Risk of Lameness in Dairy Cows with Paratuberculosis Infection
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cow Selection
2.2. Statistical Analysis
2.3. Ethical Approval
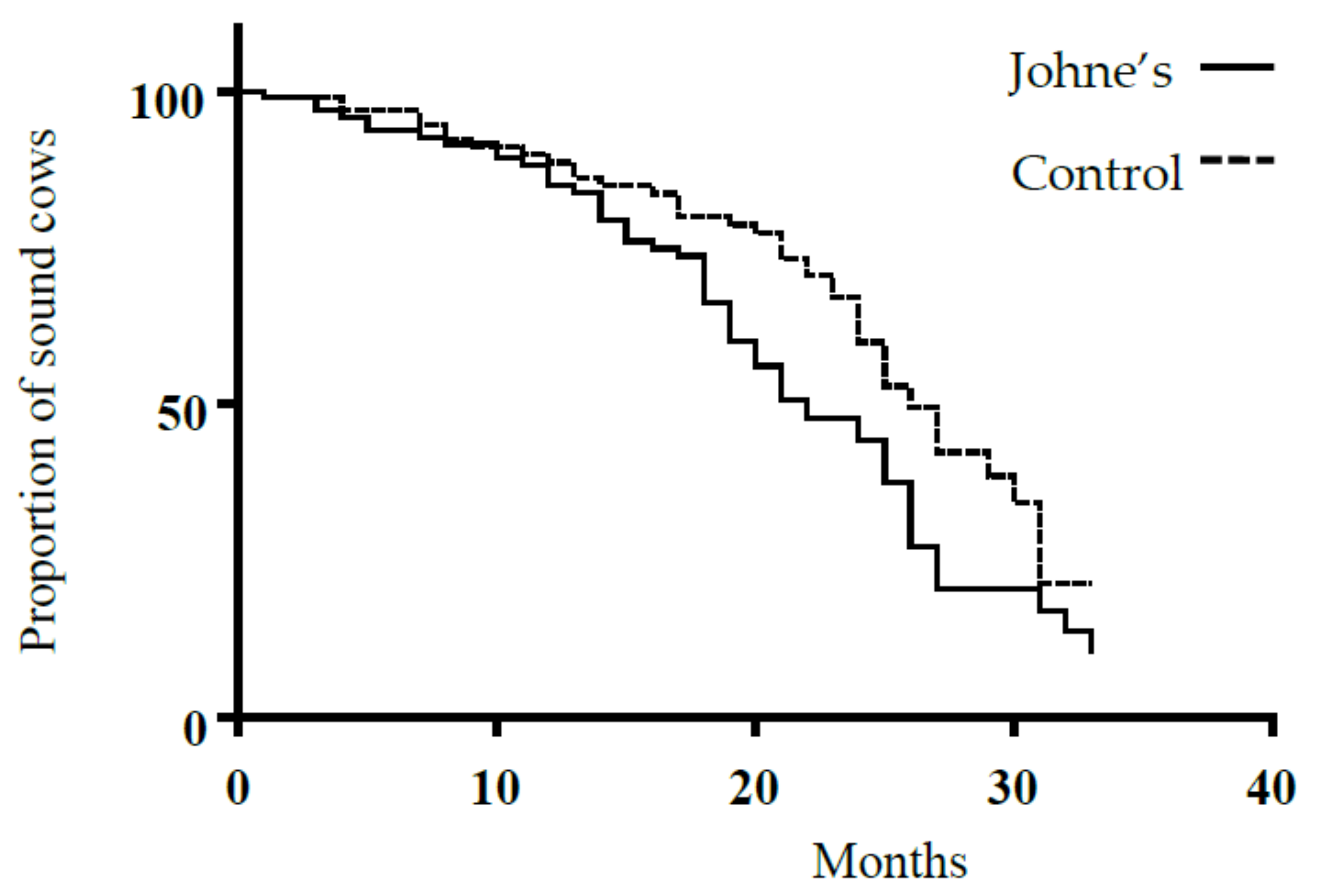
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manning, E.J.; Collins, M.T. Mycobacterium avium subsp. paratuberculosis: Pathogen, pathogenesis and diagnosis. Rev. Sci. Tech. 2001, 20, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Windsor, P.A.; Whittington, R.J. Evidence for age susceptibility of cattle to Johne’s disease. Vet. J. 2010, 184, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Harris, N.B.; Barletta, R.G. Mycobacterium avium subsp. paratuberculosis in veterinary medicine. Clin. Microb. Rev. 2001, 14, 489–512. [Google Scholar] [CrossRef] [PubMed]
- Radia, D.; Bond, K.; Limon, G.; van Winden, S.; Guitian, J. Relationship between periparturient management, prevalence of MAP and preventable economic losses in UK dairy herds. Vet. Rec. 2013, 173, 343. [Google Scholar] [CrossRef] [PubMed]
- Velasova, M.; Damaso, A.; Prakashbabu, B.C.; Gibbons, J.; Wheelhouse, N.; Longbottom, D.; Van Winden, S.; Green, M.; Guitian, J. Herd-level prevalence of selected endemic infectious diseases of dairy cows in Great Britain. J. Dairy Sci. 2017, 100, 9215–9233. [Google Scholar] [CrossRef] [PubMed]
- Cetinkaya, B.; Egan, K.; Harbour, D.; Morgan, K. An abattoir-based study of the prevalence of subclinical Johne’s disease in adult cattle in south west England. Epidemiol. Infect. 1996, 116, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Whitlock, R.H.; Buergelt, C. Preclinical and clinical manifestations of Paratuberculosis (including pathology). Vet. Clin. N. Am. Food Anim. Prac. 1996, 12, 345–356. [Google Scholar] [CrossRef]
- Cetinkaya, B.; Erdogan, H.M.; Morgan, K.L. Prevalence, incidence and geographical distribution of Johne’s disease in cattle in England and the Welsh borders. Vet. Rec. 1998, 143, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.L.; Wells, S.J.; Wagner, B.A. Herd-level economic losses associated with Johne’s disease on US dairy operations. Prev. Vet. Med. 1999, 40, 179–192. [Google Scholar] [CrossRef]
- Coussens, P.M. Mycobacterium paratuberculosis and the bovine immune system. Anim. Health Res. Rev. 2001, 2, 141–161. [Google Scholar] [CrossRef] [PubMed]
- Storset, A.K.; Hasvold, H.J.; Valheim, M.; Brun-Hansen, H.; Berntsen, G.; Whist, S.K.; Djønne, B.; Press, C.M.; Holstad, G.; Larsen, H.J.S. Subclinical paratuberculosis in goats following experimental infection: An immunological and microbiological study. Vet. Immunol. Immunopathol. 2001, 80, 271–287. [Google Scholar] [CrossRef]
- Coussens, P.M. Model for immune responses to Mycobacterium avium subspecies paratuberculosis in cattle. Infect. Immun. 2004, 72, 3089–3096. [Google Scholar] [CrossRef] [PubMed]
- Stabel, J. Host responses to Mycobacterium avium subsp. paratuberculosis: A complex arsenal. Anim. Health Res. Rev. 2006, 7, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.T. Diagnosis of Paratuberculosis. Vet. Clin. N. Am. Food Anim. Pract. 1996, 12, 357–371. [Google Scholar] [CrossRef]
- Meyer, A.; Bond, K.; Van Winden, S.; Green, M.; Guitian, J. A probabilistic approach to the interpretation of milk antibody results for diagnosis of Johne’s disease in dairy cattle. Prev. Vet. Med. 2018, 150, 30–37. [Google Scholar] [CrossRef] [PubMed]
- McKenna, S.L.B.; Keefe, G.P.; Tiwari, A.; VanLeeuwen, J.; Barkema, H.W. Johne’s disease in Canada Part II: Disease impacts, risk factors, and control programs for dairy producers. Can. Vet. J. 2006, 47, 1089–1099. [Google Scholar]
- Merkal, R.S.; Larsen, A.B.; Booth, G.D. Analysis of the effect of inapparent bovine paratuberculosis. Am. J. Vet. Res. 1975, 36, 837–838. [Google Scholar]
- Johnson-Ifearulundu, Y.J.; Kaneene, J.B.; Sprecher, D.J.; Gardiner, J.C.; Lloyd, J.W. The effect of subclinical Mycobacterium paratuberculosis infection on days open in Michigan, USA, dairy cows. Prev. Vet. Med. 2000, 46, 171–181. [Google Scholar] [CrossRef]
- Raizman, E.A.; Fetrow, J.; Wells, S.J.; Godden, S.M.; Oakes, M.J.; Vazquez, G. The association between Mycobacterium avium subsp. paratuberculosis fecal shedding or clinical Johne’s disease and lactation performance on two Minnesota, USA dairy farms. Prev. Vet. Med. 2007, 78, 179–195. [Google Scholar] [CrossRef]
- Villarino, M.; Jordan, E. Production impact of sub-clinical manifestations of bovine paratuberculosis in dairy cattle. In Proceedings of the 8th International Colloquium on Paratuberculosis, Copenhagen, Denmark, 14–17 August 2005; International Association for Paratuberculosis: Chester County, PA, USA, 2005. [Google Scholar]
- Esslemont, R.; Kossaibati, M. Incidence of production diseases and other health problems in a group of dairy herds in England. Vet. Rec. 1996, 139, 486–490. [Google Scholar] [CrossRef]
- Blowey, R. Common diseases of the foot and treatments. In Cattle Lameness and Hoofcare, 3rd ed.; 5M Enterprises: Sheffield, UK, 2015; p. 54. [Google Scholar]
- Leach, K.A.; Whay, H.R.; Maggs, C.M.; Barker, Z.E.; Paul, E.S.; Bell, A.K.; Main, D.C.J. Working towards a reduction in cattle lameness: 1. Understanding barriers to lameness control on dairy farms. Res. Vet. Sci. 2010, 89, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, M.; Downham, D.; Faull, W.; Hughes, J.; Manson, F.; Merritt, J.; Murray, R.; Russell, W.; Sutherst, J.; Ward, W. Incidence and prevalence of lameness in dairy cattle. Vet. Rec. 1996, 138, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Archer, S.C.; Bell, N.; Huxley, J. Lameness in UK dairy cows: A review of the current status. Practice 2010, 32, 492–504. [Google Scholar] [CrossRef]
- AHDB Dairy Mobility Score. Available online: https://dairy.ahdb.org.uk/non_umbraco/download.aspx?media=1349 (accessed on 18 Apirl 2019).
- Bartlett, B.; Pearse, H. Integrating milk recording data and disease test results to provide a system for the management of paratuberculosis in UK dairy herds. In Proceedings of the ICAR 2012 Conference, Cork, Ireland, 28 May–1 June 2012. Available online: https://www.icar.org/wp-content/uploads/2015/09/Bartlett.pdf (accessed on 3 Apirl 2019).
- Case Control Odds Ratios. Available online: http://www.sjsu.edu/faculty/gerstman/StatPrimer/case-control.pdf (accessed on 3 Apirl 2019).
- Bicalho, R.C.; Machado, V.S.; Caixeta, L.S. Lameness in dairy cattle: A debilitating disease or a disease of debilitated cattle? A cross-sectional study of lameness prevalence and thickness of the digital cushion. J. Dairy Sci. 2009, 92, 3175–3184. [Google Scholar] [CrossRef] [PubMed]
- Espejo, L.A.; Endres, M.I.; Salfer, J.A. Prevalence of lameness in high-producing Holstein cows housed in freestall barns in Minnesota. J. Dairy Sci. 2006, 89, 3052–3058. [Google Scholar] [CrossRef]
- Rowlands, G.; Russell, A.; Williams, L. Effects of stage of lactation, month, age, origin and heart girth on lameness in dairy cattle. Vet. Rec. 1985, 117, 576–580. [Google Scholar] [CrossRef] [PubMed]
- Stabel, J.; Bannantine, J.; Hostetter, J. Mycobacterium avium subsp. paratuberculosis infection, immunology and pathology of livestock. In Tuberculosis, Leprosy and Mycobacterial Diseases of Man and Animals: The Many Hosts of Mycobacteria; Mukundan, H., Chambers, M.A., Waters, W.R., Larsen, M.H., Eds.; CABI: Oxfordshire, UK, 2015; pp. 513–537. [Google Scholar]
- Green, L.E.; Huxley, J.N.; Banks, C.; Green, M.J. Temporal associations between low body condition, lameness and milk yield in a UK dairy herd. Prev. Vet. Med. 2014, 113, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Randall, L.V.; Green, M.J.; Chagunda, M.G.G.; Mason, C.; Archer, S.C.; Green, L.E.; Huxley, J.N. Low body condition predisposes cattle to lameness: An 8-year study of one dairy herd. J. Dairy Sci. 2015, 98, 3766–3777. [Google Scholar] [CrossRef] [PubMed]
- Brett, E. Johne’s disease: An economic evaluation of control options for the New Zealand livestock industries. In Report Prepared for Agriculture New Zealand; Agriculture New Zealand: Wellington, New Zealand, 1998; pp. 1–73. [Google Scholar]
- Marshall, R.C. Nail, claw, hoof and horn keratin. In Biology of the Integument: 2 Vertebrates; Bereiter-Hahn, J., Matoltsy, A.G., Richards, K.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 722–738. [Google Scholar]
- McGregor, H.; Abbott, K.A.; Whittington, R.J. Effects of Mycobacterium avium subsp. paratuberculosis infection on serum biochemistry, body weight and wool growth in Merino sheep: A longitudinal study. Small Rumin. Res. 2015, 125, 146–153. [Google Scholar] [CrossRef]
- Webster, S.; Mullen, J.D. Animal disease economics: The case of ovine Johne’s disease in New South Wales. In Proceedings of the Australian Agricultural and Resource Economics Society 44th Annual Conference, Sydney, Australia, 23–25 January 2000. [Google Scholar]
- Bergsten, C.; Greenough, P.R.; Gay, J.M.; Seymour, W.M.; Gay, C.C. Effects of biotin supplementation on performance and claw lesions on a commercial dairy farm. J. Dairy Sci. 2003, 86, 3953–3962. [Google Scholar] [CrossRef]
- Fitzgerald, T.; Norton, B.W.; Elliott, R.; Podlich, H.; Svendsen, O.L. The influence of long-term supplementation with biotin on the prevention of lameness in pasture fed dairy cows. J. Dairy Sci. 2000, 83, 338–344. [Google Scholar] [CrossRef]
- Hedges, J.; Blowey, R.W.; Packington, A.J.; O’Callaghan, C.J.; Green, L.E. A longitudinal field trial of the effect of biotin on lameness in dairy cows. J. Dairy Sci. 2001, 84, 1969–1975. [Google Scholar] [CrossRef]
- McDowell, L.R. Biotin. In Vitamins in Animal and Human Nutrition, 2nd ed.; Iowa State University Press: Iowa City, IA, USA, 2008; pp. 445–478. [Google Scholar]
- Willshire, J.A.; Bell, N.J. An economic review of cattle lameness. Cattle Pract. 2009, 17, 136–141. [Google Scholar]
| Lameness Status | Negative Cows (n = 98) (<20% S/P) | Medium Positive Cows (n = 49) (20% < S/P < 30%) | High Positive Cows (n = 49) (>30% S/P) |
|---|---|---|---|
| Lame cows (of which chronic) | 46 (13) | 27 (10) | 30 (7) |
| Before/after Johne’s positive | NA | 13/14 | 9/21 |
| Sound cows | 52 | 22 | 19 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smith, J.; van Winden, S. Risk of Lameness in Dairy Cows with Paratuberculosis Infection. Animals 2019, 9, 339. https://doi.org/10.3390/ani9060339
Smith J, van Winden S. Risk of Lameness in Dairy Cows with Paratuberculosis Infection. Animals. 2019; 9(6):339. https://doi.org/10.3390/ani9060339
Chicago/Turabian StyleSmith, Joshua, and Steven van Winden. 2019. "Risk of Lameness in Dairy Cows with Paratuberculosis Infection" Animals 9, no. 6: 339. https://doi.org/10.3390/ani9060339
APA StyleSmith, J., & van Winden, S. (2019). Risk of Lameness in Dairy Cows with Paratuberculosis Infection. Animals, 9(6), 339. https://doi.org/10.3390/ani9060339





