Cecal Infusion of Sodium Propionate Promotes Intestinal Development and Jejunal Barrier Function in Growing Pigs
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and methods
2.1. Experimental Procedures (Ethic)
2.2. Animals, Experimental Design and Treaments
2.3. Slaughter Procedure and Sampling
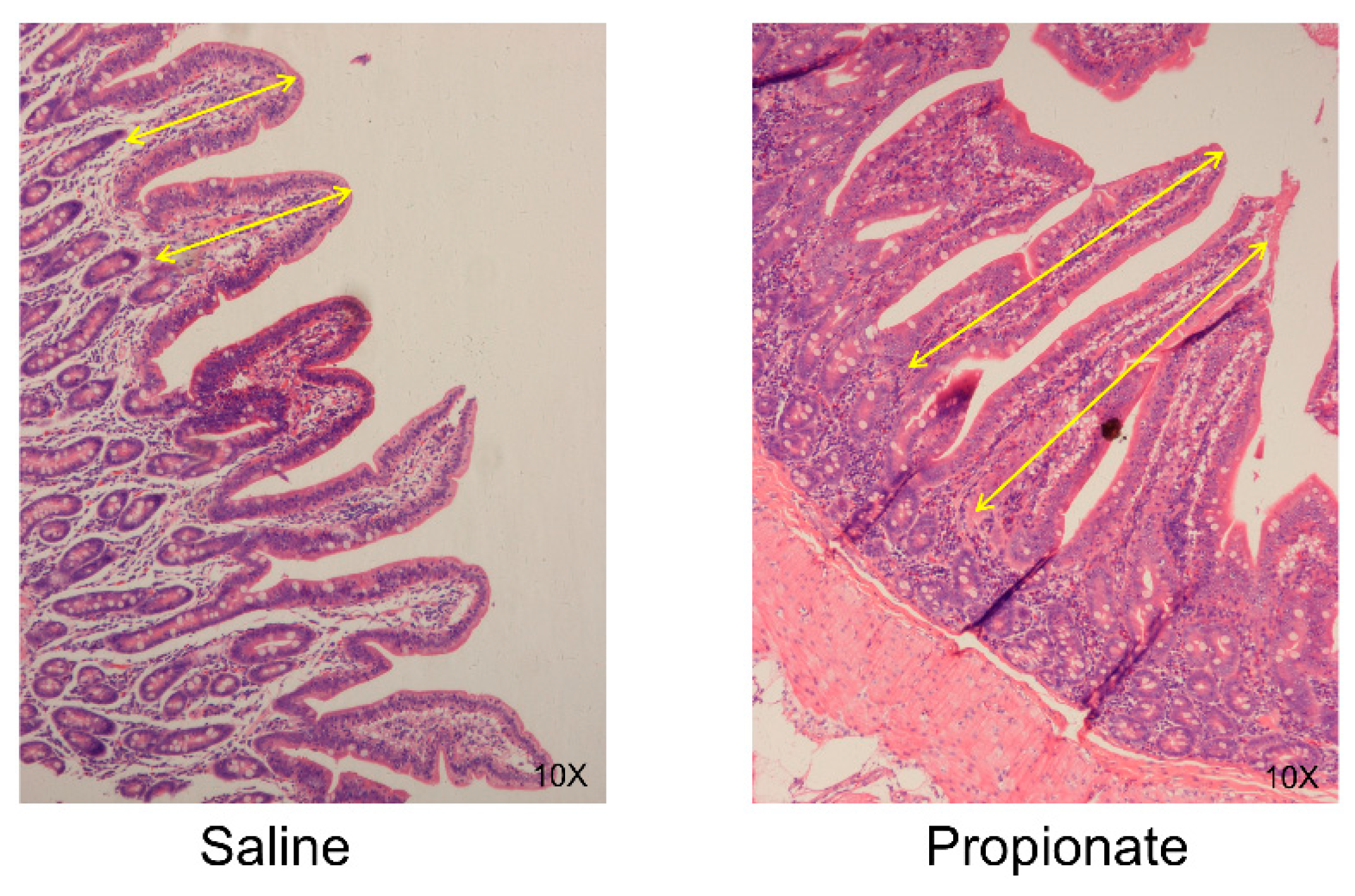
2.4. Intestinal Morphology Analysis
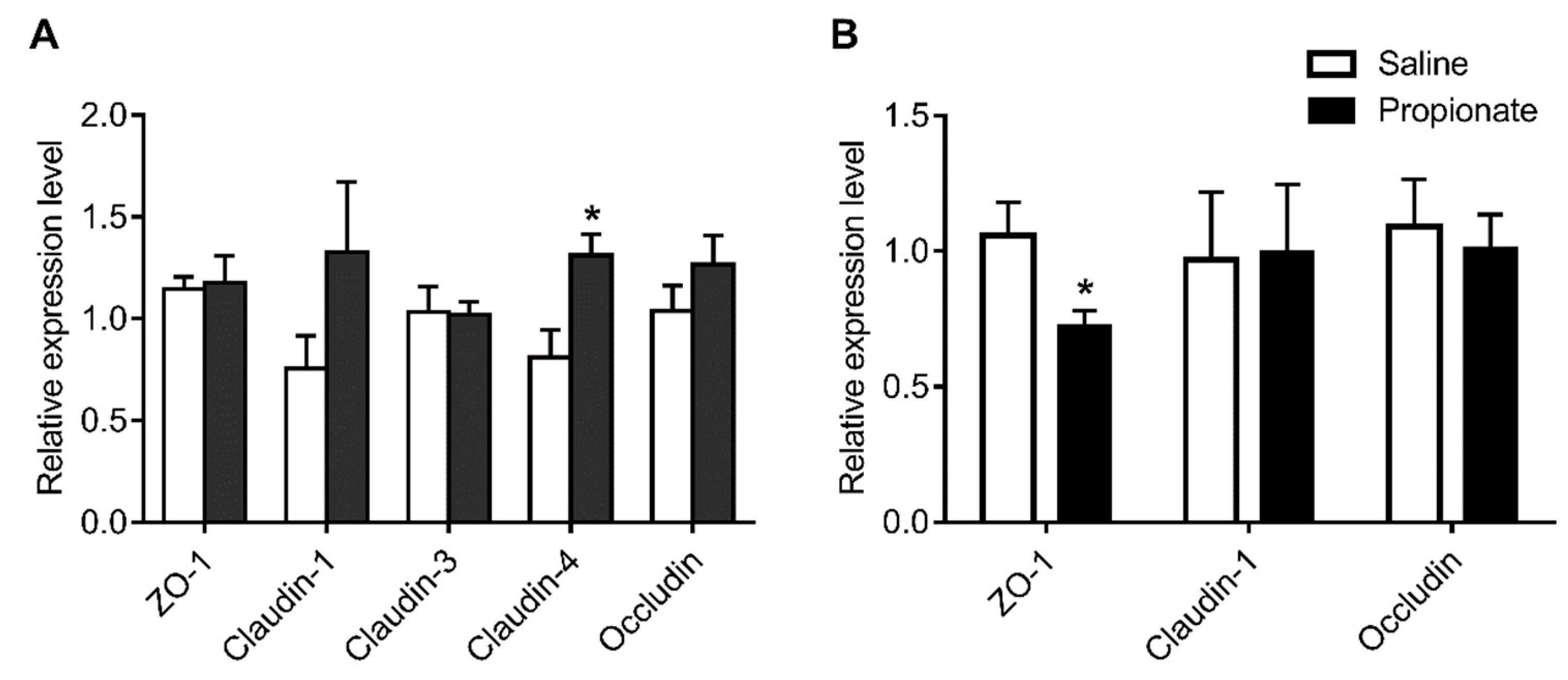
2.5. RNA Isolation and Quantitative RT-PCR
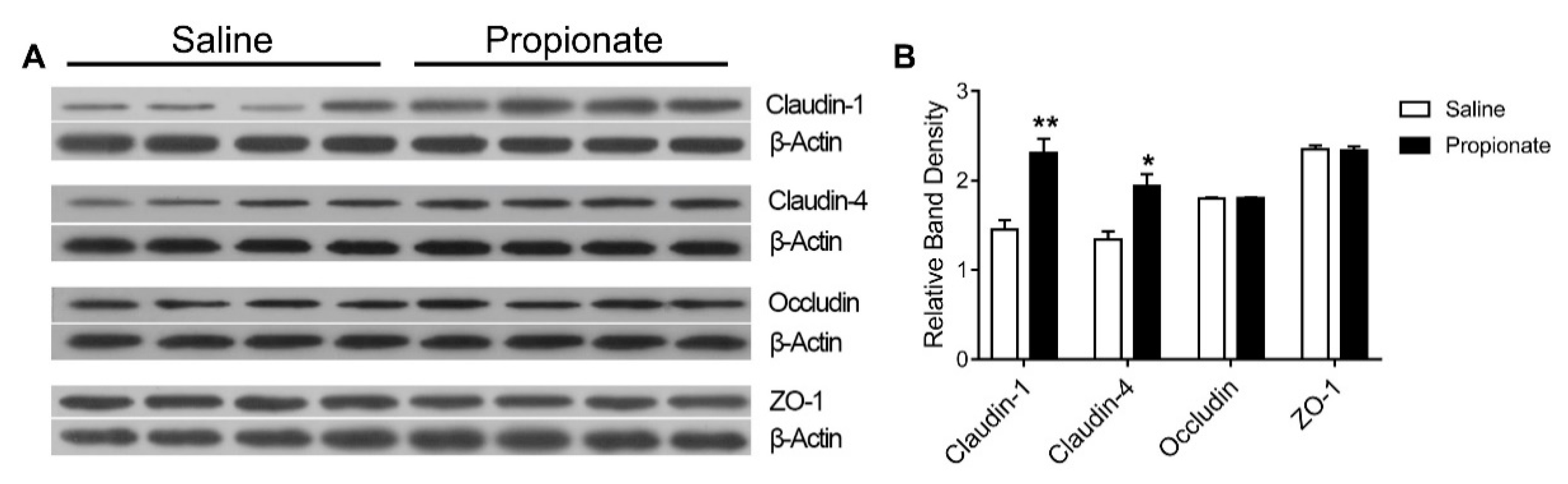
2.6. Total Protein Extraction and Western Blotting
2.7. Statistical Analysis
3. Results
3.1. The Intestinal Length and Intestinal Index
3.2. Intestinal Morphology
3.3. Tight Junction Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Postler, T.S.; Ghosh, S. Understanding the Holobiont: How Microbial Metabolites Affect Human Health and Shape the Immune System. Cell Metab. 2017, 26, 110–130. [Google Scholar] [CrossRef] [Green Version]
- Levy, M.; Blacher, E.; Elinav, E. Microbiome, metabolites and host immunity. Curr. Opin. Microbiol. 2017, 35, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilan, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takagi, T.; Naito, Y.; Higashimura, Y.; Ushiroda, C.; Mizushima, K.; Ohashi, Y.; Yasukawa, Z.; Ozeki, M.; Tokunaga, M.; Okubo, T.; et al. Partially hydrolysed guar gum ameliorates murine intestinal inflammation in association with modulating luminal microbiota and SCFA. Br. J. Nutr. 2016, 116, 1199–1205. [Google Scholar] [CrossRef] [Green Version]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheppach, W. Effects of short chain fatty acids on gut morphology and function. Gut 1994, 35, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Topping, D.L.; Clifton, P.M. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 2001, 81, 1031–1064. [Google Scholar] [CrossRef]
- Devadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kripke, S.A.; Fox, A.D.; Berman, J.M.; Settle, R.G.; Rombeau, J.L. Stimulation of intestinal mucosal growth with intracolonic infusion of short-chain fatty acids. J. Parenter Enter. Nutr. 1989, 13, 109–116. [Google Scholar] [CrossRef]
- Koruda, M.J.; Rolandelli, R.H.; Bliss, D.Z.; Hastings, J.; Rombeau, J.L.; Settle, R.G. Parenteral nutrition supplemented with short-chain fatty acids: Effect on the small-bowel mucosa in normal rats. Am. J. Clin. Nutr. 1990, 51, 685–689. [Google Scholar] [CrossRef]
- Chen, X.; Xu, J.; Su, Y.; Zhu, W. Effects of Intravenous Infusion With Sodium Butyrate on Colonic Microbiota, Intestinal Development-and Mucosal Immune-Related Gene Expression in Normal Growing Pigs. Front. Microbiol. 2018, 9, 1652. [Google Scholar] [CrossRef]
- Kien, C.L.; Blauwiekel, R.; Bunn, J.Y.; Jetton, T.L.; Frankel, W.L.; Holst, J.J. Cecal infusion of butyrate increases intestinal cell proliferation in piglets. J. Nutr. 2007, 137, 916–922. [Google Scholar] [CrossRef]
- Ashida, H.; Ogawa, M.; Kim, M.; Mimuro, H.; Sasakawa, C. Bacteria and host interactions in the gut epithelial barrier. Nat. Chem. Biol. 2011, 8, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Guilloteau, P.; Martin, L.; Eeckhaut, V.; Ducatelle, R.; Zabielski, R.; Van Immerseel, F. From the gut to the peripheral tissues: The multiple effects of butyrate. Nutr. Res. Rev. 2010, 23, 366–384. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Kim, C.Y.; Kaur, A.; Lamothe, L.; Shaikh, M.; Keshavarzian, A.; Hamaker, B.R. Dietary fibre-based SCFA mixtures promote both protection and repair of intestinal epithelial barrier function in a Caco-2 cell model. Food Funct. 2017, 8, 1166–1173. [Google Scholar] [CrossRef]
- Diao, H.; Jiao, A.R.; Yu, B.; He, J.; Yu, J.; Zheng, P.; Huang, Z.Q.; Luo, Y.H.; Luo, J.Q.; Mao, X.B. Stimulation of intestinal growth with distal ileal infusion of short-chain fatty acid: A reevaluation in a pig model. RSC Adv. 2017, 7, 30792–30806. [Google Scholar] [CrossRef]
- Feng, W.; Wu, Y.; Chen, G.; Fu, S.; Li, B.; Huang, B.; Wang, D.; Wang, W.; Liu, J. Sodium Butyrate Attenuates Diarrhea in Weaned Piglets and Promotes Tight Junction Protein Expression in Colon in a GPR109A-Dependent Manner. Cell. Physiol Biochem. 2018, 47, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Ajuwon, K.M. Butyrate modifies intestinal barrier function in IPEC-J2 cells through a selective upregulation of tight junction proteins and activation of the Akt signaling pathway. PLoS ONE 2017, 12. [Google Scholar] [CrossRef]
- Xia, Z.; Han, Y.; Wang, K.; Guo, S.; Wu, D.; Huang, X.; Li, Z.; Zhu, L. Oral administration of propionic acid during lactation enhances the colonic barrier function. Lipids Health Dis. 2017, 16, 62. [Google Scholar] [CrossRef]
- Tong, L.C.; Wang, Y.; Wang, Z.B.; Liu, W.Y.; Sun, S.; Li, L.; Su, D.F.; Zhang, L.C. Propionate Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress. Front. Pharmacol. 2016, 7, 253. [Google Scholar] [CrossRef] [PubMed]
- Sauer, W.C.; Jørgensen, H.; Berzins, R. A modified nylon bag technique for determining apparent digestibilitie. Can. J. Anim. Sci. 1983, 63, 233–237. [Google Scholar] [CrossRef]
- Li, S.; Sauer, W.C.; Fan, M.Z. The Effect of Dietary Crude Protein Level on Ileal and Fecal Amino-Acid Digestibility in Early-Weaned Pigs. J. Anim. Physiol. Anim. Nutr. 1993, 70, 117–128. [Google Scholar] [CrossRef]
- Gao, K.; Pi, Y.; Mu, C.L.; Peng, Y.; Huang, Z.; Zhu, W.Y. Antibiotics-induced modulation of large intestinal microbiota altered aromatic amino acid profile and expression of neurotransmitters in the hypothalamus of piglets. J. Neurochem. 2018, 146, 219–234. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yu, K.; Chen, H.; Su, Y.; Zhu, W. Caecal infusion of the short-chain fatty acid propionate affects the microbiota and expression of inflammatory cytokines in the colon in a fistula pig model. Microb. Biotechnol. 2018, 11, 859–868. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Reynolds, L.P.; Redmer, D.A.; Caton, J.S.; Crenshaw, J.D. Effects of dietary fiber on intestinal growth, cell proliferation, and morphology in growing pigs. J. Anim. Sci. 1994, 72, 2270–2278. [Google Scholar] [CrossRef]
- Wosten, M.M.; van Dijk, L.; Parker, C.T.; Guilhabert, M.R.; van der Meer-Janssen, Y.P.; Wagenaar, J.A.; van Putten, J.P. Growth phase-dependent activation of the DccRS regulon of Campylobacter jejuni. J. Bacteriol. 2010, 192, 2729–2736. [Google Scholar] [CrossRef]
- Wang, L.N.; Chen, X.L.; Li, X.G.; Shu, G.; Yan, H.C.; Wang, X.Q. Evaluation of adrenocorticotropin regulated glucocorticoid synthesis pathway in adrenal of different breeds of pigs. Livest. Sci. 2014, 169, 185–191. [Google Scholar] [CrossRef]
- Zhou, X.L.; Kong, X.F.; Lian, G.Q.; Blachier, F.; Geng, M.M.; Yin, Y.L. Dietary supplementation with soybean oligosaccharides increases short-chain fatty acids but decreases protein-derived catabolites in the intestinal luminal content of weaned Huanjiang mini-piglets. Nutr. Res. 2014, 34, 780–788. [Google Scholar] [CrossRef]
- Liu, W.; Mi, S.; Ruan, Z.; Li, J.; Shu, X.; Yao, K.; Jiang, M.; Deng, Z. Dietary Tryptophan Enhanced the Expression of Tight Junction Protein ZO-1 in Intestine. J. Food Sci. 2017, 82, 562–567. [Google Scholar] [CrossRef]
- Sakata, T. Stimulatory Effect of Short-Chain Fatty-Acids on Epithelial-Cell Proliferation in the Rat Intestine —A Possible Explanation for Trophic Effects of Fermentable Fiber, Gut Microbes and Luminal Trophic Factors. Br. J. Nutr. 1987, 58, 95–103. [Google Scholar] [CrossRef]
- Kaiko, G.E.; Ryu, S.H.; Koues, O.I.; Collins, P.L.; Solnica-Krezel, L.; Pearce, E.J.; Pearce, E.L.; Oltz, E.M.; Stappenbeck, T.S. The Colonic Crypt Protects Stem Cells from Microbiota-Derived Metabolites. Cell 2016, 165, 1708–1720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.; Kotani, T.; Konno, T.; Setiawan, J.; Kitamura, Y.; Imada, S.; Usui, Y.; Hatano, N.; Shinohara, M.; Saito, Y.; et al. Promotion of Intestinal Epithelial Cell Turnover by Commensal Bacteria: Role of Short-Chain Fatty Acids. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Scott, K.P.; Duncan, S.H.; Flint, H.J. Understanding the effects of diet on bacterial metabolism in the large intestine. J. Appl. Microbiol. 2007, 102, 1197–1208. [Google Scholar] [CrossRef] [PubMed]
- Sakata, T.; Yajima, T. Influence of short chain fatty acids on the epithelial cell division of digestive tract. Q. J. Exp. Physiol. 1984, 69, 639–648. [Google Scholar] [CrossRef]
- Marsman, K.E.; McBurney, M.I. Dietary fiber and short-chain fatty acids affect cell proliferation and protein synthesis in isolated rat colonocytes. J. Nutr. 1996, 126, 1429–1437. [Google Scholar] [CrossRef]
- Frankel, W.L.; Zhang, W.; Singh, A.; Klurfeld, D.M.; Don, S.; Sakata, T.; Modlin, I.; Rombeau, J.L. Mediation of the trophic effects of short-chain fatty acids on the rat jejunum and colon. Gastroenterology 1994, 106, 375. [Google Scholar] [CrossRef]
- Reilly, K.J.; Frankel, W.L.; Bain, A.M.; Rombeau, J.L. Colonic short chain fatty acids mediate jejunal growth by increasing gastrin. Gut 1995, 37, 81–86. [Google Scholar] [CrossRef]
- Desmoulin, F.; Canioni, P.; Cozzone, P.J. Glutamate-glutamine metabolism in the perfused rat liver: 13 C-NMR study using (2–13 C)-enriched acetate. FEBS Lett. 1985, 185, 29–32. [Google Scholar] [CrossRef]
- Cross, T.A.; Pahl, C.; Oberh?Nsli, R.; Aue, W.P.; Keller, U.; Seelig, J. Ketogenesis in the living rat followed by 13C NMR spectroscopy. Biochemistry 1984, 23, 6398–6402. [Google Scholar] [CrossRef]
- Windmueller, H.G.; Spaeth, A.E. Respiratory fuels and nitrogen metabolism in vivo in small intestine of fed rats. Quantitative importance of glutamine, glutamate, and aspartate. J. Biol. Chem. 1980, 255, 107–112. [Google Scholar] [PubMed]
- Tome, D. The Roles of Dietary Glutamate in the Intestine. Ann. Nutr. Metab. 2018, 73, 15–20. [Google Scholar] [CrossRef]
- Larsson, L.I.; Rehfeld, J.F. Distribution of gastrin and CCK cells in the rat gastrointestinal tract. Evidence for the occurrence of three distinct cell types storing COOH-terminal gastrin immunoreactivity. Histochemistry 1978, 58, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Moghimzadeh, E.; Ekman, R.; Hakanson, R.; Yanaihara, N.; Sundler, F. Neuronal gastrin-releasing peptide in the mammalian gut and pancreas. Neuroscience 1983, 10, 553–563. [Google Scholar] [CrossRef]
- Hung, T.V.; Suzuki, T. Dietary Fermentable Fiber Reduces Intestinal Barrier Defects and Inflammation in Colitic Mice. J. Nutr. 2016, 146, 1970–1979. [Google Scholar] [CrossRef]
| Genes | Forward Primer (5’-3’) | Reverse Primer (5’-3’) | Reference |
|---|---|---|---|
| β-actin | F:CCACGAAACTACCTTCAACTC | R:TGATCTCCTTCTGCATCCTGT | [28] |
| ZO-1 | F: GAGGATGGTCACCGTGGT | R: GGAGGATGCTGTTGTCTCGG | [29] |
| Claudin-1 | F: AGATTTACTCCTACGCTGGT | R: GCACCTCATCATCTTCCAT | [30] |
| Claudin-3 | F:CCTACGACCGCAAGGACTAC | R:GACTGGTCTCGGATGCAAGG | [30] |
| Claudin-4 | F:CGTACCGACAAGCCCTACTC | R:GCAGTCCAGGGAGAAACCAA | [30] |
| Occludin | F:ATGCTTTCTCAGCCAGCGTA | R:AAGGTTCCATAGCCTCTCGGTC | [29] |
| Item | Saline | Propionate | p-Value |
|---|---|---|---|
| Jejunum (cm) | 752.50 ± 49.05 | 795.63 ± 47.69 | 0.54 |
| Ileum (cm) | 719.38 ± 65.67 | 712.50 ± 71.21 | 0.94 |
| Colon (cm) | 227.50 ± 8.40 | 258.13 ± 8.45 | 0.02 |
| Item | Saline | Propionate | p-Value |
|---|---|---|---|
| Relative weight of stomach (%) | 0.66 ± 0.02 | 0.68 ± 0.03 | 0.54 |
| Relative weight of Ileum (%) | 1.61 ± 0.12 | 1.52 ± 0.14 | 0.60 |
| Relative weight of cecum (%) | 0.23 ± 0.03 | 0.19 ± 0.01 | 0.25 |
| Relative weight of colon (%) | 1.29 ± 0.07 | 1.35 ± 0.08 | 0.57 |
| Item | Saline | Propionate | p-Value |
|---|---|---|---|
| Jejunum | |||
| Villi length (µm) | 365.56 ± 24.87 | 524.75 ± 40.95 | 0.03 |
| Crypt depth (µm) | 207.18 ± 54.58 | 170.03 ± 11.07 | 0.57 |
| Villi/Crypt | 1.80 ± 0.50 | 3.10 ± 0.40 | 0.04 |
| Ileum | |||
| Villi length (µm) | 355.79 ± 83.27 | 456.43 ± 13.41 | 0.44 |
| Crypt depth (µm) | 132.51 ± 53.40 | 154.95 ± 35.61 | 0.74 |
| Villi/Crypt | 3.50 ± 2.04 | 3.41 ± 1.02 | 0.97 |
| Colon | |||
| Mucosa thickness (µm) | 439.20 ± 30.83 | 423.24 ± 11.13 | 0.64 |
| Crypt depth (µm) | 204.42 ± 21.42 | 168.70 ± 25.00 | 0.30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Chen, H.; Zhu, W.; Yu, K. Cecal Infusion of Sodium Propionate Promotes Intestinal Development and Jejunal Barrier Function in Growing Pigs. Animals 2019, 9, 284. https://doi.org/10.3390/ani9060284
Zhang Y, Chen H, Zhu W, Yu K. Cecal Infusion of Sodium Propionate Promotes Intestinal Development and Jejunal Barrier Function in Growing Pigs. Animals. 2019; 9(6):284. https://doi.org/10.3390/ani9060284
Chicago/Turabian StyleZhang, Yanan, Huizi Chen, Weiyun Zhu, and Kaifan Yu. 2019. "Cecal Infusion of Sodium Propionate Promotes Intestinal Development and Jejunal Barrier Function in Growing Pigs" Animals 9, no. 6: 284. https://doi.org/10.3390/ani9060284
APA StyleZhang, Y., Chen, H., Zhu, W., & Yu, K. (2019). Cecal Infusion of Sodium Propionate Promotes Intestinal Development and Jejunal Barrier Function in Growing Pigs. Animals, 9(6), 284. https://doi.org/10.3390/ani9060284





