Simple Summary
During early lactation, cows face metabolic challenges. They experience a negative energy balance as energy intake increases more slowly than energy output with milk rises. To compensate for that energy deficit, higher amounts of concentrate are offered. Additionally, cows are able to extract energy from body fat by lipid mobilization. Excessive body fat mobilization, however, leads to metabolic disorders. Therefore, high-conditioned cows are suggested to have a more pronounced lipid mobilization. The intention of the present study was to examine the change of various fat depots during the transition period depending on body condition and energy supply with ultrasonic measurements. Body condition loss after calving usually interpreted as mobilization of subcutaneous adipose tissue was not different between cows with a higher or lower body condition score. However, ultrasonic measurements detected a more pronounced mobilization of subcutaneous adipose tissue in higher conditioned animals. In contrast, inner fat depots were mobilized similarly between cows. Higher concentrate feed proportions led to a less pronounced negative energy balance. A less pronounced negative energy balance would have been expected to decrease lipid mobilization. However, this relation could not be verified in the present study. This demonstrates that sonography-based methods provide a clearer picture of metabolic conditions.
Abstract
The aim of this study was to evaluate energy metabolism and lipid mobilization via ultrasonic measurements (USM), considering inner fat depots, in lactating dairy cows differing in body condition score (BCS) and fed rations with low (35% at dry matter basis; C35) or high (60% at dry matter basis; C60) concentrate feed proportions postpartum. Sixty pluriparous German Holstein cows were arranged in a 2 × 2 factorial design from d 42 antepartum (relative to calculated calving) until d 120 postpartum. Animals were divided into a group with a lower (initial BCS = 3.1 ± 0.38 SD; BCSL) and a group with a higher (initial BCS = 3.83 ± 0.41 SD; BCSH) BCS. Due to higher dry matter intake C60 groups reached the positive energy balance earlier, whereas C35 groups had a more pronounced negative energy balance. Although this would suggest a more pronounced mobilization of C35 groups the USM revealed no differences between feeding groups. Differences in BCS between both BCS groups remained almost the same over the trial. This was not reflected in ultrasonic data, as lipid mobilization was higher in higher conditioned cows. These findings demonstrate the extended possibilities of USM to depict metabolic processes.
1. Introduction
Cows are exposed to metabolic challenges during the transition period. The imbalance between an insufficient increase of energy intake on the one hand and the high energy requirements, on the other hand, induce a negative energy balance [1,2]. The organism attempts to compensate the energy deficit by mobilizing energy from adipose tissue depots [3]. This process leads to an increase of circulating non-esterified fatty acids (NEFA) such as β-hydroxybutyrate (BHB) after a certain delay to an enlargement of ketone body concentration when hepatic NEFA utilization is insufficient [4,5]. Spreading of ketone bodies involves the risk of ketosis when exceeding critical values. Nielen et al. [6] defined BHB-values in blood serum >1.2 mM as indicator for subclinical ketosis. According to Oetzel [7] NEFA-values >0.4 mM describe a stage were higher lipid mobilization takes place and, therefore, indicate an imbalance in the energy status.
Experimental determination of adipose tissue mobilization usually requires expensive comparative slaughter techniques, which limits the investigation of kinetics of mobilization of individual cows. Using the slaughter technique, Von Soosten et al. [8] determined a mean mobilization of 20 kg during the first 42 days (d) after parturition in heifers. Drackley et al. [9] measured values of 26.5–48.3 kg for mobilization of visceral adipose tissues and 31.8–57.2 kg for abdominal adipose tissues in dry cows. However, little is known about the quantity of adipose tissue mobilization in high lactating, pluriparous cows during the transition period. Using an ultrasound-based technique which was calibrated with simultaneously slaughter-based fat depots, facilitates tracking the development of fat depot masses and consequently of their mobilization and accretion on an individual basis [10]. Thus, this technique was used in the present study to characterize the dynamics of adipose tissue metabolism of transit cows.
Furthermore, the literature reveals a discrepancy about whether a higher body condition increases the body fat mobilization in ruminants [11,12] or not [13]. The same applies for the concentrate proportion and therefore the starch and energy content of the ration. Studies differ in their results when high energy levels of the diet decrease the mobilization of adipose tissues [12,14] or not [13,15]. Hence, a second aim of the experiment was to characterize the deflections in the dynamics of adipose tissue around parturition along with milking performance and parameters of energy metabolism as triggered by varying concentrate feed proportions and different body condition.
2. Materials and Methods
The experiment was performed in compliance with the German legislation on animal protection (Animal Welfare Act) and approved by the Lower Saxony State Office for Consumer Protection and Food Safety (LAVES, Oldenburg, Germany) in consultation with an independent ethics committee (AZ 33.19-42502-04-15/1858).
2.1. Experimental Design
Sixty pluriparous German Holstein cows were involved in the study from 42 d antepartum (a.p.) relative to calculated calving until 120 d postpartum (p.p.). The experimental design was a 2 × 2 factorial layout with body condition score (BCS) and concentrate proportion in the diet (C) as factors. Cows were either in a high or a low BCS group (BCSH or BCSL). Further criteria for allocating the animals were milk yield and milk composition of the previous lactation as well as body weight and number of lactation. Supply of energy and nutrients was ensured based on the recommendations of the Society of Nutrition Physiology [16]. Before parturition, both groups received the same total mixed ration (TMR) consisting of 80% silage (70% maize silage, 30% grass silage on dry matter DM basis) and 20% concentrate on a DM basis. After parturition, the diet changed to two partial mixed rations (PMR) consisting of 48% maize silage, 20% grass silage and 32% concentrate. To subdivide the BCS groups, diets varied in C. Rations for the groups with lower concentrate proportion (C35) contained 35% concentrate and an energy content of 6.9 MJ NEL/kg DM. The lower C was chosen to stimulate lipolysis by means of an energetic undersupply [4]. For the groups with a higher amount of concentrate (C60), C increased from 35–60% during the first three weeks p.p. The C60 ration contained an energy content of 7.3 MJ NEL/kg DM. Additional concentrate was provided by automatic feeding stations (Insentec, B.V., Marknesse, The Netherlands) until the required amounts were achieved. All cows remained in treatment until day (d) 120 in milk (DIM). Cows in the BCSH group started with an average BCS of 3.83 (±0.41 standard deviation SD). The BCSL group had an average BCS of 3.1 (±0.38 SD). Cows of group BCSH/C60 (n = 15) had an average parity of 3.4 (±1.1 SD). For the BCSH/C35 (n = 15) group the average parity was 3.3 (±1.2 SD). Cows of the BCSL/C60 (n = 15) group had an average parity of 2.6 (±0.9 SD) and for the BCSL/C35 (n = 15) group the average parity was 2.5 (±0.6 SD). TMR and PMR were provided ad libitum by self-feeding stations (RIC, Insentec B.V., Marknese, The Netherlands). The components and the chemical compositions of the feedstuffs are presented in Table 1 and Table 2.

Table 1.
Composition of concentrates during the dry and the lactating periods.

Table 2.
Chemical components of concentrates and roughage during the experimental period from day 42 antepartum until day 120 postpartum.
2.2. Sample and Data Collection
Samples of the mixed rations components were taken twice weekly and then pooled to a collective sample for periods of 4 weeks. Samples of concentrate were collected once a week and pooled to a collective sample every 4 weeks, as well. Through the whole experiment, dry matter intake (DMI) was recorded individually for both PMR and concentrate provided by computerized feeding stations. Live weight was recorded weekly a.p. and twice daily p.p. after each milking. Milk yield was quantified twice daily during milking at 0530 and 1530 h by automatic milk counters (Lemmer Fullwood GmbH, Lohmar, Germany). Milk samples were taken twice a week during morning and evening milking and stored at 4 °C until analysis. BCS was determined at noon in weekly intervals on a 5-point-scale according to Edmonson et al. [17].
Blood samples were taken at determined time points, whereby deviations of 2 d were tolerated (42 d a.p., 14 d a.p., 7 d a.p., 3 d a.p., 3 d p.p., 7 d p.p., 14 d p.p., 21 d p.p., 28 d p.p., 42 d p.p., 56 d p.p., 70 d p.p. 95 d p.p. and 120 d p.p.) from the vena jugularis externa. Blood samples were centrifuged (Heraeus Varifuge 3.0R Heraeus, Osterode, Germany; 2123× g, 15 °C, 15 min) and stored at −80 °C until further analysis.
Ultrasonic measurements (USM) of adipose tissues took place at defined points in time, too (d 3 p.p., d 28 p.p., d 70 p.p. and d 120 p.p.). Back fat thickness (BFT) was estimated according to Staufenbiel [18], while rib fat thickness (RFT), subcutaneous (SAT), retroperitoneal (RAT), mesenteric (MAT) and omental (OAT) adipose tissues were assessed according to Raschka et al. [10]. For this purpose, double measurement of each tissue were performed using a Mindray M5 Vet (Mindray, Shenzhen, China) diagnostic ultrasound system with a linear (g MHz, Mindray 6LE5Vs) and a convex probe (3 MHz, Mindray 3C5s). The thickness of the measured fat tissues was sized in millimeters. To calculate the adipose tissue depot masses in kg the measuring points established by Raschka et al. [10] were used (Table A1). For calculating the mobilization of adipose tissue depots, the experiment was divided in periods (period 1: weeks 1–4 postpartum, period 2: weeks 5–10 postpartum, period 3: weeks 11–17 postpartum), which were also used for analyzing the remaining parameters to receive a consistent statistical design.
One animal could not be evaluated due to erroneous, implausible values.
2.3. Analyses
PMR components and concentrate were analyzed for DM, crude ash, crude protein, ether extract, crude fiber, neutral detergent fiber (NDFom) and acid detergent fiber (ADFom) according to the standard methods of the Association of German Agricultural Analysis and Research Centres [19] (Table 2). Milk samples were analyzed for fat, protein and lactose by an infrared milk analyzer (Milkoscan FT 6000; Foss Electric, Hillerød, Denmark). Using an automatic photometric measurement system (Eurolyser, Type VET CCA, Salzburg, Austria) serum samples were analyzed for β-hydroxybutyrat (BHB), non-esterified fatty acids (NEFA), triglycerides and glucose. According to the classification by Nielen et al. [6] BHB-values in blood serum >1.2 mM were used as indicator for subclinical ketosis.
2.4. Calculations
Weekly means of DMI, net energy intake (NEI), net energy balance (NEB), milk yield, and milk components were used for further calculations. Computations of net energy requirements for maintenance (NEM) and lactation (NEL) as well as for milk energy concentration were based on equations published by the Society of Nutrition Physiology [16]:
NEM (MJ of NEL/d) = 0.294 × BW0.75
NEL (MJ of NEL/d) = [milk energy concentration (MJ of NEL/d) + 0.086] × milk yield (kg/d)
Milk energy (MJ/kg) = 0.38 × milk fat (%) + 0.21 × milk protein (%) + 0.95
The equation published by Gaines [20] was used to calculate the fat corrected milk (FCM):
4% FCM (kg/d) = {[milk fat (%) × 0.15] + 0.4} × milk yield (kg/d)
Energy corrected milk (ECM) was calculated based on the equation by Sjaunja et al. [21]:
ECM (kg/d) = milk yield (kg/d) × {[38.3 × milk fat (g/kg) + 24.2 × milk protein (g/kg) + 16.54 × milk lactose (g/kg) + 20.7]/3140}
In order to calculate the energy intake per day, the energy content of the feedstuffs was multiplied by DMI.
To calculate the NEB, the following equation was used:
NEB (MJ of NEL/d) = NEI (MJ of NEL/d) − NEM (MJ of NEL/d) − NEL (MJ of NEL/d)
To regard the gestational requirements in the NEB 13 MJ of NEL/d were subtracted from week 6-3 a.p. During the last 3 weeks until calving the requirements were assessed with 18 MJ of NEL/d.
For determination of the different adipose tissue depot masses, equations published by Raschka et al. [10] were used based on the collected data of the ultrasonic measurements:
Subcutaneous adipose tissue (SAT, kg) = −6.66 + 0.72 × R12 + 0.31 × AW3c
Retroperitoneal adipose tissue (RAT, kg) = −9.55 + 0.62 × R12 + 0.06 × KD3b
Omental adipose tissue (OAT, kg) = −2.32 + 0.55 × BFT + 0.37 × AW3b
Mesenteric adipose tissue (MAT, kg) = −12.8 + 0.38 × AW1b + 1.73 × AW3b − 1.45 × AW3c + 0.07 × KD2c
Sum of mobilized adipose tissues (SoM, kg) = SAT + RAT + OAT + MAT
Time-dependent changes in the individual and total adipose tissue depots were calculated by the differences of the fat masses between different time points.
It is assumed that 1 g of body fat corresponds to 39.8 kJ gross energy [22] of which 16% is lost as heat when fat is mobilized [23]. The following equation was used to estimate the energy mobilized from the adipose tissue depots:
Mobilized energy = Mobilized fat (kg) × 39.8 MJ/kg × 0.84
We used the following equations according to Hurley et al. [24] to calculate the efficiency parameters feed efficiency (FE), energy conversion efficiency (ECE), metabolic efficiency (MEff) and residual energy intake (REI):
FE = ECM/DMI (kg/kg)
ECE = Energy excretion with milk (MJ)/Energy intake (MJ NEL)
MEff = [Energy intake (MJ NEL) − Energy excretion with milk (MJ)]/Body weight0.75 (kg)
REI = Energy intake (MJ NEL) − expected energy intake (MJ NEL)
Variables of each of the three periods were finally summarized according to the mobilization of the adipose tissue depots, the experimental weeks of the performance, the milk and blood parameters, as well as the results of the calculated efficiency.
2.5. Statistical Analyses
As animals were fed similar diets before parturition, we evaluated the data for the postpartum period only.
The statistical analyses were performed by utilizing the statistical software SAS (version 9.4; SAS Institute Inc., Cary, NC, USA). Performance parameters, blood values, mobilization of adipose tissue depot masses and efficiency parameters were analyzed by using the MIXED procedure for repeated measures with a compound symmetry structure [25]. BCS classification (BCSH, BCSL), C (C35, C60) and period (1, 2, 3) were applied as fixed effects, as well as the interactions between them. Each cow within treatment was considered a random effect. The period of sampling was regarded to be a repeated measure. Milk parameters were analyzed with the first measured value in week 1 as covariate. For the remaining parameters, the first measured value before calving was used as covariate. p-values ≤ 0.05 were declared to be statistically significant and p-values ≤ 0.01 considered highly significant. For calculating correlations between parameters, we employed the statistical software TIBCO Statistica (Version 13.3, TIBCO Software Inc., Palo Alto, CA, USA) by using Pearson’s correlation. The correlation coefficient (r) was considered statistically significant, when p ≤ 0.05, and highly significant, when p ≤ 0.01. In the following, results are presented as LSMean ± Standard error of means (SEM) unless otherwise stated.
3. Results
3.1. Performance Parameters
For DMI (Figure 1A) we observed a C × period interaction (p = 0.042). The C35 groups exhibited a lower DMI than the C60 groups over time. All groups had a lower DMI in the first period compared to the following.
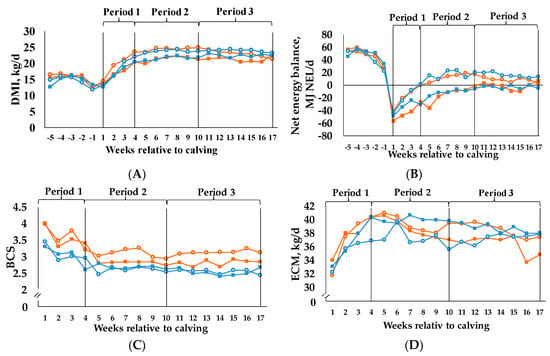
Figure 1.
Development of (A) dry matter intake (DMI), (B) net energy balance, (C) body condition score (BCS) and (D) energy corrected milk yield (ECM) in the course of the experiment. Cows were categorized in high BCS (BCSH) and low BCS (BCSL). After parturition, these two groups were divided again, each into a group with a concentrate proportion of 60% (C60) in the ration (increasing from 35–60% during the first three weeks after calving) and a group with a concentrate proportion of 35% (C35) in the ration. Thus, four groups emerged: BCSH/C60 (n = 15; ○), BCSH/C35 (n = 15; ■), BCSL/C60 (n = 15; ○), BCSL/C35 (n = 15; ■), BCS, DMI and NEB were analyzed with first measured value before calving as covariate, ECM was analyzed with first measured value from week 1 as covariate, (A) p-values: BCS = 0.761, C = 0.069, period < 0.001, BCS × C = 0.836, BCS × period = 0.232, C × period = 0.042, BCS × C × period = 0.295; (B) p-values: BCS = 0.540, C < 0.001, period < 0.001, BCS × C = 0.985, BCS × period = 0.639, C × period < 0.001, BCS × C × period = 0.020; (C) p-values: BCS = 0.004, C = 0.252, period < 0.001, BCS × C = 0.998, BCS × period = 0.011. C × period = 0.182, BCS × C × period = 0.030; (D) p-values: BCS = 0.679, C = 0.579, period < 0.001, BCS × C = 0.217, BCS × period = 0.003, C × period = 0.043, BCS × C × period = 0.150.
The same is true for NEI (Table 3), where we also detected a C × period interaction (p = 0.001). C35 groups had a significantly lower NEI than C60 groups in periods 2 and 3. For NEB (Figure 1B) we observed a treatment x period interaction (p = 0.020) as it was more negative in BCSH/C35 group than in BCSH/C60 and BCSL/C60 groups in period 1. Furthermore, NEB was in a positive range for the BCSH/C60 and BCSL/C60 groups, but in a negative range for the BCSH/C35 and BCSL/C35 groups in period 2. The development of the NEB outlines that the C60 groups reached a balanced NEB after four weeks p.p. and remained relatively stable then, whereas the C35 groups increased continuously and reached the settlement only at the end of the experiment.

Table 3.
Effects of body condition, concentrate proportion in the diet (C) and period on dry matter intake (DMI), energy intake and live weight (LSM) during period 1 (weeks 1–4 postpartum), period 2 (weeks 5–10 postpartum) and period 3 (weeks 11–17 postpartum) in the treatment groups.
For BCS (Figure 1C) we proved a treatment x period interaction (p = 0.030). The BCSH/C60 differed from the BCSL/C60 and BCSL/C35 group in all three periods, due to higher mean values. Moreover, the BCSH/C35 showed higher means in comparison to the BCSL/C35 group in period 1.
Live weight (Table 3) decreased from period 1 to 2, whereas it increased from period 2 to 3 within the C35 and C60 groups. We found a C × period interaction (p = 0.018), but no differences within one period.
3.2. Milk Parameters
For milk yield (Table 4 and Table 5) we detected two interactions, BCS × period (p = 0.023) and C × period (p < 0.001). However, neither BCS nor C had an influence within the same period. For milk fat content (Table 4 and Table 5) the same interactions were determined (BCS × period: p < 0.001, C × period: p = 0.001). The higher BCS led to a higher milk fat content in period 1, the same did a lower C in periods 2 and 3.

Table 4.
Effects of body condition, concentrate proportion in the diet (C) and period on milk parameters (LSM) during period 1 (weeks 1–4 postpartum), period 2 (weeks 5–10 postpartum) and period 3 (weeks 11–17 postpartum) in the treatment groups.

Table 5.
p-values of effects of body condition, concentrate proportion in the diet (C), period and interactions between them on milk parameters.
This is similar to the C × period interaction (p = 0.001) for milk fat yield (Table 4 and Table 5), where lower C also led to higher means in period 2. For milk protein content (Table 4 and Table 5), we found a C × period interaction (p = 0.021), too. There were no differences within the same period. Period (p < 0.001) had an effect on milk protein yield (Table 4 and Table 5), as period 1 differed from periods 2 and 3 due to higher milk protein yields for the latter two periods in all four groups. We discovered a BCS × C × period interaction (p = 0.013) for milk lactose content (Table 4 and Table 5). In period 1 the BCSL/C60 group differed from the BCSL/C35 group with regard to a higher mean. The same interaction was determined for milk lactose yield (BCS × C × period: p = 0.005, Table 4 and Table 5), although the groups exhibited no differences within the same period. We observed a C × period interaction for milk fat:protein ratio (Table 4 and Table 5), where lower C again led to higher values in all three periods. For milk energy concentration (Table 4 and Table 5), we observed a BCS × C × period interaction (p = 0.017). In periods 2 and 3 the BCSH/C35 group differed from the BCSL/C60 group regarding a higher energy concentration. The BCSL/C60 group and the BCSL/C35 group exhibited different means in period 3 only, whereby the former showed a higher energy concentration. We determined a BCS × period interaction (p = 0.003) and a C × period interaction (p = 0.040) for milk energy output (Table 4 and Table 5). However, no differences within the same periods were found. The same is true for the 4% FCM (Table 4 and Table 5). We determined the same interactions (BCS × period: p = 0.008, C × period: p = 0.044), but did not observe any differences within same periods. We found those two interactions (BCS × period: p = 0.003, C × period: p = 0.040) again for ECM (Figure 1D), but once more there were no differences within the same periods quantifiable.
3.3. Mobilization of Adipose Tissue Depots and Energy
BCS influenced BFT (Table 6) over time as we found a BCS × period interaction (p = 0.005). However, we could not determine differences between groups within one period. For RFT (Table 6) a time effect (p < 0.001) was observed. In all groups RFT was more degraded in period 1 than in periods 2 and 3.

Table 6.
Effect of body condition, C (Concentrate proportion in the diet) and period on change of back fat thickness and rib fat thickness (LSM) during period 1 (weeks 1–4 postpartum), period 2 (weeks 5–10 postpartum) and period 3 (weeks 11–17 postpartum) in the treatment groups. Negative values represent accretion of adipose tissue, positive values describe mobilization.
The same effect is true for OAT (Figure 2A, pperiod < 0.001) and RAT (Figure 2B, pperiod < 0.001). In contrast, BCS had an influence over time concerning SAT (Figure 2C), as we detected a BCS × period interaction (p = 0.002). In period 1 we observed higher mobilization for BCSH/C60 and BCSH/C35 than for BCSL/C60 and BCSL/C35. Apart from that we found a BCS × C interaction (p = 0.028) for MAT (Figure 2D).
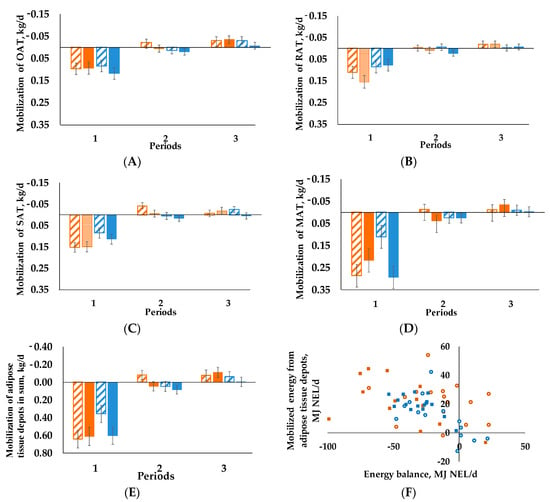
Figure 2.
Mobilization of the single adipose tissues and the sum of the (A) omental (OAT), (B) retroperitoneal (RAT), (C) subcutaneous (SAT), (D) mesenteric (MAT), (E) adipose tissues, negative values represent accretion of adipose tissue, positive values describe mobilization, LSMeans) of the experimental groups during the three periods, period 1: weeks 1–4 postpartum, period 2: weeks 5–10 postpartum, period 3: weeks 11–17 postpartum); as well as the (F) correlation of mobilized energy from adipose tissues and energy balance for each individual cow in period 1 (correlation coefficient = −0.4634, p < 0.05). Cows were categorized in high BCS (BCSH) and low BCS (BCSL). After parturition, these two groups were divided again, each into a group with a concentrate proportion of 60% (C60) in the ration (increasing from 35–60% during the first three weeks after calving) and a group with a concentrate proportion of 35% (C35) in the ration. Thus, four groups emerged: BCSH/C60 (n = 15; A–E orange striped bars, F ○) BCSH/C35 (n = 14; A–E orange bars, F ■), BCSL/C60 (n = 15; A–E blue striped bars, F ○), BCSL/C35 (n = 15; A–E blue bars, F ■). Error bars indicate SEM. Mixed models are analyzed with first measured value before calving as covariate, (A) p-values: BCS = 0.216, C = 0.213, period < 0.001, BCS × C = 0.536, BCS × period = 0.734, C × period = 0.953, BCS × C × period = 0.382; (B) p-values: BCS = 0.428, C = 0.381, period < 0.001, BCS × C = 0.648, BCS × period = 0.089, C × period = 0.526, BCS × C × period = 0.382; (C) p-values: BCS = 0.740, C = 0.250, period < 0.001, BCS × C = 0.549, BCS × period = 0.002, C × period = 0.788, BCS × C × period = 0.244; (D) p-values: BCS = 0.703, C = 0.163, period < 0.001, BCS × C = 0.034, BCS × period = 0.480, C × period = 0.366, BCS × C × period = 0.065; (E) p-values: BCS = 0.984, C = 0.138, period < 0.001, BCS × C = 0.311, BCS × period = 0.087, C × period = 0.595, BCS × C × period = 0.232.
Neither C nor BCS had an influence on SoM (Figure 2E), only a time effect (pperiod < 0.001) was visible. Period 1 differed from periods 2 and 3 concerning higher mobilization in all groups. For period 2 the BCSH/C60 group exhibited accretion, which is also true for all groups in period 3.
3.4. Blood Parameters
Results for BHB (Figure 3A) and NFEA (Figure 3B) are presented in d, as the measuring points were determined in d relative to calving. Nevertheless, in accordance with the other data, the statistical analyses are calculated in periods and start at calving.
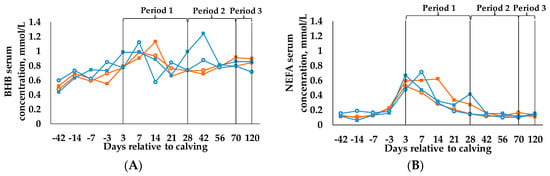
Figure 3.
Concentration of (A) β-hydroxybutyrat (BHB) and (B) non-esterified fatty acids (NEFA) in blood serum (LSM) from d 42 antepartum until d 120 postpartum. Statistical analysis starts at calving (period 1: weeks 1–4 postpartum, period 2: weeks 5–10 postpartum, period 3: weeks 11–17 postpartum). Cows were categorized in high BCS (BCSH) and low BCS (BCSL). After parturition, these two groups were divided again, each into a group with a concentrate proportion of 60% (C60) in the ration (increasing from 35–60% during the first three weeks after calving) and a group with a concentrate proportion of 35% (C35) in the ration. Thus, four groups emerged: BCSH/C60 (n = 15; ○), BCSH/C35 (n = 15; ■), BCSL/C60 (n = 15; ○), BCSL/C35 (n = 15; ■), Parameters are analyzed with first measured value before calving as covariate, (A) p-values: BCS = 0.747, C = 0.346, period = 0.906, BCS × C = 0.621, BCS × period = 0.272, C × period = 0.762, BCS × C × period = 0.422; (B) p-values: BCS = 0.877, C = 0.208, period < 0.001, BCS × C = 0.909, BCS × period = 0.557, C × period = 0.131, BCS × C × period = 0.069.
Neither BCS and C, nor time affected the concentration of BHB in blood serum. Only the BCSL/C35 group exceeded the threshold of 1.2 mmol/L declared as an indicator for subclinical ketosis according to Nielen et al. [6] in period 2.
For NEFA, we observed a time effect (p < 0.001) as concentration in blood serum was highest in period 1 and decreased in periods 2 and 3.
The same is true for glucose (Table A2) as a time effect (p < 0.001) was determined. Like in the case of NEFA, the concentration of glucose in blood serum was lower in period 1 than in periods 2 and 3, regardless of BCS and C.
Triglycerides (Table A2) were influenced by C, as C60 groups presented higher values than C35 groups. Furthermore, triglycerides exhibited a BCS × period interaction (p = 0.034). However, there were no differences within the same period. In accordance to the preceding parameter, the concentration of triglycerides was lowest in period 1 in all experimental groups.
3.5. Efficiency Parameters
To compare the different energy and feed efficiencies between groups, efficiency variables were calculated. The C affected FE (Table A3 and Table A4), as we observed a C effect (p = 0.003) as well as a time effect (p = 0.001). Groups with a lower concentrate proportion were more efficient. In period 1 all groups showed the highest FE compared to periods 2 and 3. The same is true for ECE (Table A3 and Table A4) were we determined the same effects (pC < 0.001, pperiod < 0.001). Again, groups with lower concentrate proportion had higher ECE values because of a higher efficiency. In accordance with the FE, ECE values were higher in period 1 than in periods 2 and 3 in all groups.
3.6. Correlations
Relations of elevated parameters were computed using correlations. The following values were considered: total mobilization of adipose tissue depots both in sum and separately as well as NEB, BHB, NEFA and efficiency parameter FE.
For all animals NEB correlated negatively with BFT(r = −0.466, p ≤ 0.001), RAT (r = −0.349, p ≤ 0.01), SAT (r = −0.397, p ≤ 0.01), MAT (r = −0.271, p ≤ 0.05) and SoM (r = −0.375, p ≤ 0.01), as well as with BHB (r = −0.502, p ≤ 0.001), NEFA (r = −0.402, p ≤ 0.01) and FE (r = −0.867, p ≤ 0.001). By contrast BCS, RFT and OAT did not correlate. The mobilized energy from adipose tissue depots and NEB (Figure 2F) showed a significant negative correlation, as correlation coefficient was −0.4634 (p < 0.05) for period 1.
4. Discussion
The aim of this study was to investigate the body fat depot mobilization and energy metabolism of pluriparous cows during the first weeks after parturition depending on body condition before calving and on different amounts of concentrate in the ration after calving by combining a feeding trial with ultrasound-based estimation of various depot fat depots.
One outcome of our investigation was that the amount of concentrate in the ration influenced the DMI, as groups with higher supply of concentrate consumed more DM in period 2. This result is comparable to other studies. Schmitz et al. [26] showed that a high proportion of concentrates in the ration enhanced DMI and Gruber et al. [27] proposed an increase between 0.4–0.6 kg per kg additional concentrate, which was linked to a subsequent roughage displacement. In contrast, other findings pointed out that there were no differences in feed intake between cows fed rations with different proportions of concentrates during the lactation period [28]. A reason for these controversial results might be caused by other feeding factors, such as composition, energy density and quality of roughage, as well as NDF content.
The higher DMI of C60 groups of our trial also resulted in a higher energy intake compared to C35 groups. These relations might explain why low concentrate groups suffered from a more pronounced negative EB and reached the positive EB much later than high concentrate groups. As described in Dänicke et al. [29] the energetic dilution of the C35 ration leads to a qualitative decrease of NEB.
Differences in BCS between both BCS groups and between individuals remained more or less the same over the whole transition period, whilst higher concentrate feed supply after calving (C60) failed to counterbalance the BCS loss observed in group with lower concentrate allowance (C35). Other studies agree with our findings, that feeding has little effect on BCS loss after parturition [9,30]. As NEI was higher in group C60 and NEB less negative, the question was whether the mobilization of the internal fat depots was less pronounced. However, the individual groups of our trial did not differ when SoM was evaluated collectively. Having a closer look at individual fat depots, it becomes obvious that SAT was more extensively mobilized in BCSH groups compared to their BCSL counterparts. This finding is surprising as no corresponding differences in BCS losses were noticed and BCS changes are usually regarded to represent the changes in SAT [31]. This significant effect of BCS on SAT mobilization exclusively occurred in period 1 where mobilization of all measured fat depots was most pronounced. The metabolic circumstances and the pronounced negative EB during this time explain these expectable results, which are also proved by the negative correlation of mobilized energy from body fat and NEB. Tamminga et al. [3] share our findings. With regard to the effect of body condition on mobilization of SAT Chilliard et al. [11] have shown that fat mobilization was physiologically related to body fatness. Nevertheless, we could not demonstrate the expected correlation between BCS and NEB, which would have indicated the higher potential of high-conditioned cows to mobilize body fat. This non-existent relationship could also explain why we determined no further association between BCS and mobilization. That is in line with Pedernera et al. [14] who had already pointed out, that BCS should not be used as an overall parameter to explain the energetic condition and metabolism of cows. It becomes apparent that USM show differences in lipid mobilization, which remain concealed by simply determining the BCS.
Due to the limited capability of BCS changes to indicate fat mobilization comprehensively, one could hypothesize that the mobilization of adipose tissues was related to energy balance, as proven by the highly significant overall correlations between NEB and mobilized energy from body fat, as well as those of NEB and the single adipose tissue depots and SoM in all animals of our investigation. Consequently, it should also depend on C whereby a decline of the energy balance would expectedly increase the lipolytic potential [11]. Reversely, high amounts of concentrate would then lead to a positive EB and consequently result in a decrease of adipose tissue mobilization. Our study could, however, not support those findings and other trials, which attempted to decrease body fat mobilization by increasing energy-rich diets, had neither been successful [15]. Considering the NEB alone does not duly reflect the metabolic processes. This suggests that other factors, such as genetic regulatory mechanisms may influence the mobilization relevantly.
Blood concentration of BHB is used as an indicator for lipolysis and ketosis whereby the literature states different thresholds. Nielen et al. [6] defined a value of BHB > 1.2 mmol/L in serum as the critical level for subclinical ketosis. Oetzel [7] declared a level of NEFA > 0.4 mmol/L as a stage where high lipomobilization takes place and therefore indicates an imbalance in energy state.
In the present study, none of the four trial groups exceeded the limit value for BHB in period 1, and we could not demonstrate any time or group effects, too. NEFA values were not affected by treatment either, but showed a significant time effect and also the characteristic curve during the transition period. NEFA values are typically higher in early lactation than in mid-lactation [32] which indicates higher mobilization of adipose tissue depots due to the particular metabolic challenges during this time. All four groups of our study exceeded the threshold for higher lipomobilization (>0.4 mmol/L) in period 1 [7]. This is in line with the negative energy balance during this timeframe as this threshold indicates a negative energy state. The negative EB was more pronounced in groups with lower concentrate allowance; due to a forced lipid mobilization, we would have also expected higher serum concentrations for both BHB and NEFA. However, the sonography-based determination of the adipose tissue mobilization did not verify the differences seen in NEB. A closer look at SAT, MAT and SoM indicated that regular conditioned animals with a lower concentrate proportion in the ration mobilized as much body fat as regular conditioned cows with a high concentrate availability. Only cows with an energetic oversupply mobilize less body fat. Van der Drift [33] stated that a high BCS and higher fat depot mobilization increased the risk for ketosis and hypothesized that high-energy diets compensated the cows’ energy demand and made high mobilization of body fat unnecessary in order to avoid that. Other studies confirm this presumption [12,14,34]. Chilliard [35] pointed out that cows mobilize more body fat when their access to feed is limited. Other studies argue the converse. Cows with high-energy diets exhibited higher BHB and NEFA values compared with cows supplied with low-energy diets [4,34]. Yet other studies, in turn, go in line with our findings and could not determine any significant differences between treatments in the first weeks p.p. [26,36]. This suggests that NEB does not accurately reflect the grade of lipid mobilization. The examination of lipid mobilization by ultrasonic technology brings out physiological and metabolic relations that remain concealed by using BCS determination or NEB calculation only.
A possible explanation for our results might be the absence of lipolytic stimuli in the adipose tissue depots, for example, a low glucose level [37]. Propionate is necessary for synthesizing glucose. Presumably, in the present study, there might have been sufficient DMI and also starch and energy content in both rations to generate adequate amounts of propionate in the rumen, so that glucose concentration did not decrease and therefore no lipolytic stimuli occurred. Another reason for detecting only concentrate tendencies and a single BCS effect on fat depot mobilization could be an adequate level of oxaloacetate for introducing NEFA into the citric acid cycle which prevents an increase of BHB beyond the physiological range [38]. Van der Drift [33] indicates that not only non-genetic, but also genetic variations of BHB concentration have to be considered. Those findings reflect our perceptions concerning SoM, where we could not prove any group differences either. Different studies had already pointed out that cows vary in their potential to deal with metabolic changes and to adapt to NEB and varying dietary energy [26,33,39].
In the present study, the mobilization of protein was not examined. Protein mobilization can reduce both, fat mobilization and NEFA and BHB concentration in blood [33]. It can be hypothesized that C35 and BCSH groups mobilized sufficient protein to cover group effects in fat mobilization and serum NEFA and BHB concentration.
A positive relationship between C and milk yield would have been expected due to the additionally provided energy in the diet as had been proved in previous studies [12,26,40]. BCS had no influence on milk yield either. Other investigations underline our findings that body condition at calving has little effect on milk production of well-fed cows [35]. In the present study we failed to demonstrate a BCS effect both on DMI and on milk yield. Nevertheless, other milk parameters such as milk fat content differed between trial groups in the present study. The higher BCS might have increased the milk fat content caused by higher mobilization of body fat reserves in period 1 [12,34]. Furthermore, higher amounts of concentrates led to milk fat depression in periods 2 and 3, because of the associated decrease of the acetate-to-propionate ratio in the rumen [41,42]. Acetate is an important source for synthesis of milk fat in ruminants [43]. Lipid mobilization of BCSH groups may have concealed the concentrate effect in period 1. These results are also reflected in ECM. Relating to NEB, lower ECM yield and higher DMI led to a positive EB in C60 groups.
In our trial, the C35 groups seem to be more efficient, as their FE was higher compared to C60 groups. This is, however, related to an equal milk production level of both groups, whereby animals of C35 groups consumed less DMI containing the lower energy content. Thus, C35 groups also exhibited a longer and more pronounced negative energy balance. In this case, higher milk production was accompanied by less feed intake and more body fat mobilization. Spurlock et al. [44] proposed a genetic correlation between high FE and a more pronounced negative energy balance. We could underline this due to the highly significant correlation between NEB and FE in the present study. It indicates that efficient cows might be more endangered to develop metabolic diseases. This hypothesis is confirmed by findings of Chilliard et al. [11], where NEFA concentrations were highly correlated with NEB, which could also be proven in the present study.
5. Conclusions
The results of this study confirm the benefits of higher amounts of concentrates concerning the DMI, and therefore the energy intake, whereas milk yield was unaffected. However, higher amounts of concentrate led to milk fat depression. Due to higher energy intake and equal milk yield, C60 groups reached the positive energy balance earlier than the C35 groups. Lower DMI and equal milk yield led to an improved efficiency in C35 groups. However, the term efficiency must be critically reviewed, as it is related to a high energy deficit.
The determination of body fat mobilization by ultrasonic recording revealed the differences between groups seen in the NEB, which suggested a need for higher lipid mobilization in C35 groups.
Furthermore, we could not find differences between groups concerning BCS loss. However, BCS affected the mobilization of SAT over time, as high-conditioned cows had a more pronounced SAT mobilization. Although BCS is based on changes in SAT, the determination failed to detect them in the present study. Therefore, BCS determination and NEB calculation should be used carefully as indicators for general mobilization or overall metabolic processes and conditions. The sonography-based method revealed boundaries of BCS determination and NEB calculation and visualized physiological and metabolic relations that remain concealed in other methods. Physiological processes, such as protein mobilization and hormone levels, might also influence fat loss. Further research is necessary to clarify causal interrelations.
Author Contributions
The experiment’s conceptualization was initiated by J.H., S.D., U.M., D.v.S., K.B.; Methodology and validation of data was done by K.B., D.v.S.; Formal analysis was performed by K.B.; Investigation was done by S.K., J.F., D.v.S., K.B.; Resources were looked up by K.B.; Curation and preparation, visualization of data was done by D.v.S. and K.B.; Writing original draft preparation was performed by K.B.; Supervision and writing of the review was done by D.v.S., J.F., S.K., U.M., S.D., J.H., A.Z.; Editing was done by K.B.; Project was administrated by J.H., U.M., S.D.
Funding
The project is part of indiKuh and supported by funds of the Federal Ministry of Food and Agriculture (BMEL) based on a decision of the Parliament of the Federal Republic of Germany via the Federal Office for Agriculture and Food (BLE) under the innovation support programme [grant number FKZ 2817905915].
Acknowledgments
The authors thank the co-workers of the Institute of Animal Nutrition, and of the experimental station of the Friedrich-Loeffler-Institut (FLI) in Brunswick for their support in taking care of the animals, sample and data collection as well as analyses.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Ultrasonic measuring points and its descriptions.
Table A1.
Ultrasonic measuring points and its descriptions.
| Measuring Points | Description |
|---|---|
| R12 + | Subcutaneous fat over 12th rib |
| AW1b § | Distance from skin to distal muscle margin above the peritoneum at the point of interception of a vertical line through the last lumbar vertebra and a horizontal line through the patella |
| AW3b § | Distance from the skin to the distal muscle margin above the peritoneum at the center of the paralumbar fossa |
| AW3c § | Thickness of the abdominal wall at the center of the paralumbar fossa |
| KD3b * | Distance from the skin to the peritoneum in the intertransverse space directly cranial to intertransverse space where the caudal pole of the kidney is visible |
| KD2c * | Distance from the skin to the distal kidney margin in the intertransverse space directly cranial to KD2c |
+ R = rib, § AW = abdominal wall, * KD = kidney.

Table A2.
Effects of body condition, concentrate proportion in the diet (C) and period on glucose and triglyceride concentrations in blood serum (LSM) during period 1 (weeks 1–4 postpartum), period 2 (weeks 5–10 postpartum) and period 3 (weeks 11–17 postpartum) in the treatment groups.
Table A2.
Effects of body condition, concentrate proportion in the diet (C) and period on glucose and triglyceride concentrations in blood serum (LSM) during period 1 (weeks 1–4 postpartum), period 2 (weeks 5–10 postpartum) and period 3 (weeks 11–17 postpartum) in the treatment groups.
| Item + | Treatment § | SEM # | p-Value * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BCSH/C60 n = 15 | BCSH/C35 n = 15 | BCSL/C60 n = 15 | BCSL/C35 n = 15 | BCS | C | BCS × C | BCS × Period | C × Period | BCS × C × Period | ||
| Glucose, mmol/L | |||||||||||
| Period 1 | 2.68 | 2.72 | 2.63 | 2.71 | 0.12 | 0.439 | 0.609 | 0.276 | 0.446 | 0.425 | 0.625 |
| Period 2 | 3.06 | 2.99 | 2.85 | 2.88 | |||||||
| Period 3 | 3.23 | 2.88 | 3.04 | 3.07 | |||||||
| Triglycerides, mmol/L | |||||||||||
| Period 1 | 0.13 | 0.13 | 0.12 | 0.13 | 0.01 | 0.066 | 0.044 | 0.149 | 0.034 | 0.151 | 0.077 |
| Period 2 | 0.15 | 0.16 | 0.16 | 0.16 | |||||||
| Period 3 | 0.17 | 0.24 | 0.16 | 0.16 | |||||||
§ Before calving cows were classified in high and low body condition score (BCS)-groups (BCSH, BCSL), after calving both groups were divided again, each into a group with 35% concentrate proportion (C35) and a group with 60% concentrate proportion (C60) in the ration (increasing from 35–60% during the first three weeks after parturition). Thus, four groups emerged: BCSH/C60 (n = 15), BCSH/C35 (n = 15), BCSL/C60 (n = 15), BCSL/C35 (n = 15). Values are presented as LSMeans, * Period (p < 0.001) for all variables, + Analyzed with first measured value before calving as covariate, # Pooled standard error of means.

Table A3.
Effects of body condition, concentrate proportion in the diet (C) and period on efficiency parameters (LSM) during period 1 (weeks 1–4 postpartum), period 2 (weeks 5–10 postpartum) and period 3 (weeks 11–17 postpartum) in the treatment groups.
Table A3.
Effects of body condition, concentrate proportion in the diet (C) and period on efficiency parameters (LSM) during period 1 (weeks 1–4 postpartum), period 2 (weeks 5–10 postpartum) and period 3 (weeks 11–17 postpartum) in the treatment groups.
| Item + | Treatment § | SEM # | |||
|---|---|---|---|---|---|
| BCSH/C60 n = 15 | BCSH/C35 n = 15 | BCSL/C60 n = 15 | BCSL/C35 n = 15 | ||
| Feed efficiency †, kg/kg | |||||
| Period 1 | 1.93 | 2.27 | 1.92 | 2.11 | 0.14 |
| Period 2 | 1.58 | 1.84 | 1.54 | 1.81 | 0.14 |
| Period 3 | 1.66 | 2.07 | 1.56 | 1.71 | |
| Energy conversion efficiency #, MJ/MJ NEL | |||||
| Period 1 | 0.88 | 1.04 | 0.89 | 0.97 | 0.03 |
| Period 2 | 0.71 | 0.85 | 0.70 | 0.84 | |
| Period 3 | 0.73 | 0.80 | 0.70 | 0.79 | |
| Metabolic efficiency ‡, MJ NEl/kg body weight0.75 | |||||
| Period 1 | 0.17 c | 0.00 c | 0.16 c | 0.07 c | 0.03 |
| Period 2 | 0.38 b,AB | 0.20 b,CD | 0.43 b,A | 0.22 b,C | |
| Period 3 | 0.35 a,A | 0.30 a,B | 0.42 a,A | 0.28 a,AB | |
| Residual energy intake ¶, MJ NEL | |||||
| Period 1 | 27.9 a,A | 7.5 a,B | 21.7 a,A | 10.7 a,B | 3.8 |
| Period 2 | 18.3 b,A | −5.5 b,B | 17.1 b,A | −3.3 b,B | |
| Period 3 | 5.5 c,A | −5.2 c,B | 6.7 c,A | −6.5 c,B | |
a,b Means with different superscripts differ within columns, A,B Means with different superscripts differ within row, § Before calving cows were classified in high and low body condition score (BCS)-groups (BCSH, BCSL), after calving both groups were divided again, each into a group with 35% concentrate proportion (C35) and a group with 60% concentrate proportion (C60) in the ration (increasing from 35–60% during the first three weeks after parturition). Thus, four groups emerged: BCSH/C60 (n = 15), BCSH/C35 (n = 15), BCSL/C60 (n = 15), BCSL/C35 (n = 15). Values are presented as LSMeans, + Analyzed with first measured value from week 1 as covariate, # Pooled standard error of means, † Feed efficiency (FE) = energy-corrected milk, kg/DMI, kg, # energy conversion efficiency (ECE) = energy excretion with milk, MJ/energy intake, MJ NEL, ‡ Metabolic efficiency (MEff) = (energy intake, MJ NEL − energy in milk, MJ)/body weight0.75, kg, ¶ Residual energy intake (REI) = energy intake, MJ NEL − expected energy intake, MJ NEL.

Table A4.
p-values of effects of body condition, concentrate proportion in the diet (C), period and interaction between them on efficiency parameters.
Table A4.
p-values of effects of body condition, concentrate proportion in the diet (C), period and interaction between them on efficiency parameters.
| Item + | p-Value * | |||||
|---|---|---|---|---|---|---|
| BCS | C | BCS × C | BCS × Period | C × Period | BCS × C × Period | |
| Feed efficiency †, kg/kg | 0.240 | 0.009 | 0.509 | 0.491 | 0.997 | 0.718 |
| Energy conversion efficiency #, MJ/MJ NEL | 0.291 | <0.001 | 0.617 | 0.718 | 0.190 | 0.310 |
| Metabolic efficiency ‡, MJ NEl/kg body weight0.75 | 0.182 | <0.001 | 0.672 | 0.787 | <0.001 | 0.002 |
| Residual energy intake ¶, MJ NEL | 0.920 | <0.001 | 0.629 | 0.771 | <0.001 | 0.04 |
Before calving cows were classified in high and low body condition score (BCS)-groups (BCSH, BCSL), after calving both groups were divided again, each into a group with 35% concentrate proportion (C35) and a group with 60% concentrate proportion (C60) in the ration (increasing from 35–60% during the first three weeks after parturition). Thus, four groups emerged: BCSH/C60 (n = 15), BCSH/C35 (n = 15), BCSL/C60 (n = 15), BCSL/C35 (n = 15), * Period (p < 0.001) for all variables, + Analyzed with first measured value from week 1 as covariate, † Feed efficiency (FE) = energy-corrected milk, kg/DMI, kg, # energy conversion efficiency (ECE) = energy excretion with milk, MJ/energy intake, MJ NEL, ‡ Metabolic efficiency (MEff) = (energy intake, MJ NEL − energy in milk, MJ)/body weight0.75, kg, ¶ Residual energy intake (REI) = energy intake, MJ NEL − expected energy intake, MJ NEL.
References
- Grummer, R.R. Impact of changes in organic nutrient metabolism on feeding the transition dairy cow. J. Anim. Sci. 1995, 73, 2820–2833. [Google Scholar] [CrossRef] [PubMed]
- Veerkamp, R.; Beerda, B.; Van der Lende, T. Effects of genetic selection for milk yield on energy balance, levels of hormones, and metabolites in lactating cattle, and possible links to reduced fertility. Livest. Sci. 2003, 83, 257–275. [Google Scholar] [CrossRef]
- Tamminga, S.; Luteijn, P.; Meijer, R. Changes in composition and energy content of liveweight loss in dairy cows with time after parturition. Livest. Prod. Sci. 1997, 52, 31–38. [Google Scholar] [CrossRef]
- Schulz, K.; Frahm, J.; Meyer, U.; Kersten, S.; Reiche, D.; Rehage, J.; Dänicke, S. Effects of prepartal body condition score and peripartal energy supply of dairy cows on postpartal lipolysis, energy balance and ketogenesis: An animal model to investigate subclinical ketosis. J. Dairy Res. 2014, 81, 257–266. [Google Scholar] [CrossRef]
- Littledike, E.; Young, J.; Beitz, D. Common Metabolic Diseases of Cattle: Ketosis, Milk Fever, Grass Tetany, and Downer Cow Complex. J. Dairy Sci. 1981, 64, 1465–1482. [Google Scholar] [CrossRef]
- Nielen, M.; Aarts, M.G.; Jonkers, A.G.; Wensing, T.; Schukken, Y.H. Evaluation of two cowside tests for the detection of subclinical ketosis in dairy cows. Can. Vet. J. 1994, 35, 229–232. [Google Scholar]
- Oetzel, G.R. Monitoring and testing dairy herds for metabolic disease. Vet. Clin. Food Anim. 2004, 20, 651–674. [Google Scholar] [CrossRef] [PubMed]
- Von Soosten, D.; Meyer, U.; Piechotta, M.; Flachowsky, G.; Dänicke, S. Effect of conjugated linoleic acid supplementation on body composition, body fat mobilization, protein accretion, and energy utilization in early lactation dairy cows. J. Dairy Sci. 2012, 95, 1222–1239. [Google Scholar] [CrossRef] [PubMed]
- Drackley, J.; Wallace, R.; Graugnard, D.; Vasquez, J.; Richards, B.; Loor, J. Visceral adipose tissue mass in nonlactating dairy cows fed diets differing in energy density. J. Dairy Sci. 2014, 97, 3420–3430. [Google Scholar] [CrossRef]
- Raschka, C.; Ruda, L.; Wenning, P.; von Stemm, C.-I.; Pfarrer, C.; Huber, K.; Meyer, U.; Dänicke, S.; Rehage, J. In vivo determination of subcutaneous and abdominal adipose tissue depots in German Holstein dairy cattle. J. Anim. Sci. 2016, 94, 2821–2834. [Google Scholar] [CrossRef]
- Chilliard, Y.; Ferlay, A.; Faulconnier, Y.; Bonnet, M.; Rouel, J.; Bocquier, F. Adipose tissue metabolism and its role in adaptations to undernutrition in ruminants. Proc. Nutr. Soc. 2000, 59, 127–134. [Google Scholar] [CrossRef]
- Delaby, L.; Faverdin, P.; Michel, G.; Disenhaus, C.; Peyraud, J.L. Effect of different feeding strategies on lactation performance of Holstein and Normande dairy cows. Animal 2009, 3, 891–905. [Google Scholar] [CrossRef]
- Cowan, R.; Robinson, J.; McDonald, I. A note on the effects of body fatness and level of food intake on the rate of fat loss in lactating ewes. Anim. Sci. J. 1982, 34, 355–357. [Google Scholar] [CrossRef]
- Pedernera, M.; Garcia, S.; Horagadoga, A.; Barchia, I.; Fulkerson, W. Energy balance and reproduction on dairy cows fed to achieve low or high milk production on a pasture-based system. J. Dairy Sci. 2008, 91, 3896–3907. [Google Scholar] [CrossRef]
- Roche, J.R.; Friggens, N.C.; Kay, J.K.; Fisher, M.W.; Stafford, K.J.; Berry, D.P. Body condition score and its association with dairy cow productivity, health, and welfare. J. Dairy Sci. 2009, 92, 5769–5801. [Google Scholar] [CrossRef]
- GfE. Empfehlungen zur Energie-und Nährstoffversorgung der Milchkühe und Aufzuchtrinder; DLG-Verlags-GmbH: Frankfurt am Main, Germany, 2001. [Google Scholar]
- Edmonson, A.; Lean, I.; Weaver, L.; Farver, T.; Webster, G. A body condition scoring chart for Holstein dairy cows. J. Dairy Sci. 1989, 72, 68–78. [Google Scholar] [CrossRef]
- Staufenbiel, R. Konditionsbeurteilung von Milchkühen mit Hilfe der sonographischen Rückenfettdickenmessung. Prakt. Tierarzt Coll. Vet. 1997, 27, 87–92. [Google Scholar]
- VDLUFA. Verband Deutscher Landwirtschaftlicher Untersuchungs- und Forschungsanstalten. Handbuch der landwirtschaftlichen Versuchs- und Untersuchungsmethodik (VDLUFA-Methodenbuch), Bd. III: Die Chemische Untersuchung von Futtermitteln; VDLUFA-Verlag: Darmstadt, Germany, 2006. [Google Scholar]
- Gaines, W. An efficiency formula for dairy cows. Science 1928, 67, 353–354. [Google Scholar] [CrossRef]
- Sjaunja, L.; Baevre, L.; Junkkarinen, L.; Pedersen, J.; Setälä, J. A Nordic proposal for an energy-corrected milk (ECM) formula. In Proceedings of the 27th Session International Committee for Recording and Productivity of Milk Animals, Paris, France, 2–6 July 1990; pp. 156–157. [Google Scholar]
- Brouwer, E. Report of Sub-Comittee on Constants and Factors; EAAP Scientific Series; EAAP: Rome, Italy, 1965; pp. 441–443. [Google Scholar]
- AFRC. Agriculture and Food Research Council, Energy and Protein Requirements of Ruminant Livestock; CAB International: Wallingford, UK, 1993. [Google Scholar]
- Hurley, A.; López-Villalobos, N.; McParland, S.; Kennedy, E.; Lewis, E.; O’Donovan, M.; Burke, J.; Berry, D.P. Inter-relationships among alternative definitions of feed efficiency in grazing lactating dairy cows. J. Dairy Sci. 2016, 99, 468–479. [Google Scholar] [CrossRef]
- Littell, R.; Henry, P.; Ammerman, C. Statistical analysis of repeated measures data using SAS procedures. J. Anim. Sci. 1998, 76, 1216–1231. [Google Scholar] [CrossRef]
- Schmitz, R.; Schnabel, K.; von Soosten, D.; Meyer, U.; Spiekers, H.; Rehage, J.; Dänicke, S. The effects of energy concentration in roughage and allowance of concentrates on performance, health and energy efficiency of pluriparous dairy cows during early lactation. Arch. Anim. Nutr. 2018, 72, 100–120. [Google Scholar] [CrossRef]
- Gruber, L.; Schwarz, F.; Erdin, D.; Fischer, B.; Spiekers, H.; Steingaß, H.; Meyer, U.; Chassot, A.; Jilg, T.; Obermaier, A. Vorhersage der Futteraufnahme von Milchkühen–Datenbasis von 10 Forschungs-und Universitätsinstituten Deutschlands, Österreichs und der Schweiz. 116. VDLUFA-Kongress; VDLUFA-Verlag: Rostock, Germany, 2004; pp. 484–504. [Google Scholar]
- Tienken, R.; Kersten, S.; Frahm, J.; Meyer, U.; Locher, L.; Rehage, J.; Huber, K.; Kenéz, Á.; Sauerwein, H.; Mielenz, M.; et al. Effects of an energy-dense diet and nicotinic acid supplementation on production and metabolic variables of primiparous or multiparous cows in periparturient period. Arch. Anim. Nutr. 2015, 69, 319–339. [Google Scholar] [CrossRef]
- Dänicke, S.; Meyer, U.; Kersten, S.; Frahm, J. Animal models to study the impact of nutrition on the immune system of the transition cow. Res. Vet. Sci. 2018, 116, 15–27. [Google Scholar] [CrossRef]
- Roche, J.; Berry, D.; Kolver, E. Holstein-Friesian strain and feed effects on milk production, body weight, and body condition score profiles in grazing dairy cows. J. Dairy Sci. 2006, 89, 3532–3543. [Google Scholar] [CrossRef]
- Garnsworthy, P.; Topps, J. The effect of body condition of dairy cows at calving on their food intake and performance when given complete diets. Anim. Sci. J. 1982, 35, 113–119. [Google Scholar] [CrossRef]
- González, F.D.; Muiño, R.; Pereira, V.; Campos, R.; Benedito, J.L. Relationship among blood indicators of lipomobilization and hepatic function during early lactation in high-yielding dairy cows. J. Vet. Sci. 2011, 12, 251–255. [Google Scholar] [CrossRef]
- Van der Drift, S. Ketosis in Dairy Cows: Etiologic Factors, Monitoring, Treatment. Ph.D. Thesis, Utrecht University, Utrecht, The Netherlands, 2013. [Google Scholar]
- Drong, C.; Meyer, U.; von Soosten, D.; Frahm, J.; Rehage, J.; Breves, G.; Dänicke, S. Effect of monensin and essential oils on performance and energy metabolism of transition dairy cows. J. Anim. Physiol. Anim. Nutr. 2016, 100, 537–551. [Google Scholar] [CrossRef]
- Chilliard, Y. Physiological constraints to milk production: Factors which determine nutrient partitioning, lactation persistency and mobilization of body reserves. World Rev. Anim. Prod. 1992, 27, 19–26. [Google Scholar]
- McNamara, S.; Murphy, J.; Rath, M.; O’mara, F. Effects of different transition diets on energy balance, blood metabolites and reproductive performance in dairy cows. Livest. Prod. Sci. 2003, 84, 195–206. [Google Scholar] [CrossRef]
- Herdt, T.H. Ruminant adaptation to negative energy balance: Influences on the etiology of ketosis and fatty liver. Vet. Clin. Food Anim. 2000, 16, 215–230. [Google Scholar] [CrossRef]
- Wilke, S. Parameter des Energiestoffwechsels, Milchleistung, Fruchtbarkeit und Tiergesundheit in einer konventionellen Milchviehherde. Ph.D. Thesis, Freie Universität Berlin, Berlin, Germany, 2012. [Google Scholar]
- Kessel, S.; Stroehl, M.; Meyer, H.; Hiss, S.; Sauerwein, H.; Schwarz, F.; Bruckmaier, R. Individual variability in physiological adaptation to metabolic stress during early lactation in dairy cows kept under equal conditions. J. Anim. Sci. 2008, 86, 2903–2912. [Google Scholar] [CrossRef] [PubMed]
- Rauls, C.; Meyer, U.; Hüther, L.; Von Soosten, D.; Kinoshita, A.; Rehage, J.; Breves, G.; Dänicke, S. Effects of niacin supplementation (40 weeks) and two dietary levels of concentrate on performance, blood and fatty acid profiles of dairy cattle. S. Afr. J. Anim. Sci. 2015, 45, 395–410. [Google Scholar] [CrossRef]
- Peterson, D.G.; Matitashvili, E.A.; Bauman, D.E. Diet-induced milk fat depression in dairy cows results in increased trans-10, cis-12 CLA in milk fat and coordinate suppression of mRNA abundance for mammary enzymes involved in milk fat synthesis. J. Nutr. 2003, 133, 3098–3102. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, E.; Rezende, R.; Bertics, S.; Grummer, R. Effects of transition diets varying in dietary energy density on lactation performance and ruminal parameters of dairy cows. J. Dairy Sci. 2003, 86, 916–925. [Google Scholar] [CrossRef]
- Bauman, D.E.; Griinari, J.M. Nutritional regulation of milk fat synthesis. Annu. Rev. Nutr. 2003, 23, 203–227. [Google Scholar] [CrossRef]
- Spurlock, D.; Dekkers, J.; Fernando, R.; Koltes, D.; Wolc, A. Genetic parameters for energy balance, feed efficiency, and related traits in Holstein cattle. J. Dairy Sci. 2012, 95, 5393–5402. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).