Immune System Stimulation Reduces the Efficiency of Whole-Body Protein Deposition and Alters Muscle Fiber Characteristics in Growing Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. General Design, Housing, and Treatments
2.2. Experimental Diets and Feeding
2.3. Observations and Sampling
2.4. Analytical Procedures
2.5. Calculations and Statistical Analysis
3. Results
3.1. General Observations
3.2. Body Temperature, Hematology and Blood Chemistry
3.3. Whole-body N Metabolism
3.4. Muscle Fiber Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rakhshandeh, A.; Htoo, J.K.; Karrow, N.; Miller, S.P.; de Lange, C.F.M. Impact of immune system stimulation on the ileal nutrient digestibility and utilisation of methionine plus cysteine intake for whole-body protein deposition in growing pigs. Br. J. Nutr. 2014, 111, 101–110. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, K.; Levesque, C.L.; Htoo, J.K.; de Lange, C.F.M. Immune system stimulation reduces the efficiency of tryptophan utilization for body protein deposition in growing pigs. J. Anim. Sci. 2012, 90, 3485–3491. [Google Scholar] [CrossRef]
- National Research Council. Nutrient Requirements of Swine, 11th ed.; National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-22423-9. [Google Scholar]
- Waterlow, J.C. Measurement of whole body protein turnover by the end-product method. In Protein Turnover; CABI: Wallingford, UK, 2006; pp. 97–105. [Google Scholar]
- Obled, C. Amino acid requirements in inflammatory states. Can. J. Anim. Sci. 2003, 83, 365–373. [Google Scholar] [CrossRef]
- Johnson, R.W. Inhibition of growth by pro-inflammatory cytokines: An integrated view. J. Anim. Sci. 1997, 75, 1244–1255. [Google Scholar] [CrossRef]
- Rudar, M.; Zhu, C.L.; de Lange, C.F. Dietary Leucine Supplementation Decreases Whole-Body Protein Turnover before, but Not during, Immune System Stimulation in Pigs. J. Nutr. 2017, 147, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Daiwen, C.; Keying, Z.; Chunyan, W. Influences of lipopolysaccharide-induced immune challenge on performance and whole-body protein turnover in weanling pigs. Livest. Sci. 2008, 113, 291–295. [Google Scholar] [CrossRef]
- Breuillé, D.; Arnal, M.; Rambourdin, F.; Bayle, G.; Levieux, D.; Obled, C. Sustained modifications of protein metabolism in various tissues in a rat model of long-lasting sepsis. Clin. Sci. 1998, 94, 413–423. [Google Scholar] [CrossRef]
- Lobley, G.E. Protein turnover—What does it mean for animal production? Can. J. Anim. Sci. 2003, 83, 327–340. [Google Scholar] [CrossRef]
- Reeds, P.J.; Cadenhead, A.; Fuller, M.F.; Lobley, G.E.; McDonald, J.D. Protein turnover in growing pigs. Effects of age and food intake. Br. J. Nutr. 1980, 43, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Breuille, D.; Rose, F.; Arnal, M.; Melin, C.; Obled, C. Sepsis modifies the contribution of different organs to whole-body protein synthesis in rats. Clin. Sci. 1994, 86, 663–669. [Google Scholar] [CrossRef]
- Orellana, R.A.; Kimball, S.R.; Nguyen, H.V.; Bush, J.A.; Suryawan, A.; Thivierge, M.C.; Jefferson, L.S.; Davis, T.A. Regulation of muscle protein synthesis in neonatal pigs during prolonged endotoxemia. Pediatr. Res. 2004, 55, 442–449. [Google Scholar] [CrossRef]
- Lang, C.H.; Frost, R.A.; Vary, T.C. Regulation of muscle protein synthesis during sepsis and inflammation. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E453–E459. [Google Scholar] [CrossRef]
- Orellana, R.A.; Jeyapalan, A.; Escobar, J.; Frank, J.W.; Nguyen, H.V.; Suryawan, A.; Davis, T.A. Amino acids augment muscle protein synthesis in neonatal pigs during acute endotoxemia by stimulating mTOR-dependent translation initiation. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1416–E1425. [Google Scholar] [CrossRef]
- De Lange, C.F.; Sauer, W.C.; Mosenthin, R.; Souffrant, W.B. The effect of feeding different protein-free diets on the recovery and amino acid composition of endogenous protein collected from the distal ileum and feces in pigs. J. Anim. Sci. 1989, 67, 746–754. [Google Scholar] [CrossRef]
- Rivera-Ferre, M.G.; Aguilera, J.F.; Nieto, R. Differences in whole-body protein turnover between Iberian and Landrace pigs fed adequate or lysine-deficient diets. J. Anim. Sci. 2006, 84, 3346–3355. [Google Scholar] [CrossRef]
- Rakhshandeh, A.; de Lange, C.F.M. Evaluation of chronic immune system stimulation models in growing pigs. Animal 2012, 6, 305–310. [Google Scholar] [CrossRef]
- Petry, A.; McGilvray, W.; Rakhshandeh, A.R.; Rakhshandeh, A. Technical note: Assessment of an alternative technique for measuring body temperature in pigs. J. Anim. Sci. 2017, 95, 3270–3274. [Google Scholar] [CrossRef]
- Rakhshandeh, A.; Htoo, J.K.; de Lange, C.F.M. Immune system stimulation of growing pigs does not alter apparent ileal amino acid digestibility but reduces the ratio between whole body nitrogen and sulfur retention. Livest. Sci. 2010, 134, 21–23. [Google Scholar] [CrossRef]
- Möhn, S.; de Lange, C.F. The effect of body weight on the upper limit to protein deposition in a defined population of growing gilts. J. Anim. Sci. 1998, 76, 124–133. [Google Scholar] [CrossRef]
- McGilvray, W.D.; Wooten, H.; Rakhshandeh, A.R.; Petry, A.; Rakhshandeh, A. Immune system stimulation increases dietary threonine requirements for protein deposition in growing pigs. J. Anim. Sci. 2019, 97, 735–744. [Google Scholar] [CrossRef]
- Hewitt, D.J.; de Lange, C.F.M.; Antonick, T.; Dekkers, J.C.M.; Pendleton, A.R.; Rakhshandeh, A. Effect of divergent selection for residual feed intake on whole body protein turnover in growing gilts fed either adequate or lysine deficient diets. J. Anim. Sci. 2016, 94, 108–109. [Google Scholar] [CrossRef]
- Hergenreder, J.E.; Legako, J.F.; Dinh, T.T.N.; Spivey, K.S.; Baggerman, J.O. Zinc Methionine Supplementation Impacts Gene and Protein Expression in Calf-Fed Holstein Steers with Minimal Impact on Feedlot Performance. Biol. Trace Elem. Res. 2016, 171, 315–327. [Google Scholar] [CrossRef]
- Read, W.W.; Harrison, R.A.; Halliday, D. A resin-based method for the preparation of molecular nitrogen for 15N analysis from urinary and plasma components. Anal. Biochem. 1982, 123, 249–254. [Google Scholar] [CrossRef]
- Duggleby, S.L.; Waterlow, J.C. The end-product method of measuring whole-body protein turnover: A review of published results and a comparison with those obtained by leucine infusion. Br. J. Nutr. 2005, 94, 141–153. [Google Scholar] [CrossRef]
- Waterlow, J.C.; Garlick, P.J.; Millward, D.J. General principles of the measurement of whole-body protein turnover. In Protein Turnover in Mammalian Tissues and in the Whole Body; North-Holland Publishing Company: Amersterdam, The Netherlands, 1978; pp. 225–249. ISBN 978-0-444-80021-3. [Google Scholar]
- Stuart, W.D.; Burkey, T.E.; Gabler, N.K.; Schwartz, K.J.; Klein, D.; Dawson, J.A.; Pendleton, A.R.; de Lange, C.F.M. Immune system stimulation (ISS) induced by E. coli lipopolysaccharide (LPS) alters amino acid metabolism in growing pigs. J. Anim. Sci. 2016, 94, 51–52. [Google Scholar] [CrossRef]
- Rakhshandeh, A.; Dekkers, J.C.M.; Kerr, B.J.; Weber, T.E.; English, J.; Gabler, N.K. Effect of immune system stimulation and divergent selection for residual feed intake on digestive capacity of the small intestine in growing pigs. J. Anim. Sci. 2012, 90, 233–235. [Google Scholar] [CrossRef]
- Fuller, M.F.; McWilliam, R.; Wang, T.C.; Giles, L.R. The optimum dietary amino acid pattern for growing pigs. 2. Requirements for maintenance and for tissue protein accretion. Br. J. Nutr. 1989, 62, 255–267. [Google Scholar] [CrossRef]
- McGilvray, W.D.; Klein, D.; Wooten, H.; Dawson, J.A.; Hewitt, D.; Rakhshandeh, A.R.; de Lange, C.F.M.; Rakhshandeh, A. Immune system stimulation induced by Escherichia coli lipopolysaccharide alters plasma free amino acid flux and dietary nitrogen utilization in growing pigs. J. Anim. Sci. 2019, 97, 315–326. [Google Scholar] [CrossRef]
- De Backer, D. Lactic acidosis. Intensive Care Med. 2003, 29, 699–702. [Google Scholar] [CrossRef]
- Goyette, R.E.; Key, N.S.; Ely, E.W. Hematologic changes in sepsis and their therapeutic implications. Semin. Respir. Crit. Care Med. 2004, 25, 645–659. [Google Scholar] [CrossRef]
- Delmastro-Greenwood, M.M.; Piganelli, J.D. Changing the energy of an immune response. Am. J. Clin. Exp. Immunol. 2013, 2, 30–54. [Google Scholar]
- Bruins, M.J.; Soeters, P.B.; Deutz, N.E. Endotoxemia affects organ protein metabolism differently during prolonged feeding in pigs. J. Nutr. 2000, 130, 3003–3013. [Google Scholar] [CrossRef]
- Mosoni, L.; Patureau Mirand, P.; Houlier, M.L.; Arnal, M. Age-related changes in protein synthesis measured in vivo in rat liver and gastrocnemius muscle. Mech. Ageing Dev. 1993, 68, 209–220. [Google Scholar] [CrossRef]
- Mulvaney, D.R.; Merkel, R.A.; Bergen, W.G. Skeletal muscle protein turnover in young male pigs. J. Nutr. 1985, 115, 1057–1064. [Google Scholar] [CrossRef]
- Hosten, A.O. BUN and creatinine. In Clinical Methods: The History, Physical, and Laboratory Examinations; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990; pp. 874–878. [Google Scholar]
- Pitts, R.F.; Pilkington, L.A. The relation between plasma concentrations of glutamine and glycine and utilization of their nitrogens as sources of urinary ammonia. J. Clin. Investig. 1966, 45, 86–93. [Google Scholar] [CrossRef]
- Orellana, R.A.; O’Connor, P.M.J.; Nguyen, H.V.; Bush, J.A.; Suryawan, A.; Thivierge, M.C.; Fiorotto, M.L.; Davis, T.A. Endotoxemia reduces skeletal muscle protein synthesis in neonates. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E909–E916. [Google Scholar] [CrossRef]
- Kammoun, M.; Cassar-Malek, I.; Meunier, B.; Picard, B. A simplified immunohistochemical classification of skeletal muscle fibres in mouse. Eur. J. Histochem. 2014, 58, 2254. [Google Scholar] [CrossRef]
- Pette, D.; Staron, R.S. Transitions of muscle fiber phenotypic profiles. Histochem. Cell Biol. 2001, 115, 359–372. [Google Scholar]
- Fazarinc, G.; Vrecl, M.; Škorjanc, D.; Čehovin, T.; Čandek-Potokar, M. Dynamics of myosin heavy chain isoform transition in the longissimus muscle of domestic and wild pigs during growth: A comparative study. Animal 2017, 11, 164–174. [Google Scholar] [CrossRef]
- Kim, G.-D.; Kim, B.-W.; Jeong, J.-Y.; Hur, S.-J.; Cho, I.-C.; Lim, H.-T.; Joo, S.-T. Relationship of Carcass Weight to Muscle Fiber Characteristics and Pork Quality of Crossbred (Korean Native Black Pig × Landrace) F2 Pigs. Food Bioprocess Technol. 2013, 6, 522–529. [Google Scholar] [CrossRef]
- Davis, T.A.; Fiorotto, M.L.; Beckett, P.R.; Burrin, D.G.; Reeds, P.J.; Wray-Cahen, D.; Nguyen, H.V. Differential effects of insulin on peripheral and visceral tissue protein synthesis in neonatal pigs. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E770–E779. [Google Scholar] [CrossRef]
- Kominsky, D.J.; Campbell, E.L.; Colgan, S.P. Metabolic shifts in immunity and inflammation. J. Immunol. 2010, 184, 4062–4068. [Google Scholar] [CrossRef]
- Jacobi, S.K.; Gabler, N.K.; Ajuwon, K.M.; Davis, J.E.; Spurlock, M.E. Adipocytes, myofibers, and cytokine biology: New horizons in the regulation of growth and body composition. J. Anim. Sci. 2006, 84 (Suppl. 13), E140–E149. [Google Scholar] [CrossRef]
- Joo, S.T.; Kim, G.D.; Hwang, Y.H.; Ryu, Y.C. Control of fresh meat quality through manipulation of muscle fiber characteristics. Meat Sci. 2013, 95, 828–836. [Google Scholar] [CrossRef]
- Larzul, C.; Lefaucheur, L.; Ecolan, P.; Gogué, J.; Talmant, A.; Sellier, P.; Le Roy, P.; Monin, G. Phenotypic and genetic parameters for longissimus muscle fiber characteristics in relation to growth, carcass, and meat quality traits in large white pigs. J. Anim. Sci. 1997, 75, 3126–3137. [Google Scholar] [CrossRef]
- Berri, C.; Le Bihan-Duval, E.; Debut, M.; Santé-Lhoutellier, V.; Baéza, E.; Gigaud, V.; Jégo, Y.; Duclos, M.J. Consequence of muscle hypertrophy on characteristics of Pectoralis major muscle and breast meat quality of broiler chickens. J. Anim. Sci. 2007, 85, 2005–2011. [Google Scholar] [CrossRef]
| Ingredients and Nutrients Composition | Treatment | |
|---|---|---|
| ISS− | ISS+ | |
| Ingredient composition, (g/kg as-fed basis) | ||
| Corn | 740 | 788 |
| Soybean meal | 206 | 159 |
| Hydrogenated vegetable fat | 10 | 10 |
| Lysine HCl | 4.1 | 3.8 |
| DL-Methionine | 1.0 | 0.73 |
| L-Threonine | 0.34 | 0.38 |
| L-Tryptophan | 0.2 | 0.25 |
| Limestone | 8.8 | 8.3 |
| Dicalcium phosphate | 7.9 | 7.4 |
| Salt | 5 | 5 |
| Vitamin and mineral premix | 15 | 15 |
| Titanium dioxide | 2.5 | 2.5 |
| Calculated nutrient contents (g/kg as-fed basis) | ||
| Metabolizable energy MJ/kg | 14.0 | 14.0 |
| Cp (N × 6.25) § | 140 | 121 |
| Lysine | 10.1 | 8.8 |
| Methionine | 3.3 | 2.9 |
| Methionine + Cysteine | 5.7 | 5.0 |
| Threonine | 5.1 | 4.5 |
| Tryptophan | 1.7 | 1.5 |
| Leucine | 12.9 | 11.8 |
| Isoleucine | 5.8 | 5.0 |
| Valine | 6.6 | 5.8 |
| Phenylalanine | 6.9 | 6.1 |
| Calcium | 6.1 | 5.6 |
| STTD P * | 2.9 | 2.7 |
| Analyzed Cp and AA contents (g/kg as-fed basis) § | ||
| Cp (N × 6.25) | 160 | 141 |
| Lysine | 11.6 | 10.3 |
| Methionine | 3.3 | 2.9 |
| Methionine + Cysteine | 6.2 | 5.4 |
| Threonine | 6.5 | 5.6 |
| Tryptophan | 2.0 | 1.7 |
| Leucine | 14.5 | 13.2 |
| Isoleucine | 7.0 | 6.0 |
| Valine | 7.5 | 6.6 |
| Phenylalanine | 7.9 | 6.9 |
| Measures | Health Status | SE | p-Value | |
|---|---|---|---|---|
| ISS− | ISS+ | |||
| Animals, n | 5 | 7 | ||
| Final BW, kg BW0.60 | 8.64 | 8.14 | 0.083 | 0.018 |
| N intake, g/kg BW0.60/day | 2.84 | 2.00 | 0.077 | 0.001 |
| N excretion, g/kg BW 0.60/day | 0.59 | 0.58 | 0.070 | 0.917 |
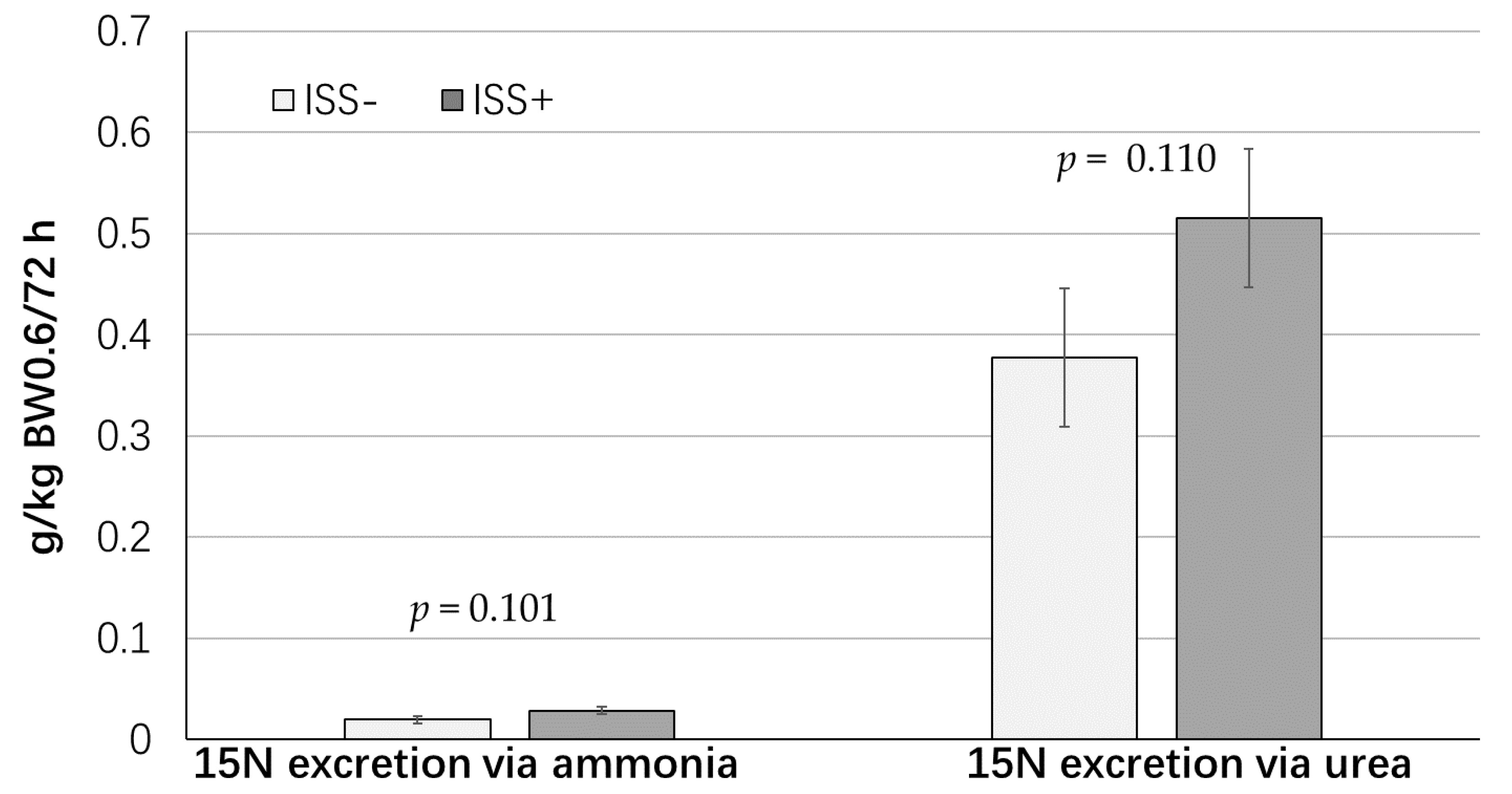
| N excretion via ammonia, g/kg BW0.60/day | 0.05 | 0.04 | 0.009 | 0.814 |
| N excretion via urea, g/kg BW0.60/day | 0.51 | 0.56 | 0.054 | 0.413 |
| 15N administered, mg/kg BW0.60 | 7.10 | 6.79 | 0.180 | 0.168 |
| N Flux 2, g/kg BW0.60/day | 12.20 | 8.65 | 0.276 | 0.001 |
| Protein synthesis, g N/kg BW0.60/day | 11.63 | 8.10 | 0.277 | 0.001 |
| Protein degradation, g N/kg BW0.60/day | 9.40 | 6.71 | 0.319 | 0.001 |
| Protein retention 3, g N/kg BW0.60/day | 2.28 | 1.42 | 0.130 | 0.001 |
| PD 4, g/kg BW0.60/day | 2.10 | 1.52 | 0.151 | 0.017 |
| N retention:N intake | 0.80 | 0.69 | 0.040 | 0.033 |
| Protein synthesis: Protein degradation | 1.24 | 1.19 | 0.024 | 0.065 |
| Protein synthesis:Protein retention | 5.12 | 5.87 | 0.240 | 0.055 |
| Muscle Fiber Characteristics | Health Status | SE | p-Value | |||
|---|---|---|---|---|---|---|
| ISS− | ISS+ | ISS | MS | ISS × MS | ||
| Nuclei density, mm2 | ||||||
| LD | 1134 | 995 | 76.7 | 0.018 | 0.001 | 0.001 |
| PM | 1367 a | 1076 b | 54.2 | |||
| ST | 1110 | 1109 | 78.0 | |||
| SV | 1065 | 1060 | 34.8 | |||
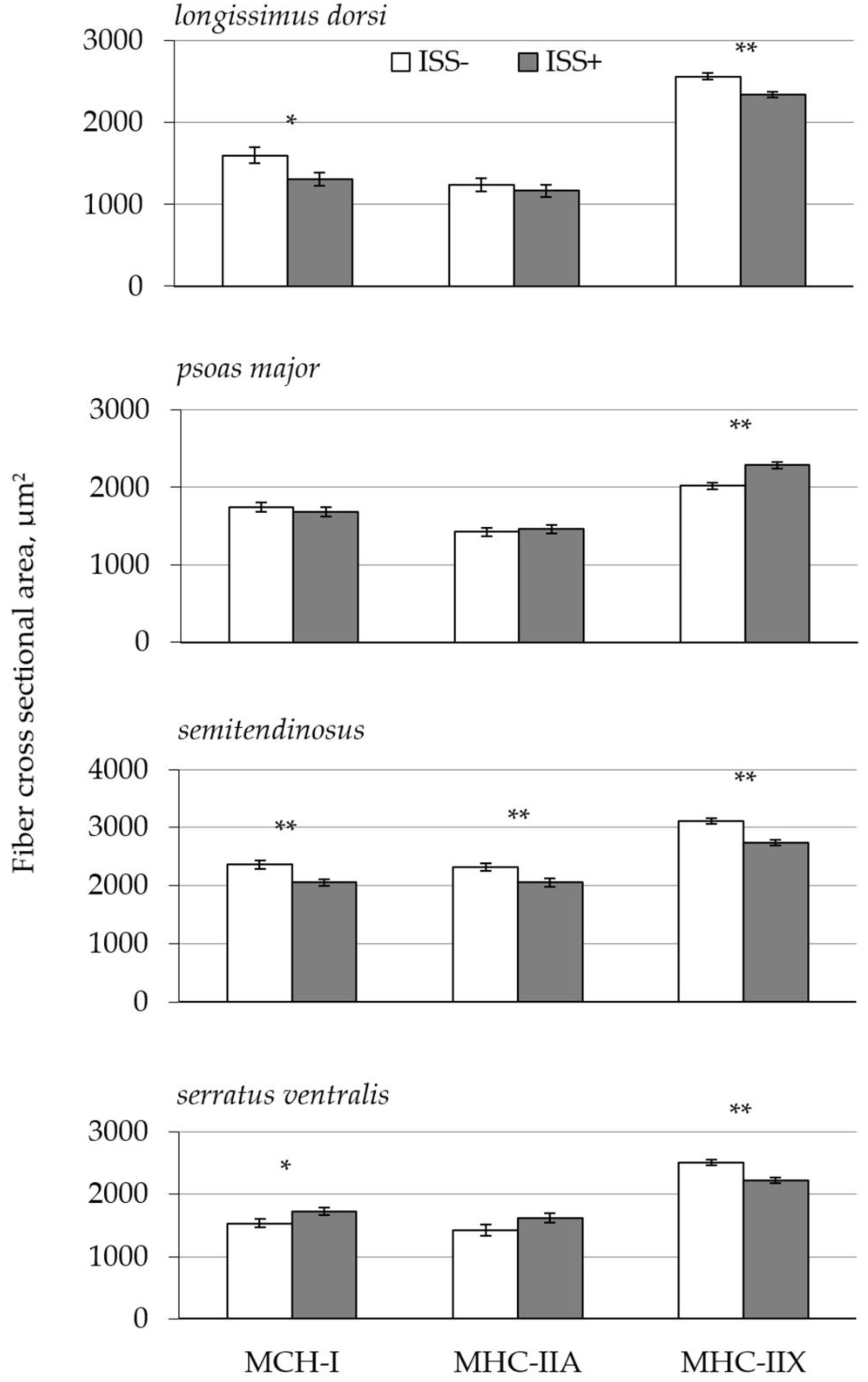
| MHC-I, % | ||||||
| LD | 8.9 b | 12.7 a | 1.10 | 0.001 | 0.001 | 0.034 |
| PM | 21.0 | 24.6 | 2.42 | |||
| ST | 20.8 | 26.0 | 4.88 | |||
| SV | 16.6 b | 28.9 a | 2.32 | |||
| MHC-IIA, % | ||||||
| LD | 17.0 | 16.5 | 1.80 | 0.048 | 0.001 | 0.160 |
| PM | 27.7 | 23.1 | 2.78 | |||
| ST | 25.7 | 18.9 | 2.41 | |||
| SV | 12.2 | 13.0 | 2.00 | |||
| MHC-IIX, % | ||||||
| LD | 72.7 a | 68.1 b | 1.53 | 0.035 | 0.01 | 0.051 |
| PM | 50.3 | 47.1 | 4.58 | |||
| ST | 52.2 | 52.3 | 6.71 | |||
| SV | 70.6 a | 55.3 b | 3.30 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGilvray, W.D.; Johnson, B.; Wooten, H.; Rakhshandeh, A.R.; Rakhshandeh, A. Immune System Stimulation Reduces the Efficiency of Whole-Body Protein Deposition and Alters Muscle Fiber Characteristics in Growing Pigs. Animals 2019, 9, 323. https://doi.org/10.3390/ani9060323
McGilvray WD, Johnson B, Wooten H, Rakhshandeh AR, Rakhshandeh A. Immune System Stimulation Reduces the Efficiency of Whole-Body Protein Deposition and Alters Muscle Fiber Characteristics in Growing Pigs. Animals. 2019; 9(6):323. https://doi.org/10.3390/ani9060323
Chicago/Turabian StyleMcGilvray, Whitney D., Bradley Johnson, Hailey Wooten, Amanda R. Rakhshandeh, and Anoosh Rakhshandeh. 2019. "Immune System Stimulation Reduces the Efficiency of Whole-Body Protein Deposition and Alters Muscle Fiber Characteristics in Growing Pigs" Animals 9, no. 6: 323. https://doi.org/10.3390/ani9060323
APA StyleMcGilvray, W. D., Johnson, B., Wooten, H., Rakhshandeh, A. R., & Rakhshandeh, A. (2019). Immune System Stimulation Reduces the Efficiency of Whole-Body Protein Deposition and Alters Muscle Fiber Characteristics in Growing Pigs. Animals, 9(6), 323. https://doi.org/10.3390/ani9060323





