913 | 12.09 | 0.227 | 0.585 | 0.215 |
| FCR | 1.84 c | 1.75 b | 1.86 c | 1.72 b | 1.71 b | 1.64 a | 1.73 b | 1.73 b | 1.68 a,b | 0.005 | <0.001 | 0.007 | <0.001 |
| RGR | 192.03 | 192.30 | 191.90 | 192.08 | 192.42 | 192.62 | 192.18 | 192.21 | 192.62 | 0.17 | 0.740 | 0.775 | 0.926 |
| SeS | Met-Se | Nano-Se | SEM | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 | 0.45 | 0.6 | 0.3 | 0.45 | 0.6 | 0.3 | 0.45 | 0.6 | Se Source | Se Level | Se Source × Level | ||
| Se concentration | |||||||||||||
| Serum Se, mg/L | 0.17 f | 0.32 e | 0.42 d | 0.20 f | 0.48 c | 0.64 a | 0.20 f | 0.54 b | 0.63 a | 0.005 | <0.001 | <0.001 | <0.001 |
| Liver Se, mg/kg | 0.32 f | 0.94 d | 1.16 c | 0.52 e | 1.27 c | 1.52 b | 0.59 e | 1.55 b | 1.83 a | 0.011 | <0.001 | <0.001 | <0.001 |
| Muscle Se, mg/kg | 0.16 g | 0.27 e | 0.32 c,d | 0.28 d,e | 0.41 b | 0.75 a | 0.22 f | 0.35 c | 0.77 a | 0.005 | <0.001 | <0.001 | <0.001 |
| Plasma biochemistry | |||||||||||||
| AST U/L | 83.50 | 84.28 | 84.78 | 83.59 | 83.63 | 83.71 | 83.43 | 84.12 | 84.17 | 0.12 | 0.173 | 0.049 | 0.559 |
| ALT U/L | 152.91 | 152.94 | 155.06 | 152.81 | 152.84 | 152.91 | 152.81 | 152.59 | 153.18 | 0.028 | 0.175 | 0.097 | 0.320 |
| Creatinine mg/dL | 5.68 b | 5.84 a,b | 6.50 a | 5.70 a,b | 5.58 b | 6.04 a,b | 5.94 a,b | 5.92 a,b | 6.20 a,b | 0.009 | 0.162 | 0.002 | 0.521 |
| SeS | Met-Se | Nano-Se | SEM | p-Value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.3 | 0.45 | 0.6 | 0.3 | 0.45 | 0.6 | 0.3 | 0.45 | 0.6 | Se Source | Se Level | Se Source × Level | ||
| pH, 0.5 h | 6.37 d,e | 6.49 c,d | 6.32 e | 6.56 c | 6.67 b | 6. 80 a | 6.78 a | 6.82 a | 6.83 a | 0.007 | <0.001 | <0.001 | <0.001 |
| pH, 24 h | 5.46 d | 5.48 c,d | 5.41 d | 5.54 b,c | 5.56 b,c | 5.67 a | 5.59 b | 5.62 a,b | 5.70 a | 0.005 | <0.001 | <0.001 | <0.001 |
| Drip loss, % | 2.74 a | 2.62 b | 2.83 a | 2.38 c | 2.17 d | 2.13 d,e | 2.35 c | 2.16d | 2.04 e | 0.007 | <0.001 | <0.001 | <0.001 |
| Cooking loss % | 14.04 b | 12.98 c | 14.60 a | 12.96 c | 12.64 c,d | 12.16 e | 12.86c | 12.18 d,e | 12.07 e | 0.03 | <0.001 | <0.001 | <0.001 |
| Breast TBRAS, mg/kg 3 h | 0.17 a | 0.17 a | 0.14 a | 0.14 a | 0.11 a,b | 0.04 c | 0.11 a | 0.05 b,c | 0.03c | 0.02 | <0.001 | <0.001 | <0.038 |
| Breast TBRAS, mg/kg 2 W | 0.47 a | 0.38 b,c | 0.42 a,b | 0.36 b,c | 0.30 c,d | 0.26 d | 0.39 a,b,c | 0.22 d,e | 0.15 e | 0.02 | <0.001 | <0.001 | <0.001 |
| Breast TBRAS, mg/kg 4 W | 0.83 a | 0.78 a,b | 0.77 a,b | 0.71 b,c | 0.71 b,c | 0.68 c,d | 0.64 d | 0.53 e | 0.50 e | 0.02 | <0.001 | <0.001 | <0.007 |
| Thigh TBRAS, mg/kg 3 h | 0.23 a | 0.26 a | 0.24 a | 0.25 a | 0.21 a,b | 0.13 c | 0.23 a | 0.16 b,c | 0.11c | 0.02 | <0.001 | <0.001 | <0.001 |
| Thigh TBRAS, mg/kg 2 W | 0.48 a | 0.40 b | 0.47 a | 0.35 b,c | 0.29 c,d | 0.31 c | 0.39 b | 0.23 d,e | 0.18 e | 0.02 | <0.001 | <0.001 | <0.001 |
| Thigh TBRAS, mg/kg 4 W | 0.86 a | 0.80 a | 0.80 a | 0.72 b | 0.72 b | 0.69 b,c | 0.65 c | 0.56 d | 0.54d | 0.02 | <0.001 | <0.001 | <0.002 |
| Breast ABTS, 3 h | 2.30 e | 2.47 d | 2.09 f | 3.47 c | 3.60 c | 3.75 b | 3.87 b | 4.20 a | 4.21 a | 0.03 | <0.001 | <0.001 | <0.001 |
| Breast ABTS, 4 week | 5.37 f | 5.59 e | 5.27 g | 6.31 d | 6.52 c | 6.93 b | 6.92 b | 8.14 a | 8.21 a | 0.02 | <0.001 | <0.001 | <0.001 |
| Thigh ABTS, 3 h | 6.47 f | 6.73 d,e | 6.93 c | 6.70 e | 7.04 b,c | 6.95 c | 6.90 c,d | 7.18 b | 8.18 a | 0.05 | <0.001 | <0.001 | <0.001 |
| Thigh ABTS, 4 week | 7.47 e | 8.06 b,c | 7.94 c | 7.70 d | 7.73 d | 7.95 c | 7.90 c | 8.18 a,b | 9.18 a | 0.04 | <0.001 | <0.001 | <0.001 |
| Breast DPPH, 3 h | 5.91 e | 6.19 d | 5.81 f | 5.98 e | 6.29 c,d | 6.30 c,d | 6.34 c | 6.74 b | 7.13 a | 0.04 | <0.001 | <0.001 | <0.001 |
| Breast DPPH, 4 week | 6.39 d | 6.71 c | 6.35 d | 6.48 d | 6.79 c | 6.80 c | 6.85 c | 7.34 b | 6.77 a | 0.05 | <0.001 | <0.001 | <0.001 |
| Thigh DPPH, 3 h | 7.14 d,e | 7.48 a,b | 7.01 e | 7.21 c,d,e | 7.20 c,d,e | 7.36 b,c,d | 7.42 a,b,c | 7.45 a,b | 7.61 a | 0.05 | <0.001 | <0.03 | <0.001 |
| Thigh DPPH, 4 week | 8.54 f | 8.82 e | 8.83 e | 9.23 d | 9.39 c | 9.58 b | 9.41 c | 9.58 b | 9.72 a | 0.02 | <0.001 | <0.001 | <0.02 |
| Breast FRAP, 3 h | 0.15 d | 0.22 b,c | 0.16 c,d | 0.22 b,c | 0.32 a | 0.33 a | 0.25 b | 0.33 a | 0.37 a | 0.01 | <0.001 | <0.001 | <0.001 |
| Breast FRAP, 4 week | 0.28 c | 0.38 b | 0.30 c | 0.37 b | 0.43 a,b | 0.42 a,b | 0.40 a,b | 0.43 a,b | 0.45 a | 0.01 | <0.001 | <0.001 | <0.012 |
| Thigh FRAP, 3 h | 1.06 f,g | 1.13 e,f | 1.03 g | 1.15 e | 1.19 d,e | 1.35 a,b | 1.26 c,d | 1.30 b,c | 1.40 a | 0.02 | <0.001 | <0.01 | <0.001 |
| Thigh FRAP, 4 week | 1.24 a | 1.28 a | 1.26 a | 1.31 a | 1.40 c | 1.52 a,b | 1.39 c | 1.50 b | 1.59 a | 0.02 | <0.001 | <0.001 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, D.; Kishawy, A.T.Y.; Khater, S.I.; Hamed Arisha, A.; Mohammed, H.A.; Abdelaziz, A.S.; Abd El-Rahman, G.I.; Elabbasy, M.T. Effect of Dietary Modulation of Selenium Form and Level on Performance, Tissue Retention, Quality of Frozen Stored Meat and Gene Expression of Antioxidant Status in Ross Broiler Chickens. Animals 2019, 9, 342. https://doi.org/10.3390/ani9060342
Ibrahim D, Kishawy ATY, Khater SI, Hamed Arisha A, Mohammed HA, Abdelaziz AS, Abd El-Rahman GI, Elabbasy MT. Effect of Dietary Modulation of Selenium Form and Level on Performance, Tissue Retention, Quality of Frozen Stored Meat and Gene Expression of Antioxidant Status in Ross Broiler Chickens. Animals. 2019; 9(6):342. https://doi.org/10.3390/ani9060342
Chicago/Turabian StyleIbrahim, Doaa, Asmaa T.Y. Kishawy, Safaa I. Khater, Ahmed Hamed Arisha, Haiam A. Mohammed, Ahmed Shaban Abdelaziz, Ghada I. Abd El-Rahman, and Mohamed Tharwat Elabbasy. 2019. "Effect of Dietary Modulation of Selenium Form and Level on Performance, Tissue Retention, Quality of Frozen Stored Meat and Gene Expression of Antioxidant Status in Ross Broiler Chickens" Animals 9, no. 6: 342. https://doi.org/10.3390/ani9060342
APA StyleIbrahim, D., Kishawy, A. T. Y., Khater, S. I., Hamed Arisha, A., Mohammed, H. A., Abdelaziz, A. S., Abd El-Rahman, G. I., & Elabbasy, M. T. (2019). Effect of Dietary Modulation of Selenium Form and Level on Performance, Tissue Retention, Quality of Frozen Stored Meat and Gene Expression of Antioxidant Status in Ross Broiler Chickens. Animals, 9(6), 342. https://doi.org/10.3390/ani9060342







