Finding Meanings in Low Dimensional Structures: Stochastic Neighbor Embedding Applied to the Analysis of Indri indri Vocal Repertoire
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Acoustical Analysis
2.3. Acoustic Embedding and Classification Procedure
3. Results
3.1. t-SNE Mapping
3.2. Call Recognition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Valletta, J.J.; Torney, C.; Kings, M.; Thornton, A.; Madden, J. Applications of machine learning in animal behaviour studies. Anim. Behav. 2017, 124, 203–220. [Google Scholar] [CrossRef]
- Zheng, J.; Qiu, H.; Xu, X.; Wang, W.; Huang, Q. Fast Discriminative Stochastic Neighbor Embedding Analysis. Comput. Math. Methods Med. 2013, 2013, 106867. [Google Scholar] [CrossRef]
- Piles, M.; Díez, J.; del Coz, J.J.; Montañés, E.; Quevedo, J.R.; Ramon, J.; Rafel, O.; López-Béjar, M.; Tusell, L. Predicting fertility from seminal traits: Performance of several parametric and non-parametric procedures. Livest Sci. 2013, 155, 137–147. [Google Scholar] [CrossRef]
- Cox, M.A.; Cox, T.F. Multidimensional Scaling; Chapman & Hall/CRC: Boca Raton, FL, USA, 2001. [Google Scholar]
- Jolliffe, I.T. Principal Component Analysis; Springer: New York, NY, USA, 1986. [Google Scholar] [CrossRef]
- Tenenbaum, J.B.; Silva, V.; Langford, J.C. A Global Geometric Framework for Nonlinear Dimensionality Reduction. Science 2000, 290, 2319. [Google Scholar] [CrossRef]
- Cook, J.A.; Sutskever, I.; Mnih, A.; Hinton, G.E. Visualizing similarity data with a mixture of maps. In Proceedings of the 11th International Conference on Artificial Intelligence and Statistics, San Juan, Puerto Rico, 21–24 March 2007; pp. 67–74. [Google Scholar]
- Hinton, G.; Roweis, S. Stochastic neighbor embedding. Adv. Neural Inf. Process. Syst. 2002, 15, 833–840. [Google Scholar]
- Roweis, S.T.; Saul, L.K. Nonlinear Dimensionality Reduction by Locally Linear Embedding. Science 2000, 290, 2323. [Google Scholar] [CrossRef]
- Van der Maaten, L.J.P.; Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 2008, 9, 2579–2605. [Google Scholar]
- Van der Maaten, L.J.P.; Postma, E.O. Proceedings of the SPIE Optical Engineering and Applications. Tescher, A., Ed.; The International Society for Optical Engineering: San Diego, CA, USA, 2010; Volume 7798. [Google Scholar]
- Platzer, A. Visualization of SNPs with t-SNE. PLoS ONE 2013, 8, e56883. [Google Scholar] [CrossRef] [PubMed]
- Jamieson, A.R.; Giger, M.L.; Drukker, K.; Li, H.; Yuan, Y.; Bhooshan, N. Exploring nonlinear feature space dimension reduction and data representation in breast CADx with Laplacian Eigenmaps and t-SNE. Med. Phys. 2010, 37, 339–351. [Google Scholar] [CrossRef] [PubMed]
- Acuff, N.V.; Linden, J. Using Visualization of t-Distributed Stochastic Neighbor Embedding to Identify Immune Cell Subsets in Mouse Tumors. J. Immunol. 2017, 198, 4539–4546. [Google Scholar] [CrossRef]
- Bittner, R.M.; Salamon, J.; Bosch, J.J.; Bello, J.P. Pitch Contours as a Mid-Level Representation for Music Informatics. In Proceedings of the Audio Engineering Society Conference: 2017 AES International Conference on Semantic Audio, Erlangen, Germany, 22–24 June 2017. [Google Scholar]
- Cho, K.; van Merriënboer, B.; Gulcehre, C.; Bougares, F.; Schwenk, H.; Bahdanau, D.; Bengio, Y. Learning Phrase Representations using RNN Encoder–Decoder for Statistical Machine Translation. In Proceedings of the Conference on Empirical Methods in Natural Language Processing (EMNLP), Doha, Qatar, 25–29 October 2014; pp. 1724–1734. [Google Scholar]
- Hamel, P.; Eck, D. Learning features from music audio with deep belief networks. In Proceedings of the 11th International Society for Music Information Retrieval Conference (ISMIR 2010), Utrecht, The Netherlands, 9–13 August 2010. [Google Scholar]
- Panteli, M.; Bittner, R.; Bello, J.P.; Dixon, S. Towards the characterization of singing styles in world music. In Proceedings of the IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), New Orleans, LA, USA, 5–9 March 2017. [Google Scholar]
- Charan, R.; Manisha, A.; Karthik, R.; Kumar, R.M. A text-independent speaker verification model: A comparative analysis. In Proceedings of the IEEE International Conference on Intelligent Computing and Control (I2C2), Tamil Nadu, India, 23–24 June 2017. [Google Scholar]
- Berman, G.J.; Choi, D.M.; Bialek, W.; Shaevitz, J.W. Mapping the stereotyped behaviour of freely moving fruit flies. J. R. Soc. Interface 2014, 11, 20140672. [Google Scholar] [CrossRef]
- Gamba, M.; Friard, O.; Riondato, I.; Righini, R.; Colombo, C.; Miaretsoa, L.; Torti, V.; Nadhurou, B.; Giacoma, C. Comparative analysis of the vocal repertoire of Eulemur: A dynamic time warping approach. Int. J. Primatol. 2015, 36, 894–910. [Google Scholar] [CrossRef]
- Green, S. Communication by a graded vocal system in Japanese monkeys. In Primate Behaviour; Rosenblum, L.A., Ed.; Academic Press: New York, NY, USA, 1975; pp. 1–102. [Google Scholar]
- Hammerschmidt, K.; Fischer, J. The vocal repertoire of Barbary macaques: A quantitative analysis of a graded signal system. Ethology 1998, 104, 203–216. [Google Scholar] [CrossRef]
- Zuberbühler, K.; Noë, R.; Seyfarth, R.M. Diana monkey long-distance calls: Messages for conspecifics and predators. Anim. Behav. 1997, 53, 589–604. [Google Scholar] [CrossRef]
- Arnold, K.; Zuberbühler, K. The alarm calling system of adult male putty-nosed monkeys (Cercopithecus nictitans martini). Anim. Behav. 2006, 72, 643–653. [Google Scholar] [CrossRef]
- Marler, P. The structure of animal communication sounds. In Recognition of Complex Acoustic Signals; Bullock, T.H., Evans, E.F., Eds.; Dahlem Konferenzen: Berlin, Germany, 1977; pp. 17–35. [Google Scholar]
- Kenaan, S.; Lemasson, A.; Zuberbühler, K. Graded or discrete? A quantitative analysis of Campbell’s monkey alarm calls. Anim. Behav. 2013, 85, 109–118. [Google Scholar] [CrossRef]
- Peckre, L.; Kappeler, P.M.; Fichtel, C. Clarifying and expanding the social complexity hypothesis for communicative complexity. Behav. Ecol. Sociobiol. 2019, 73, 11. [Google Scholar] [CrossRef]
- Wadewitz, P.; Hammerschmidt, K.; Battaglia, D.; Witt, A.; Wolf, F.; Fischer, J. Characterizing vocal repertoires—Hard vs. soft classification approaches. PLoS ONE 2015, 10, e0125785. [Google Scholar] [CrossRef]
- Bouchet, H.; Blois-Heulin, C.; Lemasson, A. Social complexity parallels vocal complexity: A comparison of three non-human primate species. Front. Psychol. 2013, 4, 390. [Google Scholar] [CrossRef]
- Manser, M.B.; Seyfarth, R.M.; Cheney, D.L. Suricate alarm calls signal predator class and urgency. Trends Cogn. Sci. 2002, 6, 55–57. [Google Scholar] [CrossRef]
- Fischer, J.; Wadewitz, P.; Hammerschmidt, K. Structural variability and communicative complexity in acoustic communication. Anim. Behav. 2016, 134, 229–237. [Google Scholar] [CrossRef]
- McCowan, B. A New Quantitative Technique for Categorizing Whistles Using Simulated Signals and Whistles from Captive Bottlenose Dolphins (Delphinidae, Tursiops truncatus). Ethology 1995, 100, 177–193. [Google Scholar] [CrossRef]
- Snowdon, C.T.; Elowson, A.M.; Roush, R.S. Social influences on vocal development in new world primates. In Social Influences on Vocal Development; Snowdon, C.T., Elowson, A.M., Eds.; Cambridge University Press: Cambridge, UK, 1997; pp. 234–248. [Google Scholar]
- Lemasson, A.; Hausberger, M. Acoustic variability and social significance of calls in female Campbell’s monkeys (Cercopithecus campbelli campbelli). J. Acoust. Soc. Am. 2011, 129, 3341–3352. [Google Scholar] [CrossRef] [PubMed]
- McComb, K.; Semple, S. Coevolution of vocal communication and sociality in primates. Biol. Lett. 2005, 1, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef] [PubMed]
- Boersma, P.; Weenink, D. Praat: Doing Phonetics by Computer [Computer Program]. Version 6.0.28. 2017. Available online: http://www.praat.org/ (accessed on 23 March 2017).
- Maretti, G.; Sorrentino, V.; Finomana, A.; Gamba, M.; Giacoma, C. Not just a pretty song: An overview of the vocal repertoire of Indri indri. J. Anthropol. Sci. 2010, 88, 151–165. [Google Scholar] [PubMed]
- Gamba, M.; Torti, V.; Estienne, V.; Randrianarison, R.M.; Valente, D.; Rovara, P.; Bonadonna, G.; Friard, O.; Giacoma, C. Indris have got rhythm! Timing and pitch variation of a primate song examined between sexes and age Classes. Front. Neurosci. 2016, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Macedonia, J.; Stanger, K. Phylogeny of the Lemuridae Revisited: Evidence from Communication Signals. Folia Primatol. 1994, 63, 1–43. [Google Scholar] [CrossRef]
- Krijthe, J.H. Rtsne: T-Distributed Stochastic Neighbor Embedding Using a Barnes-Hut Implementation. 2015. Available online: https://github.com/jkrijthe/Rtsne (accessed on 8 February 2019).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 23 April 2018).
- MacQueen, J.B. Some Methods for classification and Analysis of Multivariate Observations. In Proceedings of the 5th Berkeley Symposium on Mathematical Statistics and Probability; University of California Press: Berkeley, CA, USA; Los Angeles, CA, USA, 1967; Volume 1, pp. 281–297. [Google Scholar]
- Frank, E.; Hall, M.A.; Witten, I.H. The WEKA Workbench. Online Appendix for Data Mining: Practical Machine Learning Tools and Techniques, 4th ed.; Morgan Kaufmann: Burlington, MA, USA, 2016. [Google Scholar]
- Hornik, K.; Stinchcombe, M.; White, H. Multi-layer feedforward networks are universal approximators. Neural Netw. 1989, 2, 359–366. [Google Scholar] [CrossRef]
- Schalkoff, R. Pattern Recognition: Statistical, Structural and Neural Approaches; John Wiley & Sons: New York, NY, USA, 1992; p. 364. [Google Scholar]
- Sueur, J.; Aubin, T.; Simonis, C. Seewave: A free modular tool for sound analysis and synthesis. Bioacoustics 2008, 18, 213–226. [Google Scholar] [CrossRef]
- Sueur, J. What Is Sound? In Sound Analysis and Synthesis with R; Use R! Series; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Pozzi, L.; Gamba, M.; Giacoma, C. The Use of Artificial Neural Networks to Classify Primate Vocalizations: A Pilot Study on Black Lemurs. Am. J. Primatol. 2010, 72, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Mosavi, M.R.; Khishe, M.; Naseri, M.J.; Parvizi, G.R.; Ayat, M. Multi-Layer Perceptron Neural Network Utilizing Adaptive Best-Mass Gravitational Search Algorithm to Classify Sonar Dataset. Arch. Acoust. 2019, 44, 137–151. [Google Scholar]
- Petter, J.J.; Charles-Dominique, P. Vocal communication in prosimians. In The Study of Prosimian Behaviour; Doyle, G.A., Martin, R.D., Eds.; New York Academic Press: New York, NY, USA, 1979; pp. 272–282. [Google Scholar]
- Bouchet, H.; Blois-Heulin, C.; Pellier, A.S.; Zuberbühler, K.; Lemasson, A. Acoustic variability and individual distinctiveness in the vocal repertoire of red-capped mangabeys (Cercocebus torquatus). J. Comp. Psychol. 2012, 126, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, K.; Fischer, J. Baboon vocal repertoires and the evolution of primate vocal diversity. J. Hum. Evol. 2018, 126, 1–13. [Google Scholar] [CrossRef]
- Pollock, J.I. Field observations on Indri indri: A preliminary report. In Lemur Biology; Tattersall, I., Sussman, R., Eds.; Plenum Press: New York, NY, USA, 1975; pp. 28–31. [Google Scholar]
- Hauser, M.D. Vocal communication in macaques: Causes of variation. In Evolutionary Ecology and Behavior of Macaques; Fa, J.E., Lindburg, D., Eds.; Cambridge University Press: Cambridge, UK, 1996; pp. 551–578. [Google Scholar]
- Fuller, J.L. The Vocal Repertoire of Adult Male Blue Monkeys (Cercopithecus mitis stulmanni): A Quantitative Analysis of Acoustic Structure. Am. J. Primatol. 2014, 76, 203–216. [Google Scholar] [CrossRef]
- Fichtel, C.; Kappeler, P.M. Anti-predator behavior of group-living Malagasy primates: Mixed evidence for a referential alarm call system. Behav. Ecol. Sociobiol. 2002, 51, 262–275. [Google Scholar] [CrossRef]
- Cäsar, C.; Zuberbühler, K. Referential alarm calling behaviour in New World primates. Curr. Zool. 2012, 58, 680–697. [Google Scholar] [CrossRef][Green Version]
- Price, T.; Wadewitz, P.; Cheney, D.; Seyfarth, R.; Hammerschmidt, K.; Fischer, J. Vervet revisited: A quantitative analysis of alarm call structure and context specificity. Sci. Rep. 2015, 5, 13220. [Google Scholar] [CrossRef] [PubMed]
- Riondato, I.; Cissello, E.; Papale, E.; Friard, O.; Gamba, M.; Giacoma, C. Unsupervised Acoustic Analysis of the Vocal Repertoire of the Gray-Shanked Douc Langur (Pygathrix cinerea). J. Comput. Acoust. 2017, 25, 1750018. [Google Scholar] [CrossRef]
- Bonadonna, G.; Torti, V.; Randrianarison, R.M.; Martinet, N.; Gamba, M.; Giacoma, C. Behavioral correlates of extra-pair copulation in Indri indri. Primates 2014, 55, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Tecot, S.R. Seasonality and Predictability: The Hormonal and Behavioral Responses of the Red-Bellied Lemur, Eulemur rubriventer, in Southeastern Madagascar. Ph.D. Dissertation, University of Texas, Austin, TX, USA, 2008. [Google Scholar]
- Mitani, J.C. Comparative field studies of African ape vocal behavior. In Great Ape Societies; McGrew, W., Marchant, L., Nishida, T., Eds.; Cambridge University Press: Cambridge, UK, 1996; pp. 241–254. [Google Scholar] [CrossRef]
- Riondato, I.; Giuntini, M.; Gamba, M.; Giacoma, C. Vocalization of red- and grey-shanked douc langurs (Pygathrix nemaeus and P. cinerea). Vietnam. J. Primatol. 2013, 2, 75–82. [Google Scholar]
- Kawabe, M.; Mano, T. Ecology and behavior of the wild proboscis monkey, Nasalis larvatus (Wurmb), in Sabah, Malaysia. Primates 1972, 13, 213–227. [Google Scholar] [CrossRef]
- Röper, K.M.; Scheumann, M.; Wiechert, A.B.; Nathan, S.; Goossens, B.; Owren, M.K.; Zimmermann, E. Vocal acoustics in the endangered proboscis monkey (Nasalis larvatus). Am. J. Primatol. 2014, 76, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Marler, P. On the origin of speech from animal sounds. In The Role of Speech in Language; Kavanagh, J.F., Cutting, J., Eds.; MIT Press: Cambridge, MA, USA, 1975; pp. 11–37. [Google Scholar]
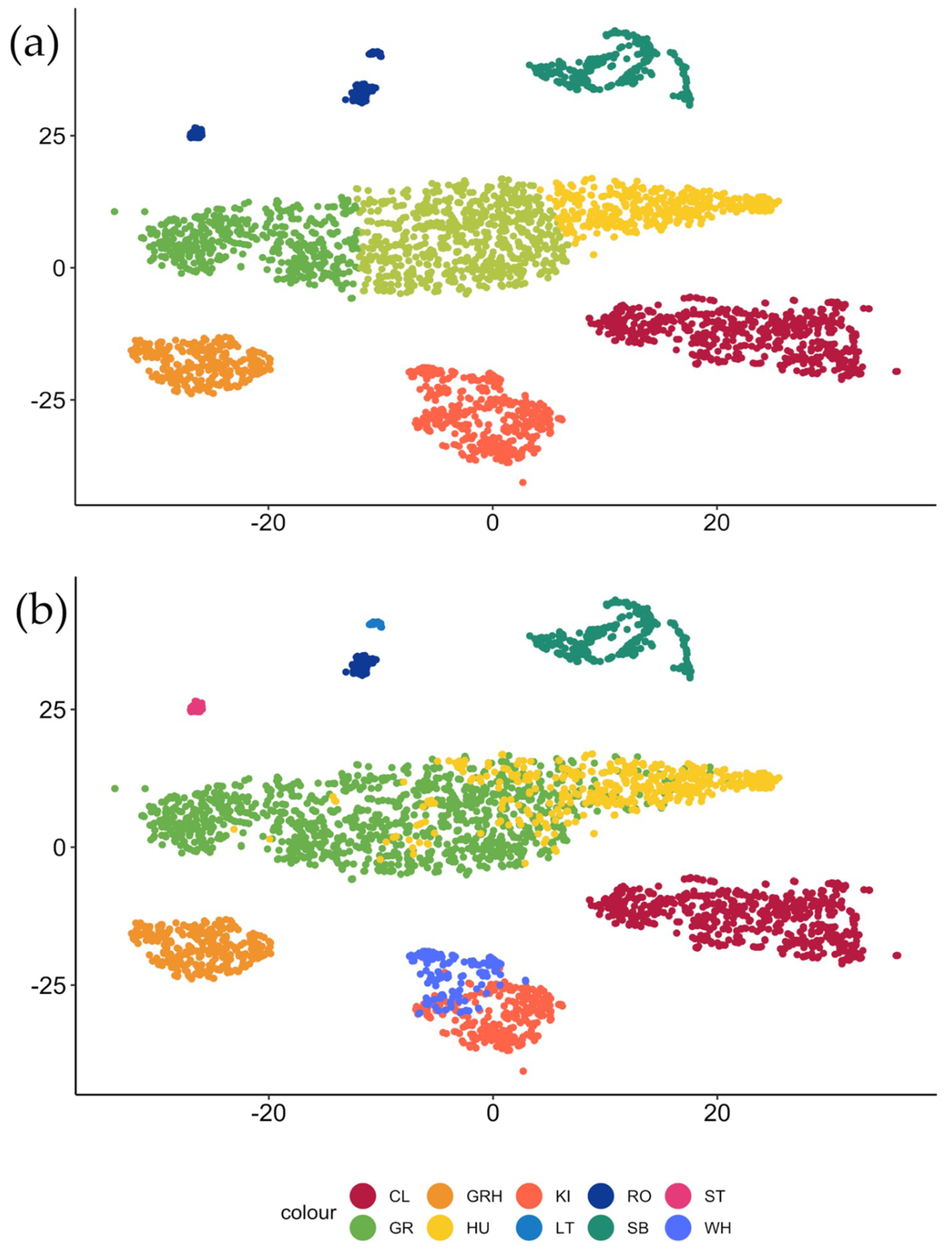
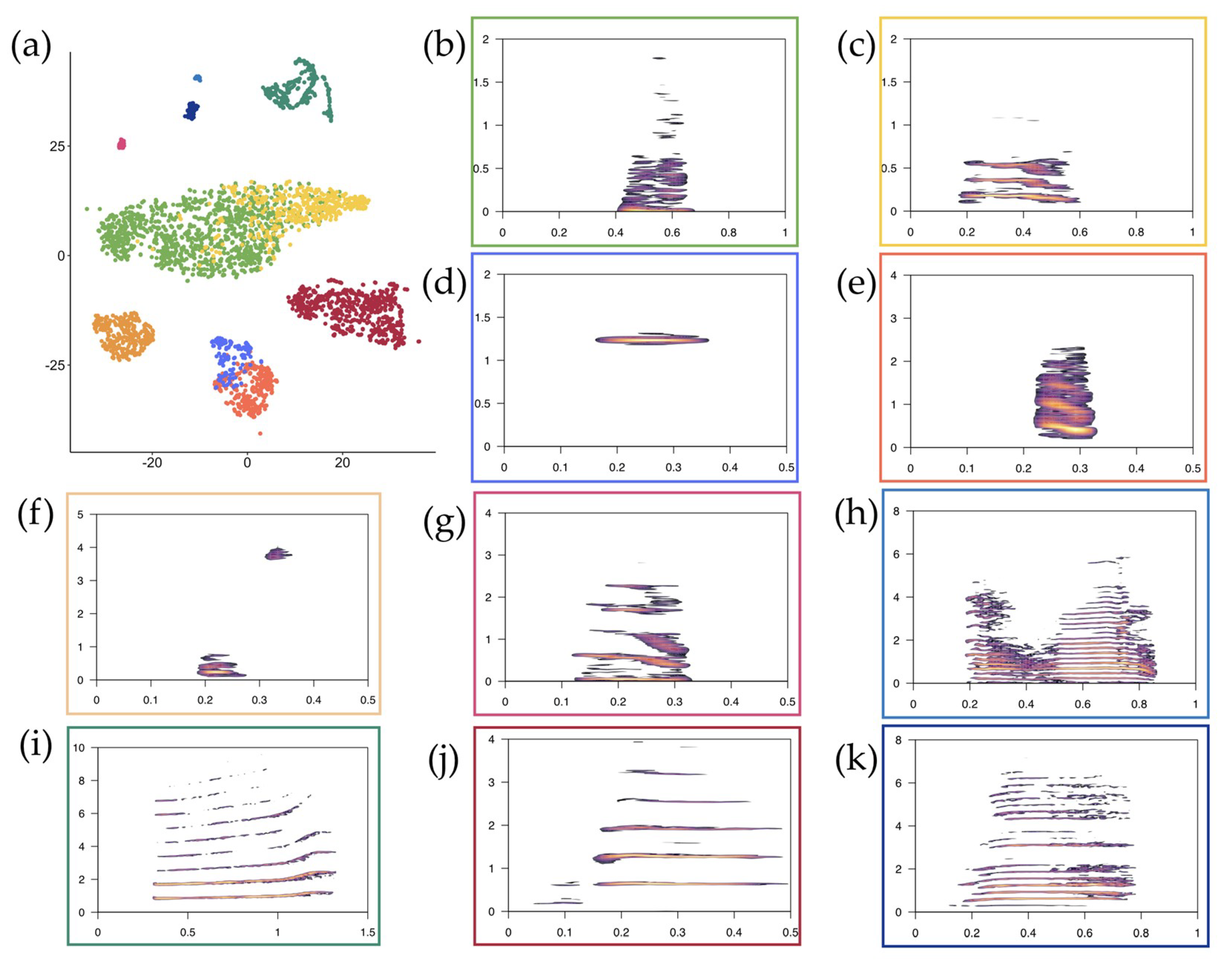
| Cluster | CL | GR | WG | HU | KI | LT | RO | SB | ST | WH |
|---|---|---|---|---|---|---|---|---|---|---|
| 1st | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 2nd | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 |
| 3rd | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 4th | 0.00 | 85.04 | 0.00 | 14.96 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 5th | 0.00 | 0.00 | 0.00 | 0.00 | 66.37 | 0.00 | 0.00 | 0.00 | 0.00 | 33.63 |
| 6th | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 22.63 | 45.26 | 0.00 | 32.12 | 0.00 |
| 7th | 0.00 | 99.00 | 0.00 | 1.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 8nd | 0.00 | 17.94 | 0.00 | 82.06 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Vocal Type | TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area |
|---|---|---|---|---|---|---|---|---|
| CL | 0.99 | 0.00 | 0.99 | 0.99 | 0.99 | 0.988 | 1.00 | 1.00 |
| GR | 0.82 | 0.08 | 0.84 | 0.82 | 0.83 | 0.74 | 0.94 | 0.88 |
| GRH | 0.71 | 0.04 | 0.59 | 0.71 | 0.64 | 0.61 | 0.96 | 0.65 |
| HU | 0.83 | 0.02 | 0.85 | 0.83 | 0.84 | 0.81 | 0.98 | 0.90 |
| KI | 0.79 | 0.02 | 0.78 | 0.79 | 0.78 | 0.76 | 0.98 | 0. 87 |
| LT | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| RO | 0.81 | 0.00 | 1.00 | 0.81 | 0.90 | 0.90 | 1.00 | 0.98 |
| SB | 1.00 | 0.02 | 0.98 | 1.00 | 0.99 | 0.99 | 1.00 | 1.00 |
| ST | 0.69 | 0.00 | 0.75 | 0.70 | 0.72 | 0.72 | 0.98 | 0.76 |
| WH | 0.75 | 0.01 | 0.79 | 0.75 | 0.77 | 0.76 | 0.95 | 0.84 |
| Weighted Average | 0.86 | 0.04 | 0.86 | 0.86 | 0.86 | 0.82 | 0.97 | 0.90 |
| Classified As | A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|---|
| CL | 99.03 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 4.76 |
| GR | 0.00 | 84.25 | 38.14 | 14.09 | 10.53 | 0.00 | 0.00 | 0.00 | 0.00 | 4.76 |
| GRH | 0.00 | 4. 99 | 58.76 | 0.00 | 4.21 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| HU | 0.00 | 6.30 | 1.03 | 84.56 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 2.38 |
| KI | 0.00 | 3.15 | 1.03 | 0.67 | 77.89 | 0.00 | 0.00 | 0.00 | 16.67 | 9.52 |
| LT | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| RO | 0.97 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 | 2.00 | 0.00 | 0.00 |
| SB | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 98.00 | 0.00 | 0.00 |
| ST | 0.00 | 0.79 | 0.00 | 0.67 | 0.00 | 0.00 | 0.00 | 0.00 | 75.00 | 0.00 |
| WH | 0.00 | 0.52 | 1.03 | 0.00 | 7.37 | 0.00 | 0.00 | 0.00 | 8.33 | 78.57 |
| Cluster | TP Rate | FP Rate | Precision | Recall | F-Measure | MCC | ROC Area | PRC Area |
|---|---|---|---|---|---|---|---|---|
| 3rd | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1st | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 4th | 0.96 | 0.05 | 0.85 | 0.96 | 0.90 | 0.88 | 0.99 | 0.97 |
| 7th | 0.83 | 0.00 | 0.97 | 0.83 | 0.90 | 0.88 | 1.00 | 0.98 |
| 8th | 0.88 | 0.01 | 0.95 | 0.88 | 0.92 | 0.91 | 1.00 | 0.98 |
| 5th | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 6th | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 2nd | 1.00 | 0.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Weighted Average | 0.95 | 0.01 | 0.96 | 0.95 | 0.95 | 0.95 | 1.00 | 0.99 |
| Classified as | A | B | C | D | E | F | G | H |
|---|---|---|---|---|---|---|---|---|
| 3rd | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1st | 0.00 | 100.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 4th | 0.00 | 0.00 | 85.35 | 3.08 | 5.00 | 0.00 | 0.00 | 0.00 |
| 7th | 0.00 | 0.00 | 9.16 | 96.92 | 0.00 | 0.00 | 0.00 | 0.00 |
| 8th | 0.00 | 0.00 | 5.49 | 0.00 | 95.00 | 0.00 | 0.00 | 0.00 |
| 5th | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 | 0.00 | 0.00 |
| 6th | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 | 0.00 |
| 2nd | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 100.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valente, D.; De Gregorio, C.; Torti, V.; Miaretsoa, L.; Friard, O.; Randrianarison, R.M.; Giacoma, C.; Gamba, M. Finding Meanings in Low Dimensional Structures: Stochastic Neighbor Embedding Applied to the Analysis of Indri indri Vocal Repertoire. Animals 2019, 9, 243. https://doi.org/10.3390/ani9050243
Valente D, De Gregorio C, Torti V, Miaretsoa L, Friard O, Randrianarison RM, Giacoma C, Gamba M. Finding Meanings in Low Dimensional Structures: Stochastic Neighbor Embedding Applied to the Analysis of Indri indri Vocal Repertoire. Animals. 2019; 9(5):243. https://doi.org/10.3390/ani9050243
Chicago/Turabian StyleValente, Daria, Chiara De Gregorio, Valeria Torti, Longondraza Miaretsoa, Olivier Friard, Rose Marie Randrianarison, Cristina Giacoma, and Marco Gamba. 2019. "Finding Meanings in Low Dimensional Structures: Stochastic Neighbor Embedding Applied to the Analysis of Indri indri Vocal Repertoire" Animals 9, no. 5: 243. https://doi.org/10.3390/ani9050243
APA StyleValente, D., De Gregorio, C., Torti, V., Miaretsoa, L., Friard, O., Randrianarison, R. M., Giacoma, C., & Gamba, M. (2019). Finding Meanings in Low Dimensional Structures: Stochastic Neighbor Embedding Applied to the Analysis of Indri indri Vocal Repertoire. Animals, 9(5), 243. https://doi.org/10.3390/ani9050243






