Whole-Life or Fattening Period Only Broiler Feeding Strategies Achieve Similar Levels of Omega-3 Fatty Acid Enrichment Using the DHA-Rich Protist, Aurantiochytrium limacinum
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
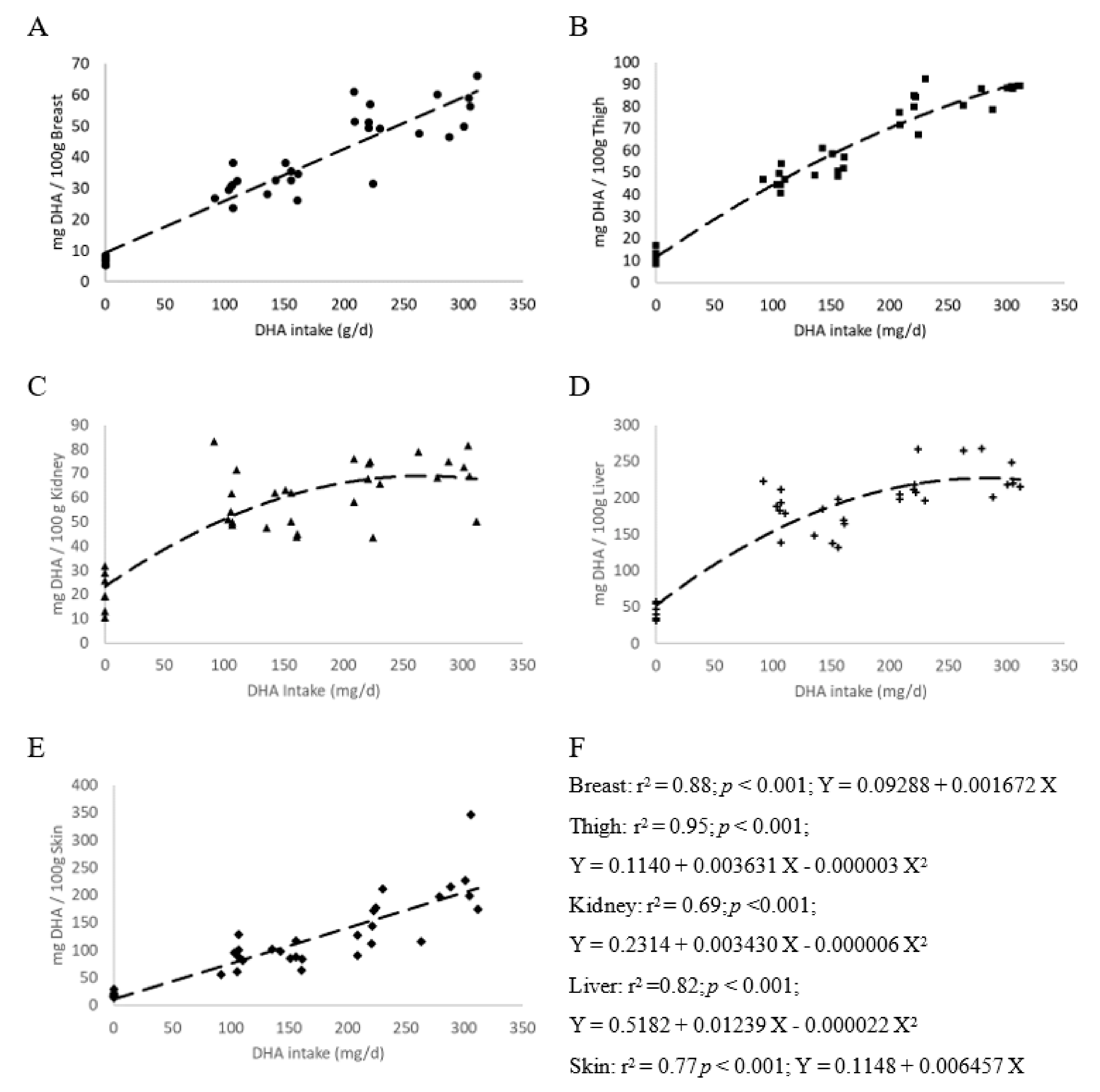
3.1. Bird Health and Performance
3.2. Meat Fatty Acid Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McManus, A.; Merga, M.; Newton, W. Omega-3 fatty acids. What consumers need to know. Appetite 2011, 57, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Taneja, A.; Singh, H. Challenges for the Delivery of Long-Chain n-3 Fatty Acids in Functional Foods. Annu. Rev. Food Sci. Technol. 2012, 3, 105–123. [Google Scholar] [CrossRef] [PubMed]
- González-Esquerra, R.; Leeson, S. Alternatives for enrichment of eggs and chicken meat with omega-3 fatty acids. Can. J. Anim. Sci. 2001, 81, 295–305. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Omega-3 Polyunsaturated Fatty Acids and Their Health Benefits. Annu. Rev. Food Sci. Technol. 2018, 9, 345–381. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority. Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J. 2010, 8, 1461. [Google Scholar] [CrossRef]
- Papanikolaou, Y.; Brooks, J.; Reider, C.; Fulgoni, V.L.U.S. Adults are not meeting recommended levels for fish and omega-3 fatty acid intake: Results of an analysis using observational data from NHANES 2003–2008. Nutr. J. 2014, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Givens, D.I.; Kliem, K.E.; Gibbs, R.A. The role of meat as a source of n-3 polyunsaturated fatty acids in the human diet. Meat Sci. 2006, 74, 209–218. [Google Scholar] [CrossRef]
- Howe, P.; Meyer, B.; Record, S.; Baghurst, K. Dietary intake of long-chain ω-3 polyunsaturated fatty acids: Contribution of meat sources. Nutrition 2006, 22, 47–53. [Google Scholar] [CrossRef]
- Gibbs, R.A.; Rymer, C.; Givens, D.I. Fatty acid composition of cooked chicken meat and chicken meat products as influenced by price range at retail. Food Chem. 2013, 138, 1749–1756. [Google Scholar] [CrossRef]
- Ribeiro, T.; Lordelo, M.M.; Alves, S.P.; Bessa, R.J.B.; Costa, P.; Lemos, J.P.C.; Ferreira, L.M.A.; Fontes, C.M.G.A.; Prates, J.A.M. Direct supplementation of diet is the most efficient way of enriching broiler meat with n-3 long-chain polyunsaturated fatty acids. Br. Poult. Sci. 2013, 54, 753–765. [Google Scholar] [CrossRef]
- Gonzalez-Esquerra, R.; Leeson, S. Effect of feeding hens regular or deodorized menhaden oil on production parameters, yolk fatty acid profile, and sensory quality of eggs. Poult. Sci. 2000, 79, 1597–1602. [Google Scholar] [CrossRef] [PubMed]
- Bryhni, E.A.; Kjos, N.P.; Ofstad, R.; Hunt, M. Polyunsaturated fat and fish oil in diets for growing-finishing pigs: Effects on fatty acid composition and meat, fat, and sausage quality. Meat Sci. 2002, 62, 1–8. [Google Scholar] [CrossRef]
- Salem, N.; Eggersdorfer, M. Is the world supply of omega-3 fatty acids adequate for optimal human nutrition? Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Mooney, J.W.; Hirschler, E.M.; Kennedy, A.K.; Sams, A.R.; Van Elswyk, M.E. Lipid and flavour quality of stored breast meat from broilers fed marine algae. J. Sci. Food Agric. 1998, 78, 134–140. [Google Scholar] [CrossRef]
- Abril, R.; Barclay, W. Production of docosahexaenoic acid-enriched poultry eggs and meat using an algae-based feed ingredient. In The Return of w3 Fatty Acids into the Food Supply. I. Land-Based Animal Food Products and Their Health Effects; Simopoulos, A.P., Ed.; Karger: Basel, Switzerland, 1998; Vol. 83, pp. 77–88. [Google Scholar]
- Moran, C.A.; Currie, D.; Keegan, J.; Knox, A. Tolerance of Broilers to Dietary Supplementation with High Levels of the DHA-Rich Microalga, Aurantiochytrium Limacinum: Effects on Health and Productivity. Animals 2018, 8, 180. [Google Scholar] [CrossRef] [PubMed]
- Keegan, J.D.; Currie, D.; Knox, A.; Moran, C.A. Redressing the balance: Including DHA-rich Aurantiochytrium limacinum in broiler diets increases tissue omega-3 fatty acid content and lowers the n-6:n-3 ratio. Br. Poult. Sci. 2019, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Moran, C.A.; Morlacchini, M.; Keegan, J.D.; Fusconi, G. Increasing the Omega-3 Content of Hen’s Eggs through Dietary Supplementation with Aurantiochytrium limacinum Microalgae: Effect of Inclusion Rate on the Temporal Pattern of Docosahexaenoic Acid Enrichment, Efficiency of Transfer, and Egg Characteristics. J. Appl. Poult. Res. 2018, 28, 329–338. [Google Scholar] [CrossRef]
- Moran, C.A.; Morlacchini, M.; Keegan, J.D.; Fusconi, G. Dietary supplementation of finishing pigs with the docosahexaenoic acid-rich microalgae, Aurantiochytrium limacinum: Effects on performance, carcass characteristics and tissue fatty acid profile. Asian Australas. J. Anim. Sci. 2018, 31, 712–720. [Google Scholar] [CrossRef]
- Moran, C.A.; Morlacchini, M.; Keegan, J.D.; Fusconi, G. The effect of dietary supplementation with Aurantiochytrium limacinum on lactating dairy cows in terms of animal health, productivity and milk composition. J. Anim. Physiol. Anim. Nutr. (Berl). 2018, 102, 576–590. [Google Scholar] [CrossRef]
- Moran, C.A.; Morlacchini, M.; Keegan, J.D.; Delles, R.; Fusconi, G. Effects of a DHA-rich unextracted microalgae as a dietary supplement on performance, carcass traits and meat fatty acid profile in growing-finishing pigs. J. Anim. Physiol. Anim. Nutr. (Berl). 2018, 102, 1026–1038. [Google Scholar] [CrossRef]
- Dillon, G.P.; Yiannikouris, A.; Brandl, W.; Cardinall, C.; Yuan, W.; Moran, C.A. Analytical Method Assessment for the Determination of DHA and Fatty Acids Present in Unextracted Aurantiochytrium limacinum Biomass. Food Nutr. Sci. 2019, 469–483. [Google Scholar]
- Aviagen Ross Broiler Management Handbook. Available online: https://digital-library.theiet.org/content/journals/10.1049/tpe.1986.0057 (accessed on 4 June 2019).
- Aviagen Ross 308 Broiler: Nutrition Specifications. Available online: http://en.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross308BroilerNutritionSpecs2014-EN.pdf (accessed on 15 May 2019).
- Dillon, G.; Brandl, W.; Cardinall, C.; Yuan, W.; Ao, T.; Yiannikouris, A.; Moran, C.A. GC-FID method validation of DHA in layer hen feed supplemented with heterotrophically grown, dried microalgae powder (All-G-Rich). In Poultry Science Association Annual Meeting, San Antonio, USA, 23–26 July 2018; (E-suppl 1); Poultry Science: San Antonio, TX, USA, 2018; pp. 186–187. [Google Scholar]
- Dillon, G.P.; Yiannikouris, A.; Brandl, W.; Cardinall, C.; Yuan, W.; Moran, C.A. Fitness for purpose and stability assessment of long-chain polyunsaturated fatty acids in chicken tissues. Regul. Toxicol. Pharmacol. 2019, 103, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Lordelo, M.M.; Costa, P.; Alves, S.P.; Benevides, W.S.; Bessa, R.J.B.; Lemos, J.P.C.; Pinto, R.M.A.; Ferreira, L.M.A.; Fontes, C.M.G.A.; Prates, J.A.M. Effect of reduced dietary protein and supplementation with a docosahexaenoic acid product on broiler performance and meat quality. Br. Poult. Sci. 2014, 55, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Long, S.F.; Kang, S.; Wang, Q.Q.; Xu, Y.T.; Pan, L.; Hu, J.X.; Li, M.; Piao, X.S. Dietary supplementation with DHA-rich microalgae improves performance, serum composition, carcass trait, antioxidant status, and fatty acid profile of broilers. Poult. Sci. 2018, 97, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.M.; El-Senousey, H.K. Nutritional factors affecting abdominal fat deposition in poultry: A review. Asian Australas. J. Anim. Sci. 2014, 27, 1057–1068. [Google Scholar] [CrossRef] [PubMed]
- Zuidhof, M.J.; Betti, M.; Korver, D.R.; Hernandez, F.I.L.; Schneider, B.L.; Carney, V.L.; Renema, R.A. Omega-3-enriched broiler meat: 1. Optimization of a production system. Poult. Sci. 2009, 88, 1108–1120. [Google Scholar] [PubMed]
- Kanakri, K.; Carragher, J.; Hughes, R.; Muhlhausler, B.; Gibson, R. A reduced cost strategy for enriching chicken meat with omega-3 long chain polyunsaturated fatty acids using dietary flaxseed oil. Br. Poult. Sci. 2017, 58, 283–289. [Google Scholar] [CrossRef]
- Konieczka, P.; Czauderna, M.; Smulikowska, S. The enrichment of chicken meat with omega-3 fatty acids by dietary fish oil or its mixture with rapeseed or flaxseed—Effect of feeding duration: Dietary fish oil, flaxseed, and rapeseed and n-3 enriched broiler meat. Anim. Feed Sci. Technol. 2017, 223, 42–52. [Google Scholar] [CrossRef]
- European Commission Nutrition Claims. Available online: https://ec.europa.eu/food/safety/labelling_nutrition/claims/nutrition_claims_en (accessed on 12 September 2018).
- Sprecher, H. Metabolism of highly unsaturated n-3 and n-6 fatty acids. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2000, 1486, 219–231. [Google Scholar] [CrossRef]
- Kaur, G.; Cameron-Smith, D.; Garg, M.; Sinclair, A.J. Docosapentaenoic acid (22:5n-3): A review of its biological effects. Prog. Lipid Res. 2011, 50, 28–34. [Google Scholar] [CrossRef]
- Conquer, J.A.; Tierney, M.C.; Zecevic, J.; Bettger, W.J.; Fisher, R.H. Fatty acid analysis of blood plasma of patients with alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids 2000, 35, 1305–1312. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 2008, 233, 674–688. [Google Scholar] [CrossRef] [PubMed]

| Diet Ingredient | Day 0–21 | Day 21–42 | ||||
|---|---|---|---|---|---|---|
| 0%WL 0.5%FP 1.0%FP | 0.5%WL | 1.0%WL | 0%WL | 0.5%FP 0.5%WL | 1.0%FP 1.0%WL | |
| Corn meal | 46.20 | 46.50 | 46.25 | 52.96 | 52.90 | 52.90 |
| Soybean meal 48% | 36.70 | 36.58 | 36.60 | 30.12 | 30.00 | 29.88 |
| Wheat meal | 8.25 | 8.00 | 8.00 | 8.55 | 8.53 | 8.50 |
| Soybean oil | 4.50 | 4.13 | 3.90 | 4.58 | 4.28 | 3.93 |
| Dicalcium phosphate | 1.80 | 1.76 | 1.75 | 1.37 | 1.37 | 1.37 |
| Limestone | 1.00 | 0.98 | 0.97 | 0.98 | 0.98 | 0.98 |
| Sodium bicarbonate | 0.40 | 0.40 | 0.39 | 0.39 | 0.39 | 0.39 |
| Lysine HCL | - | - | - | 0.10 | 0.10 | 0.10 |
| DL - Methionine | 0.40 | 0.40 | 0.39 | 0.20 | 0.20 | 0.20 |
| Vitamins and minerals 1 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| Salt | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 | 0.15 |
| Ronomix Hiphos | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| AURA | - | 0.50 | 1.00 | - | 0.50 | 1.00 |
| Nutrient Value | Day 0–21 1 | Day 21–42 | ||||
|---|---|---|---|---|---|---|
| 0%WL 0.5%FP 1.0%FP | 0.5%WL | 1.0%WL | 0%WL | 0.5%FP 0.5%WL | 1.0%FP 1.0%WL | |
| Dry matter (%) | 89.4 | 89.0 | 89.2 | 88.9 | 88.8 | 88.9 |
| Crude protein (%) | 23.7 | 23.6 | 23.6 | 19.4 | 19.5 | 19.4 |
| Crude fibre (%) | 2.8 | 2.8 | 2.8 | 2.7 | 2.8 | 2.8 |
| Crude fat (%) | 6.5 | 6.5 | 6.9 | 6.8 | 6.5 | 6.9 |
| Ash (%) | 6.3 | 6.0 | 6.0 | 5.2 | 5.0 | 5.4 |
| Starch (%) | 40.2 | 40.5 | 39.9 | 43.5 | 43.9 | 43.5 |
| Sugar (%) | 4.6 | 4.6 | 4.6 | 4.5 | 4.6 | 4.6 |
| M.E. 2 (Kcal/kg) | 3180.5 | 3167.5 | 3169.0 | 3154.0 | 3158.0 | 3160.0 |
| Fatty Acid (mg/g) | Day 0–21 1 | Day 21–42 | ||||
|---|---|---|---|---|---|---|
| 0%WL 0.5%FP 1.0%FP | 0.5%WL | 1.0%WL | 0%WL | 0.5%FP 0.5%WL | 1.0%FP 1.0%WL | |
| C12:0 Lauric | 0.00 | 0.01 | 0.02 | 0.00 | 0.01 | 0.02 |
| C14:0 Myristic | 0.04 | 0.24 | 0.48 | 0.03 | 0.21 | 0.41 |
| C15:0 Pentadecanoic | 0.01 | 0.07 | 0.13 | 0.01 | 0.06 | 0.11 |
| C16:0 Palmitic | 6.24 | 7.91 | 10.47 | 6.44 | 7.65 | 9.89 |
| C16:1 Palmitoleic | 0.05 | 0.05 | 0.05 | 0.04 | 0.04 | 0.05 |
| C17:0 Heptadecanoic | 0.04 | 0.06 | 0.07 | 0.04 | 0.05 | 0.07 |
| C18:0 Stearic | 2.11 | 1.96 | 2.02 | 2.09 | 1.86 | 1.89 |
| C18:1n9 cis Oleic | 13.96 | 12.60 | 12.86 | 14.53 | 12.93 | 12.94 |
| C18:1 cis11 | 0.61 | 0.55 | 0.56 | 0.59 | 0.52 | 0.52 |
| C19:0 Nonadecanoic | 0.11 | 0.10 | 0.10 | 0.11 | 0.09 | 0.10 |
| C18:2n6c Linoleic | 29.38 | 25.62 | 26.53 | 29.25 | 26.38 | 26.34 |
| C18:3n3 α-Linolenic | 2.27 | 1.95 | 1.98 | 2.20 | 1.94 | 1.96 |
| C20:0 Arachidic | 0.21 | 0.20 | 0.22 | 0.22 | 0.20 | 0.21 |
| C20:1 cis-11-Eicosenoic | 0.11 | 0.10 | 0.11 | 0.11 | 0.10 | 0.09 |
| C20:5n3 EPA 2 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| C22:0 Behenic | 0.18 | 0.17 | 0.18 | 0.18 | 0.16 | 0.16 |
| C24:0 Lignoceric | 0.08 | 0.08 | 0.08 | 0.08 | 0.07 | 0.08 |
| C22:6n3 DHA 3 | 0.00 | 0.73 | 1.53 | 0.00 | 0.69 | 1.43 |
| Parameter | 0 %WL D 0–42 | 0.5%WL D 0–42 | 1.0%WL D 0–42 | 0.5%FP D 21–42 | 1.0%FP D 21–42 | Standard Error | p Value |
|---|---|---|---|---|---|---|---|
| Weight Day 0 | 37.3 | 37.1 | 36.9 | 37.0 | 37.2 | 0.27 | 0.920 |
| Weight Day 21 | 883.7 ab | 918.6 a | 848.3 b | 888.3 ab | 883.7 ab | 13.91 | 0.026 |
| Weight Day 42 | 3072.1 a | 2998.1 ab | 2809.2 b | 2861.0 ab | 2947.8 ab | 59.10 | 0.028 |
| 1 ADG D 0–21 | 40.3 ab | 42.0 a | 38.5 b | 40.5 ab | 40.3 ab | 0.65 | 0.016 |
| ADG D 21–42 | 101.9 a | 98.4 ab | 91.2 b | 93.9 ab | 97.5 ab | 2.52 | 0.051 |
| ADG D 0–42 | 70.9 a | 70.0 ab | 64.8 b | 67.2 ab | 68.8 ab | 1.43 | 0.036 |
| 2 ADFI D 0–21 | 53.5 ab | 58.9 a | 53.4 ab | 53.0 ab | 52.6 b | 1.48 | 0.031 |
| ADFI D21-42 | 162.9 a | 157.5 ab | 148.2 b | 151.2 ab | 153.2 ab | 2.95 | 0.014 |
| ADFI D0-42 | 107.9 | 107.8 | 100.6 | 102.0 | 103.0 | 1.88 | 0.026 |
| 3 FCR D0-21 | 1.3 | 1.4 | 1.4 | 1.3 | 1.3 | 0.03 | 0.144 |
| FCR D21-42 | 1.6 | 1.6 | 1.6 | 1.6 | 1.6 | 0.03 | 0.723 |
| FCR D0-42 | 1.5 | 1.5 | 1.6 | 1.5 | 1.5 | 0.02 | 0.441 |
| 4 EPEF D21 | 317.5 | 315.0 | 287.6 | 323.7 | 322.8 | 9.01 | 0.049 |
| EPEF D42 | 436.7 | 435.2 | 395.0 | 416.7 | 431.9 | 17.67 | 0.428 |
| Breast weight (g) | 604.6 | 590.3 | 556.6 | 552.1 | 575.0 | 20.39 | 0.388 |
| Thigh weight (g) | 581.4a | 552.1 ab | 525.7 b | 509.3 b | 542.4 ab | 13.46 | 0.009 |
| Dressing (%) | 86.0 | 86.2 | 86.2 | 86.3 | 86.3 | 0.44 | 0.990 |
| Breast (%) | 23.4 | 23.6 | 23.0 | 23.2 | 23.2 | 0.67 | 0.980 |
| Thigh (%) | 22.5 | 22.1 | 21.8 | 21.4 | 21.9 | 0.48 | 0.551 |
| Fatty Acid (mg/100g) | 0%WL D 0–42 | 0.5%WL D 0–42 | 1.0%WL D 0–42 | 0.5%FP D21–42 | 1.0%FP D 21–42 | Standard Error | p Value |
|---|---|---|---|---|---|---|---|
| Breast | |||||||
| C15:0 | 1.5 b | 2.2 ab | 3.2 a | 2.2 ab | 2.7 ab | 0.36 | 0.034 |
| C20:5n3 EPA 1 | 1.9 b | 2.5 b | 4.3 a | 2.7 b | 3.5 ab | 0.39 | 0.001 |
| C22:4n6 | 18.6 a | 10.9 b | 8.9 b | 11.7 b | 8.5 b | 0.87 | <0.001 |
| C22:5n3 DPA 2 | 12.1 | 11.3 | 12.4 | 11.5 | 11.7 | 0.76 | 0.841 |
| C22:6n3 DHA 3 | 6.9 c | 32.6 b | 55.2 a | 30.3 b | 50.1 a | 2.26 | <0.001 |
| Total Omega 3 | 66.8 b | 83 ab | 111.9 a | 82.8 ab | 101.1 ab | 8.82 | 0.010 |
| Total Omega 6 | 818.7 | 689.8 | 711.5 | 710.3 | 650.9 | 102.6 | 0.829 |
| Omega 6 / Omega 3 | 12.2 a | 8.2 b | 6.2 c | 8.3 b | 6.3 c | 0.4 | <0.001 |
| Thigh | |||||||
| C12:0 | 0.7 b | 1.1 ab | 1.1 ab | 0.8 ab | 1.2 a | 0.11 | 0.011 |
| C14:0 | 14.6 b | 25.3 ab | 27.8a | 19.4 ab | 30.0 a | 2.66 | 0.002 |
| C15:0 | 3.0 c | 6.4 ab | 6.9 ab | 4.8 bc | 7.4 a | 0.59 | <0.001 |
| C17:0 | 5.8 b | 8.2 a | 7.0 ab | 6.9 ab | 8.1 ab | 0.58 | 0.040 |
| C20:4n6 | 115.5 a | 97.8 b | 89.5 b | 100.1 ab | 91.6 b | 3.98 | 0.001 |
| C20:5n3 EPA | 2.9 c | 5.0 b | 8.2 a | 4.5 bc | 7.6 a | 0.44 | <0.001 |
| C22:4n6 | 25.8 a | 14.9 b | 11.9b | 15.1 b | 11.7 b | 0.87 | <0.001 |
| C22:5n3 DPA | 20.1 | 17.2 | 17.1 | 16.3 | 16.8 | 0.96 | 0.067 |
| C22:6n3 DHA | 11.7 c | 53.6 b | 86.0 a | 46.6 b | 79.6 a | 2.05 | <0.001 |
| Total Omega 3 | 140.7 c | 200.1 ab | 207.8 ab | 165 bc | 223.3 a | 13.3 | 0.001 |
| Total Omega 6 | 1788 | 2087 | 1609 | 1695 | 1953 | 162.3 | 0.251 |
| Omega 6 / Omega 3 | 12.7 a | 10.4 b | 7.7 c | 10.3 b | 8.6 c | 0.252 | <0.001 |
| Liver | |||||||
| C15:0 | 1.5 b | 2.0 ab | 2.8 a | 2.2 ab | 2.8 a | 0.22 | 0.001 |
| C20:4n6 | 346.8 a | 306.8 ab | 270.5 bc | 324.8 a | 257.2 c | 12.07 | <0.001 |
| C20:5n3 EPA | 7.1 b | 10.0 b | 15.7 a | 10.7 ab | 15.3 a | 1.25 | <0.001 |
| C22:4n6 | 37.4 a | 24.7 bc | 21.5 bc | 26.5 b | 18.2 c | 1.8 | <0.001 |
| C22:5n3 DPA | 27.7 | 23.6 | 26.9 | 29.2 | 24.1 | 1.79 | 0.153 |
| C22:6n3 DHA | 45.3 d | 161.7 c | 233.4 a | 187.6 bc | 214.4 ab | 8.82 | <0.001 |
| Total Omega 3 | 104.4 d | 215.9 c | 300.2 a | 251.4 bc | 281.5 ab | 11.16 | <0.001 |
| Total Omega 6 | 1143.9 | 1057.1 | 1074.1 | 1127.2 | 1085.2 | 59.31 | 0.820 |
| Omega 6 / Omega 3 | 11.1 a | 5.0 b | 3.6 c | 4.5 bc | 3.9 bc | 278 | <0.001 |
| Kidney | |||||||
| C20:2n6 | 35.0 a | 34.8 ab | 26.4 c | 32.3 abc | 27.9 bc | 1.72 | 0.002 |
| C20:3n3 | 5.0 a | 5.2 a | 3.6 b | 4.8 ab | 4.2 ab | 0.33 | 0.010 |
| C20:5n3 EPA | 8.1 c | 25.5 b | 37.5 a | 26.4 b | 36.8 a | 2.3 | <0.001 |
| C22:4n6 | 24.2 a | 16 b | 14.2 b | 15.8 b | 11.3 b | 1.76 | <0.001 |
| C22:5n3 DPA | 14.4 | 14.8 | 14.7 | 15.9 | 14.1 | 1.08 | 0.798 |
| C22:6n3 DHA | 21.2 c | 53.3 b | 70.8 a | 60.1 ab | 65.8 ab | 3.95 | <0.001 |
| Total Omega 3 | 122.3 b | 201.0 a | 192.6 ab | 180.7 ab | 193.8 a | 17.3 | 0.020 |
| Total Omega 6 | 1798.1 | 2217 | 1600.5 | 1750.5 | 1710.6 | 256.43 | 0.506 |
| Omega 6 / Omega 3 | 14.8 a | 10.8 b | 8.3 c | 9.3 bc | 8.7 bc | 0.591 | <0.001 |
| Skin with adhering fat | |||||||
| C12:0 | 9.5 b | 11.6 ab | 15.0 a | 11.0 b | 13.1 ab | 0.95 | 0.004 |
| C14:0 | 181.3 d | 248.5 bc | 332.7a | 220.2cd | 305.4 ab | 15.74 | <0.001 |
| C14:1 | 29.5 | 28.6 | 38.1 | 24.3 | 35.9 | 3.8 | 0.092 |
| C15:0 | 34.6 d | 59.7 bc | 79.9 a | 51.8 c | 72.1 ab | 3.93 | <0.001 |
| C18:3n6 | 101.8 | 81.5 | 79.3 | 80.6 | 90.6 | 5.78 | 0.049 |
| C20:4n6 | 251.2 a | 199.3 ab | 228.1 ab | 217.0 ab | 180.4 b | 16.35 | 0.047 |
| C20:5n3 EPA | 20.1 c | 30.7 bc | 71.6 a | 30 bc | 46.5 b | 6.05 | <0.001 |
| C22:4n6 | 57.5 a | 40.6 b | 41.6 ab | 40.9 b | 31.8 b | 4.02 | 0.002 |
| C22:5n3 DPA | 38.1 | 37.7 | 51.9 | 38.6 | 39.6 | 3.64 | 0.047 |
| C22:6n3 DHA | 18.5 c | 90.8 b | 210.1 a | 87.3 b | 147.3 b | 14.69 | <0.001 |
| Total Omega 34 | 1372.9 | 1307.8 | 1448.6 | 1231.9 | 1345.2 | 85.44 | 0.489 |
| Total Omega 65 | 19460.0 | 18141.0 | 16840.9 | 16994.7 | 17248.3 | 1029.4 | 0.368 |
| Omega 6 / Omega 3 | 14.2 a | 14.0 ab | 11.7 c | 13.8 ab | 12.9 bc | 0.317 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D. Keegan, J.; Fusconi, G.; Morlacchini, M.; A. Moran, C. Whole-Life or Fattening Period Only Broiler Feeding Strategies Achieve Similar Levels of Omega-3 Fatty Acid Enrichment Using the DHA-Rich Protist, Aurantiochytrium limacinum. Animals 2019, 9, 327. https://doi.org/10.3390/ani9060327
D. Keegan J, Fusconi G, Morlacchini M, A. Moran C. Whole-Life or Fattening Period Only Broiler Feeding Strategies Achieve Similar Levels of Omega-3 Fatty Acid Enrichment Using the DHA-Rich Protist, Aurantiochytrium limacinum. Animals. 2019; 9(6):327. https://doi.org/10.3390/ani9060327
Chicago/Turabian StyleD. Keegan, Jason, Giorgio Fusconi, Mauro Morlacchini, and Colm A. Moran. 2019. "Whole-Life or Fattening Period Only Broiler Feeding Strategies Achieve Similar Levels of Omega-3 Fatty Acid Enrichment Using the DHA-Rich Protist, Aurantiochytrium limacinum" Animals 9, no. 6: 327. https://doi.org/10.3390/ani9060327
APA StyleD. Keegan, J., Fusconi, G., Morlacchini, M., & A. Moran, C. (2019). Whole-Life or Fattening Period Only Broiler Feeding Strategies Achieve Similar Levels of Omega-3 Fatty Acid Enrichment Using the DHA-Rich Protist, Aurantiochytrium limacinum. Animals, 9(6), 327. https://doi.org/10.3390/ani9060327





