Changes in Serum Amyloid A (SAA) Concentration in Arabian Endurance Horses During First Training Season
Abstract
1. Introduction
2. Materials and Methods
2.1. Horses and Blood Sampling
2.2. Haematology and Serum Analysis
2.3. Statistical Analysis
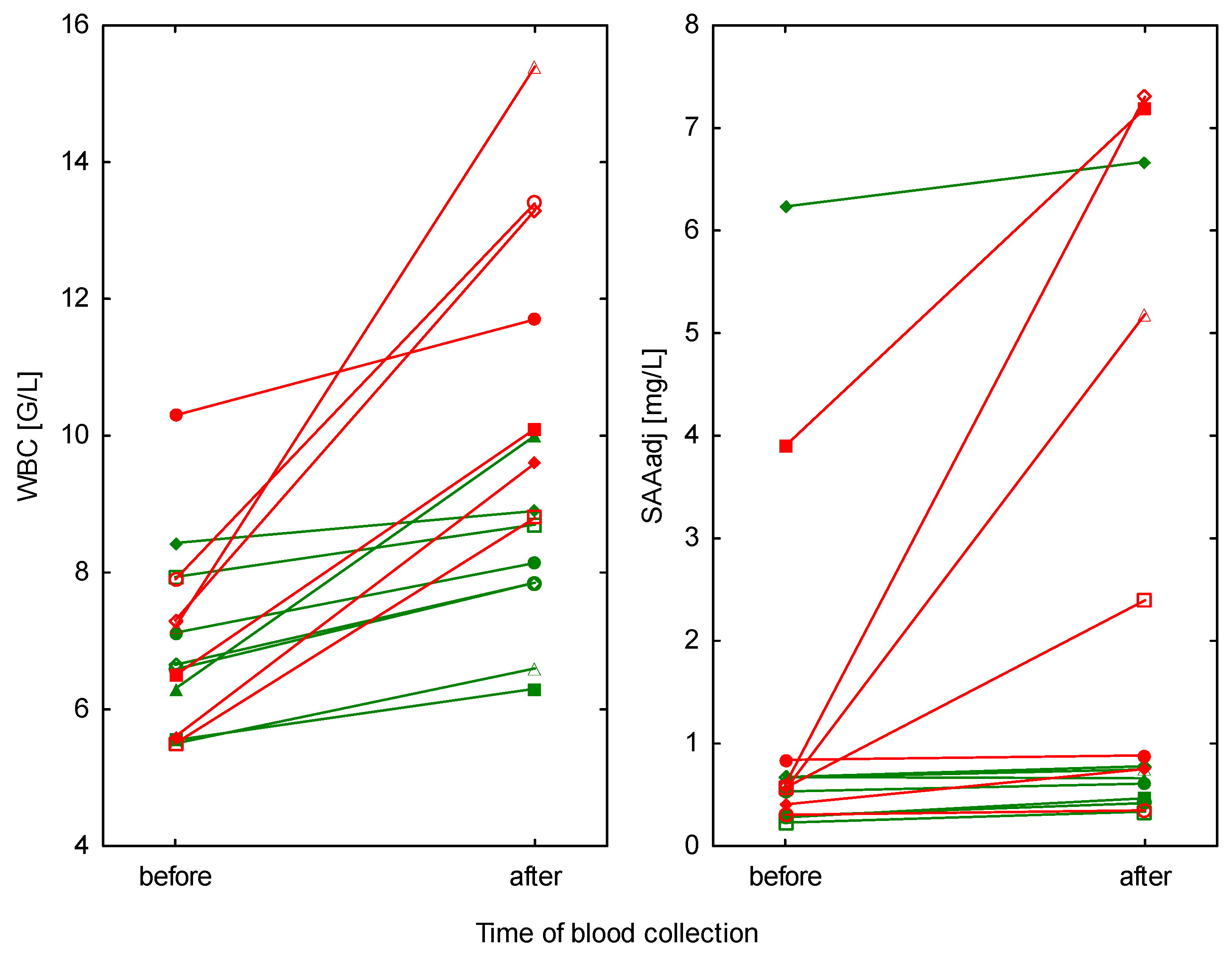
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APR | Acute Phase Response |
| APPs | Acute Phase Proteins |
| AST | Aspartate aminotransferase |
| CPK | Creatine phosphokinase |
| HGB | Hemoglobin concentration |
| IL-1 | Interleukin 1 |
| IL-6 | Interleukin 6 |
| PCV | Packed cell volume |
| RBC | Red blood cell count |
| SAA | Serum amyloid A |
| SAAadj | Serum amyloid A adjusted |
| TSP | Total serum protein concentration |
| TSP1 | Total serum protein level after exertion |
| TSP0 | Total serum protein level before exertion |
| TNF-α | Tumor necrosis factor α |
| WBC | White blood cell count |
References
- Clayton, H.M. The cardiovascular system. In Conditioning Sport Horses, 2nd ed.; Clayton, H.M., Ed.; Sport Horse Publication: Mason, Canada, 1991; Volume 2, pp. 88–91. [Google Scholar]
- Hinchcliff, K.K.; Geor, R. Exercise testing in the field. In Equine Sports Medicine and Surgery, 2nd ed.; Hinchcliff, K.K., Kanepis, A., Geor, R., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2014; Volume 1, pp. 1254–1257. [Google Scholar]
- McGowan, C. Clinical pathology in the racing horse: The role of clinical pathology in assessing fitness and performance in the racehorse. Vet. Clin. North Am. Equine Pract. 2008, 24, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Cray, C.; Zaias, J.; Altman, N.H. Acute Phase Response in Animals: A Review. Comp. Med. 2009, 59, 517–526. [Google Scholar] [PubMed]
- Cray, C. Acute Phase Proteins in Animals. Prog. Mol. Biol. Transl. Sci. 2012, 105, 113–150. [Google Scholar] [PubMed]
- Petersen, H.H.; Nielsen, J.P.; Heegaard, P.M.H. Application of acute phase protein measurements in veterinary clinical chemistry. Vet. Res. 2004, 35, 163–187. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.; Andersen, P.H. The acute phase protein serum amyloid A (SAA) as a marker of inflammation in horses. Equine vet. Educ. 2007, 19, 38–46. [Google Scholar] [CrossRef]
- Nunokawa, Y.; Fujinaga, T.; Taira, T.; Okumura, M.; Yamashita, K.; Tsunoda, N.; Hagio, M. Evaluation of serum amyloid A protein as an acute-phase reactive protein in horses. J. Vet. Med. Sci. 1993, 55, 1011–1016. [Google Scholar] [CrossRef]
- Witkowska-Piłaszewicz, O.; Żmigrodzka, M.; Winnicka, A.; Miśkiewicz, A.; Strzelec, K.; Cywińska, A. Serum amyloid A in equine health and disease. Equine Vet. J. 2018. [CrossRef]
- Fallon, K.E. The acute phase response and exercise: the ultramarathon as prototype exercise. Clin. J. Sport Med. 2001, 1, 38–43. [Google Scholar] [CrossRef]
- Weight, L.M.; Donald, A.; Jacobs, P. Strenuous exercise: analogous to the acute-phase response? Clin. Sci. 1991, 81, 677–683. [Google Scholar] [CrossRef]
- Cywinska, A.; Szarska, E.; Górecka, R.; Witkowski, L.; Hecold, M.; Bereznowski, A.; Schollenberger, A.; Winnicka, A. Acute phase protein concentrations after limited and long distance endurance rides in horses. Res. Vet. Sci. 2012, 93, 1402–1406. [Google Scholar] [CrossRef]
- Turło, A.; Cywińska, A.; Czopowicz, M.; Witkowski, L.; Jaśkiewicz, A.; Winnicka, A. Racing Induces Changes in the Blood Concentration of Serum Amyloid A in Thoroughbred Racehorses. J. Equine Vet. Sci. 2016, 36, 15–18. [Google Scholar] [CrossRef]
- Cywinska, A.; Turło, A.; Witkowski, L.; Szarska, E.; Winnicka, A. Changes in blood cytokine concentrations in horses after long-distance endurance rides. Med. Wet. 2014, 70, 568–571. [Google Scholar]
- Cywinska, A.; Witkowski, L.; Szarska, E.; Schollenberger, A.; Winnicka, A. Serum amyloid A (SAA) concentration after training sessions in Arabian race and endurance horses. BMC Vet Res. 2013, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, P.D.; Lawrence, L.M.; Danielsen, K.; Powell, D.M.; Thompson, K.N. Effect of conditioning and exercise type on serum creatine kinase and aspartate aminotransferase activity. Equine Vet. J. 1995, 18, 243–247. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Clarkson, P.M. Exercise-Induced Muscle Damage and Adaptation. Sports Med. 1989, 7, 207–234. [Google Scholar] [CrossRef] [PubMed]
- McKeever, K.H.; Hinchcliff, K.W.; Reed, S.M.; Robertin, J.T. Role of decreased plasma volume in haematocrit alterations during incremental treadmill exercise in horses. Am. J. Physiol. 1993, 265, 404–408. [Google Scholar]
- Convertino, V.A.; Brock, P.J.; Keil, L.C.; Bernauer, E.M.; Greenleaf, J.E. Exercise training-induced hypervolemia: Role of plasma albumin, renin, and vasopressin. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1980, 48, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.J.; Hodgson, D.R. Haematological and plasma biochemical parameters in endurance horses during training. Equine Vet. J. 1982, 14, 144–148. [Google Scholar] [CrossRef]
- Cywinska, A.; Gorecka, R.; Szarska, E.; Witkowski, L.; Dziekan, P.; Schollenberger, A. Serum amyloid A level as a potential indicator of the status of endurance horses. Equine Vet. J. Suppl. 2010, 38, 23–27. [Google Scholar] [CrossRef]
- Nieman, D.C.; Dumke, C.L.; Henson, D.A.; McAnulty, S.R.; Gross, S.J.; Lind, R.H. Muscle damage is linked to cytokine changes following a 160-km race. Brain Behav. Immun. 2005, 19, 398–403. [Google Scholar] [CrossRef]
- Smith, L.L. Cytokine hypothesis of overtraining: A physiological adaptation to excessive stress? Med. Sci. Sports Exerc. 2000, 32, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Febbraio, M.A.; Hiscock, N.; Sacchetti, M.; Fischer, C.P.; Pedersen, B.K. Interleukin-6 is a novel factor mediating glucose homeostasis in skeletal muscle contraction. Diabetes 2004, 53, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Liburt, N.R.; Adams, A.A.; Betancourt, A.; Horohov, D.W.; McKeever, K.H. Exercise-induced increases in inflammatory cytokines in muscle and blood of horses. Equine Vet. J. 2010, 42, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Hobo, S.; Niwa, S.; Anzai, T. Evaluation of serum amyloid A and surfactant protein D in sera for identification of horses with bacterial pneumonia. J. Vet. Med. Sci. 2007, 69, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, M.; Oue, Y.; Morita, Y.; Kanno, T.; Kinoshita, Y.; Niwa, H.; Ueno, T.; Katayama, Y.; Bannai, H.; Tsujimura, K.; et al. Experimental inoculation of equine coronavirus into Japanese draft horses. Arch. Virol. 2014, 12, 3329–3334. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, R.F.; Fernandes, W.R. Post-ride inflammatory markers in endurance horses. Cienc. Rural. 2016, 46, 1256–1261. [Google Scholar] [CrossRef][Green Version]
- Snow, D.H.; Kerr, M.G.; Nimmo, M.A.; Abbott, E.M. Alterations in blood, sweat, urine and muscle composition during prolonged exercise in the horse. Vet. Rec. 1982, 110, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Moro-Garcia, M.A.; Fernández-García, B.; Echeverría, A.; Rodríguez-Alonso, M.; Suárez-García, F.M.; Solano-Jaurrieta, J.J.; López-Larrea, C.; Alonso-Arias, R. Frequent participation in high volume exercise throughout life is associated with a more differentiated adaptive immune response. Brain Behav. Immun. 2014, 39, 61–74. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Unit | Individual Effect of the Horse | Number of Efforts in the Season | Time of Blood Collection (Before vs. After) b | Type of Effort (Training vs. Competition) b | Interaction between Type of Effort and Time of Blood Collection | |||
|---|---|---|---|---|---|---|---|---|---|
| p-Value | Standardized Regression Coefficient (β) | p-Value | Standardized Regression Coefficient (β) | p-Value | Standardized Regression Coefficient (β) | p-Value | p-Value | ||
| WBC | 109/L | 0.546 | - c | 0.264 | - c | 0.051 | - c | 0.883 | <0.001 |
| RBC | 1012/L | <0.001 | - c | 0.601 | 0.243 | 0.007 | - c | 0.388 | 0.550 |
| HGB | mmol/L | <0.001 | - c | 0.859 | 0.258 | 0.019 | - c | 0.098 | 0.525 |
| PCV | 1/1 | <0.001 | - c | 0.060 | 0.246 | 0.008 | - c | 0.930 | 0.584 |
| TSP | g/L | 0.177 | 0.412 | 0.007 | 0.371 | 0.002 | - c | 0.457 | 0.855 |
| AST | U/L | 0.106 | - c | 0.148 | 0.288 | 0.016 | - c | 0.899 | 0.767 |
| CPK | U/L | 0.022 | - c | 0.369 | 0.312 | 0.007 | - c | 0.089 | 0.108 |
| SAAadj a | mg/L | <0.001 | - c | 0.169 | - c | 0.249 | - c | 0.717 | 0.045 |
| Parameter Unit | Type of Effort | Time of Blood Collection | Before vs. After p-Value | |
|---|---|---|---|---|
| Before Effort | After Effort | |||
| WBC (109/L) mean ± SD (range) | training (n = 8) | 6.8 ± 1.0 (5.5–8.4) | 8.0 ± 1.2 (6.3–10.0) | 0.009 a |
| competition (n = 7) | 7.2 ± 1.6 (5.5–10.3) | 11.8 ± 2.4 (8.8–15.4) | 0.001 a | |
| training vs. competition p-value | 0.428 a | <0.001 a | ||
| SAAadj (mg/L) median, IQR (range) | training (n = 8) | 0.60, 0.29–0.67 (0.23–6.23) | 0.63, 0.44–0.76 (0.33–6.67) | 0.077 c |
| competition (n = 7) | 0.57, 0.41–0.84 (0.30–3.90) | 2.40, 0.75–7.19 (0.35–7.30) | 0.023 c | |
| training vs. competition p-value | 0.484 b | 0.025 b | ||
| Parameter Unit | Type of Effort | Time of Blood Collection (mean ± SD (range)) | Before vs. After p-Value a | |
|---|---|---|---|---|
| Before Effort | After Effort | |||
| RBC (1012/L) | training (n = 8) | 8.5 ± 1.0 (7.3–10.1) | 9.0 ± 1.1 (7.8–10.9) | <0.05 |
| competition (n = 7) | 8.8 ± 1.2 (7.0–10.0) | 9.5 ± 1.3 (7.6–10.7) | ||
| HGB (mmol/L) | training (n = 8) | 7.8 ± 0.9 (6.9–9.6) | 8.4 ± 1.1 (7.0–10.4) | <0.01 |
| competition (n = 7) | 7.4 ± 1.0 (5.8–8.9) | 8.1 ± 0.8 (6.8–9.1) | ||
| PCV (l/l) | training (n = 8) | 37.6 ± 4.6 (32.9–46.4) | 40.0 ± 5.0 (34.4–48.6) | ≤0.01 |
| competition (n = 7) | 38.8 ± 5.4 (30.5–45.2) | 42.2 ± 5.7 (33.4–49.1) | ||
| TSP (g/L) | training (n = 8) | 63.6 ± 4.5 (57–71) | 67.1 ± 3.6 (61–72) | ≤0.001 |
| competition (n = 7) | 64.3 ± 2.9 (61–69) | 67.9 ± 4.0 (64–75) | ||
| AST (U/L) | training (n = 8) | 278.9 ± 28.1 (231–316) | 298.7 ± 27.2 (258–328) | <0.05 |
| competition (n = 7) | 280.5 ± 37.0 (247–343) | 303.2 ± 35.5 (246–347) | ||
| CPK (U/L) | training (n = 8) | 289.6 ± 108.6 (151–429) | 369.4 ± 104.3 (225–512) | <0.05 |
| competition (n = 7) | 321.9 ± 141.9 (195–548) | 488.7 ± 181.0 (200–774) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witkowska-Piłaszewicz, O.; Bąska, P.; Czopowicz, M.; Żmigrodzka, M.; Szczepaniak, J.; Szarska, E.; Winnicka, A.; Cywińska, A. Changes in Serum Amyloid A (SAA) Concentration in Arabian Endurance Horses During First Training Season. Animals 2019, 9, 330. https://doi.org/10.3390/ani9060330
Witkowska-Piłaszewicz O, Bąska P, Czopowicz M, Żmigrodzka M, Szczepaniak J, Szarska E, Winnicka A, Cywińska A. Changes in Serum Amyloid A (SAA) Concentration in Arabian Endurance Horses During First Training Season. Animals. 2019; 9(6):330. https://doi.org/10.3390/ani9060330
Chicago/Turabian StyleWitkowska-Piłaszewicz, Olga, Piotr Bąska, Michał Czopowicz, Magdalena Żmigrodzka, Jarosław Szczepaniak, Ewa Szarska, Anna Winnicka, and Anna Cywińska. 2019. "Changes in Serum Amyloid A (SAA) Concentration in Arabian Endurance Horses During First Training Season" Animals 9, no. 6: 330. https://doi.org/10.3390/ani9060330
APA StyleWitkowska-Piłaszewicz, O., Bąska, P., Czopowicz, M., Żmigrodzka, M., Szczepaniak, J., Szarska, E., Winnicka, A., & Cywińska, A. (2019). Changes in Serum Amyloid A (SAA) Concentration in Arabian Endurance Horses During First Training Season. Animals, 9(6), 330. https://doi.org/10.3390/ani9060330





