Feeding Diets Moderate in Physically Effective Fibre Alters Eating and Feed Sorting Patterns without Improving Ruminal pH, but Impaired Liver Health in Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Feeding
2.2. Feed Sampling and Analysis
2.3. Measurement of Particle Size and Sorting Behaviour
2.4. Measurement of Chewing Activity
2.5. Measurement of the Reticuloruminal pH and Monitoring of SARA Conditions
2.6. Determination of Milk Yield, Milk Composition, and Feed Efficiency
2.7. Blood Sampling and Analyses
2.8. Statistical Analysis
3. Results
3.1. DMI, Reticuloruminal pH, and Chewing Activity
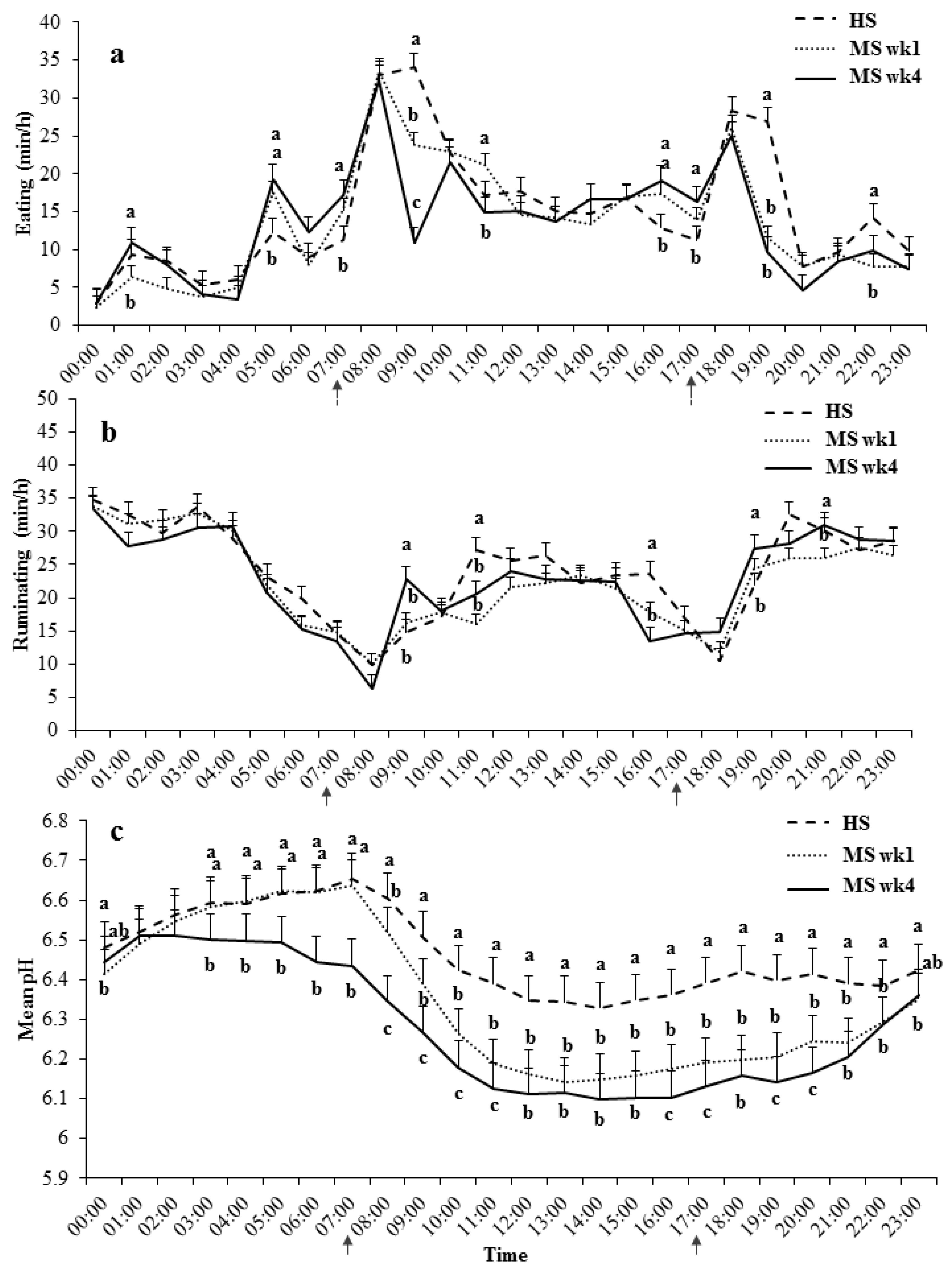
3.2. Daily Eating, Ruminating, and Mean pH Patterns
3.3. Sorting Behaviour
3.4. Milk Yield, Milk Composition, and Feed Efficiency
3.5. Blood Metabolites, Liver-Associated Variables, and Blood Minerals
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zebeli, Q.; Aschenbach, J.R.; Tafaj, M.; Boguhn, J.; Ametaj, B.N.; Drochner, W. Invited review: Role of physically effective fibre and estimation of dietary fibre adequacy in high-producing dairy cattle. J. Dairy Sci. 2012, 95, 1041–1056. [Google Scholar] [CrossRef] [PubMed]
- Humer, E.; Petri, R.; Aschenbach, J.R.; Bradford, B.; Penner, G.; Tafaj, M.; Südekum, K.H.; Zebeli, Q. Invited review: Practical feeding management recommendations to mitigate the risk of subacute ruminal acidosis in dairy cattle. J. Dairy Sci. 2018, 101, 872–888. [Google Scholar] [CrossRef]
- Enemark, J.M.D. The monitoring, prevention and treatment of sub-acute ruminal acidosis (SARA): A review. Vet. J. 2008, 176, 32–43. [Google Scholar] [CrossRef]
- German Society of Nutrition Physiology (GfE). Communications of the committee for requirement standards of the society of nutrition physiology: Evaluation of structural effectiveness of mixed rations for dairy cows—Status and perspectives. Proc. Soc. Nutr. Physiol. 2014, 23, 165–179. [Google Scholar]
- DeVries, T.J.; Dohme, F.; Beauchemin, K.A. Repeated ruminal acidosis challenges in lactating dairy cows at high and low risk for developing acidosis: Feed sorting. J. Dairy Sci. 2008, 91, 3958–3967. [Google Scholar] [CrossRef] [PubMed]
- DeVries, T.J.; Beauchemin, K.A.; Dohme, F.; Schwartzkopf-Genswein, K.S. Repeated ruminal acidosis challenges in lactating dairy cows at high and low risk for developing acidosis: Feeding, ruminating, and lying behavior. J. Dairy Sci. 2009, 92, 5067–5078. [Google Scholar] [CrossRef] [PubMed]
- Maulfair, D.D.; McIntyre, K.K.; Heinrichs, A.J. Subacute ruminal acidosis and total mixed ration preference in lactating dairy cows. J. Dairy Sci. 2013, 96, 6610–6620. [Google Scholar] [CrossRef] [PubMed]
- Kröger, I.; Humer, E.; Neubauer, V.; Reisinger, N.; Aditya, S.; Zebeli, Q. Modulation of chewing behavior and reticular pH in nonlactating cows challenged with concentrate-rich diets supplemented with phytogenic compounds and autolyzed yeast. J. Dairy Sci. 2017, 100, 9702–9714. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chang, G.; Zhang, K.; Xu, L.; Jin, D.; Bilal, M.S.; Shen, X. Rumen-derived lipopolysaccharide provoked inflammatory injury in the liver of dairy cows fed a high-concentrate diet. Oncotarget 2017, 8, 46769–46780. [Google Scholar] [CrossRef] [PubMed]
- German Society of Nutrition Physiology (GfE). Recommendations for the Supply of Energy and Nutrients to Dairy Cows and Heifers; Committee for Requirement Standards of the Society of Nutrition Physiology, DLG Verlag: Frankfurt am Main, Germany, 2001. [Google Scholar]
- Kononoff, P.J.; Heinrichs, A.J.; Buckmaster, D.R. Modification of the Penn State forage and total mixed ration particle separator and the effects of moisture content on its measurements. J. Dairy Sci. 2003, 86, 1858–1863. [Google Scholar] [CrossRef]
- VDLUFA Association of German Agricultural Analytic and Research Institutes. The Chemical Analysis of Feed, 8th ed.; VDLUFA-Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- Lammers, B.P.; Buckmaster, D.R.; Heinrichs, A.J. A simple method for the analysis of particle sizes of forage and total mixed rations. J. Dairy Sci. 1996, 79, 922–928. [Google Scholar] [CrossRef]
- Leonardi, C.; Armentano, L.E. Effect of quantity, quality, and length of alfalfa hay on selective consumption by dairy cows. J. Dairy Sci. 2003, 86, 557–564. [Google Scholar] [CrossRef]
- Kröger, I.; Humer, E.; Neubauer, V.; Kraft, N.; Ertl, P.; Zebeli, Q. Validation of a noseband sensor system for monitoring ruminating activity in cows under different feeding regimens. Livest. Sci. 2016, 193, 118–122. [Google Scholar] [CrossRef]
- Zebeli, Q.; Dijkstra, J.; Tafaj, M.; Steingass, H.; Ametaj, B.N.; Drochner, W. Modeling the adequacy of dietary fiber in dairy cows based on the responses of ruminal pH and milk fat production to composition of the diet. J. Dairy Sci. 2008, 91, 2046–2066. [Google Scholar] [CrossRef]
- Neubauer, V.; Humer, E.; Kröger, I.; Braid, T.; Wagner, M.; Zebeli, Q. Differences between pH of indwelling sensors and the pH of fluid and solid phase in the rumen of dairy cows fed varying concentrate levels. J. Anim. Physiol. Anim. Nutr. 2018, 102, 343–349. [Google Scholar] [CrossRef]
- DeVries, T.J.; Schwaiger, T.; Beauchemin, K.A.; Penner, G.B. Impact of severity of ruminal acidosis on feed-sorting behaviour of beef cattle. Anim. Prod. Sci. 2014, 54, 1238–1242. [Google Scholar] [CrossRef]
- González, L.A.; Manteca, X.; Calsamiglia, S.; Schwartzkopf-Genswein, K.S.; Ferret, A. Ruminal acidosis in feedlot cattle: Interplay between feed ingredients, rumen function and feeding behavior (a review). Anim. Feed Sci. Technol. 2012, 172, 66–79. [Google Scholar] [CrossRef]
- Tafaj, M.; Zebeli, Q.; Maulbetsch, A.; Steingaß, H.; Drochner, W. Effects of fibre concentration of diets consisting of hay and slowly degradable concentrate on ruminal fermentation and digesta particle size in mid—Lactation dairy cows. Arch. Anim. Nutr. 2006, 60, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Schirmann, K.; von Keyserlingk, M.A.G.; Weary, D.M.; Veira, D.M.; Heuwieser, W. Technical note: Validation of a system for monitoring rumination in dairy cows. J. Dairy Sci. 2009, 92, 6052–6055. [Google Scholar] [CrossRef]
- Chibisa, G.E.; Beauchemin, K.A.; Penner, G.B. Relative contribution of ruminal buffering systems to pH regulation in feedlot cattle fed either low- or high-forage diets. Animal 2016, 10, 1164–1172. [Google Scholar] [CrossRef]
- Neubauer, V.; Petri, R.; Humer, E.; Kröger, I.; Mann, E.; Reisinger, N.; Wagner, M.; Zebeli, Q. High-grain diets supplemented with phytogenic compounds or autolyzed yeast modulate ruminal bacterial community and fermentation in dry cows. J. Dairy Sci. 2018, 101, 2335–2349. [Google Scholar] [CrossRef]
- Dirksen, G.; Liebich, H.G.; Brosi, G.; Hagemeister, H.; Mayer, E. Morphology of the Rumen epithelium and fatty acid resorption in cattle—Important factors for health and performance. Zentralbl. Veterinärmed. 1984, 31, 414–430. [Google Scholar] [CrossRef]
- Qumar, M.; Khiaosa-ard, R.; Pourazad, P.; Wetzels, U.; Klevenhusen, F.; Aschenbach, J.R.; Zebeli, Q. Evidence of in vivo absorption of lactate and modulation of short chain fatty acid absorption from the reticulorumen of non-lactating cattle fed high concentrate diets. PLoS ONE 2016, 11, e0164192. [Google Scholar] [CrossRef]
- Sudweeks, E.; Ely, L.; Mertens, D.; Sisk, L. Assessing minimum amounts and form of roughages in ruminant diets: Roughage value index system. J. Anim. Sci. 1981, 53, 1406–1411. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Farr, B.I.; Rode, L.M.; Schaalje, G.B. Effects of alfalfa silage chop length and supplementary long hay on chewing and milk production of dairy cows. J. Dairy Sci. 1994, 77, 1326–1339. [Google Scholar] [CrossRef]
- Mertens, D.R. Creating a system for meeting the fiber requirements of dairy cows. J. Dairy Sci. 1997, 80, 1463–1481. [Google Scholar] [CrossRef]
- Plaizier, J.C.; Krause, D.O.; Gozho, G.N.; McBride, B.W. Subacute ruminal acidosis in dairy cows: The physiological causes, incidence and consequences. Vet. J. 2008, 176, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Chiquette, J.; Lagrost, J.; Girard, C.L.; Talbot, G.; Li, S.; Plaizier, J.C.; Hindrichsen, I.K. Efficacy of the direct-fed microbial enterococcus faecium alone or in combination with saccharomyces cerevisiae or lactococcus lactis during induced subacute ruminal acidosis. J. Dairy Sci. 2015, 98, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Chehreh, H.; Fartashvand, M. Evaluation of hepatic function markers of serum in dairy cattle with lactic acidosis. Indian J. Fundam. Appl. Life Sci. 2014, 4, 455–460. [Google Scholar]
- Zebeli, Q.; Dunn, S.; Ametaj, B.N. Strong associations among rumen endotoxin and acute phase proteins with plasma minerals in lactating cows fed graded amounts of concentrate. J. Anim. Sci. 2010, 88, 1545–1553. [Google Scholar] [CrossRef]
- Eckel, E.F.; Ametaj, B.N. Invited review: Role of bacterial endotoxins in the etiopathogenesis of periparturient diseases of transition dairy cows. J. Dairy Sci. 2016, 99, 5967–5990. [Google Scholar] [CrossRef]
- Kraft, W.; Dürr, U. Reference Values. Clinical Laboratory Diagnostics in Veterinary Medicine; Schattauer: Stuttgart, Germany, 2005; p. 512. [Google Scholar]
- Qumar, M.; Khiaosa-ard, R.; Klevenhusen, F.; Plaizier, J.C.; Zebeli, Q. Gastrointestinal endotoxin and metabolic responses in cows fed and recovered from two different grain-rich challenges. Livest. Sci. 2017, 203, 120–123. [Google Scholar] [CrossRef]
- Pourazad, P.; Khiaosa-ard, R.; Qumar, M.; Wetzels, S.U.; Klevenhusen, F.; Metzler-Zebeli, B.U.; Zebeli, Q. Transient feeding of a concentrate-rich diet increases the severity of subacute ruminal acidosis in dairy cattle. J. Anim. Sci. 2016, 94, 726–738. [Google Scholar] [CrossRef]
- Zebeli, Q.; Dunn, S.M.; Ametaj, B.N. Perturbations of plasma metabolites correlated with the rise of rumen endotoxin in dairy cows fed diets rich in easily degradable carbohydrates. J. Dairy Sci. 2011, 94, 2374–2382. [Google Scholar] [CrossRef]
- Wille, S.; Simon, A.; Platen, M.; Oertel, C. Factors influencing the activity of liver enzymes of clinically healthy dairy cows under field conditions. Züchtungskunde 2010, 82, 155–164. [Google Scholar]
- Nolan, J.P. The role of intestinal endotoxin in liver injury. Hepatology 2010, 52, 1829–1835. [Google Scholar] [CrossRef] [PubMed]
- Bossaert, P.; Trevisi, E.; Opsomer, G.; Bertoni, G.; De Vliegher, S.; Leroy, J.L.M.R. The association between indicators of inflammation and liver variables during the transition period in high-yielding dairy cows: An observational study. Vet. J. 2012, 192, 222–225. [Google Scholar] [CrossRef]
- DeAndrade, K.Q.; Moura, F.A.; Marques, J. Oxidative stress and inflammation in hepatic diseases: Therapeutic possibilities of n-acetylcysteine. Int. J. Mol. Sci. 2015, 16, 30269–30308. [Google Scholar] [CrossRef] [PubMed]
- Abaker, J.A.; Xu, T.L.; Jin, D.; Chang, G.J.; Zhang, K.M.; Shen, X.Z. Lipopolysaccharide derived from the digestive tract provokes oxidative stress in the liver of dairy cows fed a high-grain diet. J. Dairy Sci. 2017, 100, 666–678. [Google Scholar] [CrossRef]
| Item | HS | MS |
|---|---|---|
| Ingredient (% of DM) | ||
| Grass silage | 48.0 | 32.0 |
| Meadow hay | 12.0 | 8.0 |
| Barley grain | 25.2 | 37.8 |
| Soybean meal | 6.0 | 9.0 |
| Corn | 3.6 | 5.4 |
| Rapeseed meal | 3.2 | 4.8 |
| Beet pulp | 1.3 | 1.9 |
| Mineral–vitamin premix 1 | 0.4 | 0.6 |
| Monocalcium phosphate | 0.2 | 0.3 |
| Sodium chloride | 0.1 | 0.2 |
| Nutrient composition (% of DM unless otherwise stated) | ||
| DM, % of fresh matter | 46.1 | 47.6 |
| Organic matter | 91.0 | 92.3 |
| Crude protein | 16.2 | 17.2 |
| Ether extract | 2.1 | 2.5 |
| aNDFom 2 | 40.0 | 32.5 |
| ADF 3 | 24.1 | 18.6 |
| Starch | 18.8 | 27.7 |
| NEL 4 (MJ/kg DM) | 5.87 | 7.53 |
| Particle size distribution (% of DM) | ||
| >19 mm | 45.5 ± 8.76 | 35.4 ± 4.83 |
| 8.0–19.0 mm | 15.6 ±4.32 | 18.5 ± 8.52 |
| 1.18–8.0 mm | 34.4 ± 4.67 | 41.6 ± 4.76 |
| Pan (<1.18 mm) | 4.5 ± 1.23 | 4.5 ± 1.16 |
| pef 5 > 8 | 0.61 | 0.54 |
| pef > 1.18 | 0.96 | 0.96 |
| PeNDF 6 > 8 (% of DM) | 24.4 | 17.6 |
| peNDF > 1.18 (% of DM) | 38.4 | 31.2 |
| Item | Feeding Phase | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| HS | MS wk 1 | MS wk 2 | MS wk 3 | MS wk 4 | Phase | ||
| DMI (kg/d) | 22.3 d | 23.6 c | 23.8 b,c | 24.6 a,b | 24.8 a | 0.65 | <0.01 |
| Milk yield (kg/d) | 33.1 c,d | 34.0 b,c | 35.2 a | 35.1 a,b | 34.4 b | 1.1 | <0.01 |
| Time pH <6.0 (min/d) | 182 b | 344 a | 308 a | 353 a | 358 a | 81.4 | <0.01 |
| Mean pH | 6.46 a | 6.34 b | 6.33 b | 6.29 c | 6.27 c | 0.06 | <0.01 |
| Diurnal variation of pH 1 | 0.169 c | 0.239 a | 0.239 a | 0.243 a | 0.219 b | 0.0078 | <0.01 |
| Minimum pH | 6.09 a | 5.87 b | 5.82 b,c | 5.79 c | 5.81 c | 0.06 | <0.01 |
| Maximum pH | 6.81 a | 6.83 a | 6.78 b | 6.76 b | 6.70 c | 0.05 | <0.01 |
| Item | Feeding Phase 1 | SEM | p-Value | ||
|---|---|---|---|---|---|
| HS | MS wk 1 | MS wk 4 | Phase | ||
| Eating | |||||
| Min/d | 356 a | 320 b | 314 b | 16.8 | <0.01 |
| Chews/g of DMI 2 | 1.2 a | 1.0 b | 0.84 c | 0.07 | <0.01 |
| Min/kg DMI | 16.4 a | 14.4 b | 12.4 c | 0.88 | <0.01 |
| Min/kg total NDF 3 intake | 41.2 a,b | 44.0 a | 39.2 b | 2.57 | 0.10 |
| Min/kg peNDF 4 >8 | 66.9 b | 86.1 a | 68.3 b | 4.42 | <0.01 |
| Min/kg peNDF>1.18 | 44.6 | 45.7 | 41.3 | 1.81 | 0.15 |
| Ruminating | |||||
| Min/d | 568 a | 532 b | 547 a,b | 11.9 | 0.02 |
| Chews/g of DMI | 1.7 a | 1.5 b | 1.3 c | 0.04 | <0.01 |
| Min/kg DMI | 26.9 a | 23.7 b | 21.3 c | 0.67 | <0.01 |
| Min/kg total NDF intake | 67.7 b | 72.6 a | 67.9 b | 2.04 | 0.01 |
| Min/kg peNDF>8 | 110.0 c | 142.8 a | 118.1 b | 4.28 | <0.01 |
| Min/kg peNDF>1.18 | 74.2 | 74.2 | 72.0 | 2.18 | 0.68 |
| Ruminating boli | 597 a | 542 b | 557 b | 16.9 | <0.01 |
| Ruminating chews/bolus | 59.0 | 59.8 | 60.2 | 1.42 | 0.35 |
| Total chewing | |||||
| Min/d | 926 a | 853 b | 860 b | 19.1 | <0.01 |
| Chews/g of DMI | 2.9 a | 2.5 b | 2.2 c | 0.09 | <0.01 |
| Min/kg DMI | 43.1 a | 38.1 b | 33.8 c | 1.20 | <0.01 |
| Min/kg total NDF intake | 109 b | 117 a | 107 b | 3.54 | <0.01 |
| Min/kg peNDF>8 | 177 b | 229 a | 186 b | 6.62 | <0.01 |
| Min/kg peNDF>1.18 | 119 | 120 | 113 | 3.28 | 0.22 |
| Total chews/min | 70.6 a | 68.2 b | 67.9 b | 0.94 | <0.01 |
| Item | Feeding Phase | SEM | p-Value | ||
|---|---|---|---|---|---|
| HS | MS wk 1 | MS wk 4 | Phase | ||
| Sorting index 1, | |||||
| Long | 95.2 a,b | 97.2 a | 90.0 b | 2.31 | 0.09 |
| Medium | 102.1 b | 101.5 b | 108.8 a | 1.69 | 0.01 |
| Fine | 105.3 | 102.4 | 105.2 | 1.08 | 0.12 |
| Very fine | 98.6 a | 84.9 a,b | 70.3 b | 6.18 | 0.01 |
| Actual intake (kg/d) 2 | |||||
| Long | 20.3 a | 15.4 b | 16.7 b | 0.97 | <0.01 |
| Medium | 8.75 b | 11.4 a | 13.5 a | 0.79 | <0.01 |
| Fine | 17.3 b | 21.6 a | 21.1 a | 0.76 | <0.01 |
| Very fine | 1.71 a | 1.91 a | 1.07 b | 0.15 | <0.01 |
| Item | Feeding Phase | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| HS | MS wk 2 | MS wk 3 | MS wk 4 | Phase | ||
| ECM 1 (kg/d) | 30.7 b | 33.4 a,b | 36.0 a | 32.9 a,b | 1.57 | 0.02 |
| ECM/DMI 2 (kg/kg) | 1.45 b | 1.37 b | 1.63 a | 1.37 b | 0.071 | 0.02 |
| Fat (%) | 3.9 | 3.7 | 3.9 | 3.6 | 0.21 | 0.38 |
| Fat yield (kg/d) | 1.22 | 1.27 | 1.48 | 1.24 | 0.099 | 0.12 |
| Protein (%) | 3.3 c | 3.5 b | 3.6 b | 3.6 a | 0.06 | <0.01 |
| Protein yield (kg/d) | 1.05 b | 1.21 a | 1.23 a | 1.23 a | 0.040 | <0.01 |
| Fat: Protein | 1.2 | 1.1 | 1.1 | 1.0 | 0.08 | 0.17 |
| Lactose (%) | 4.8 a | 4.7 b | 4.7 b | 4.7 b | 0.04 | 0.01 |
| Lactose yield (kg/d) | 1.51 b | 1.64 a | 1.64 a | 1.61 a | 0.059 | <0.01 |
| SCC 3 (log10/mL) | 4.5 | 4.6 | 4.8 | 4.6 | 11.94 | 0.33 |
| MUN 4 (mg/dl) | 24.3 a | 17.9 c | 20.6 b | 23.3 a | 1.01 | <0.01 |
| pH | 6.7 a | 6.6 b | 6.6 b | 6.6 b | 0.01 | <0.01 |
| NDM 5 (%) | 8.9 b,c | 8.9 b | 9.0 a,b | 9.1 a | 0.08 | <0.01 |
| Item | Feeding Phase | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| HS | MS wk 2 | MS wk 3 | MS wk 4 | Phase | ||
| Glucose (mg/dl) | 57.4 b | 66.1 a | 65.9 a | 63.5 a | 1.44 | <0.01 |
| Cholesterol (mg/dl) | 198 a | 187 b | 178 b | 177 b | 8.32 | <0.01 |
| BHB 1 (mmol/l) | 0.59 a | 0.34 b | 0.29 b | 0.33 b | 0.03 | <0.01 |
| NEFA 2 (mmol/l) | 0.23 a | 0.10 b | 0.10 b | 0.09 b | 0.02 | <0.01 |
| Minerals 3 | ||||||
| Ca (mmol/l) | 2.54 a | 2.47 a,b | 2.44 b | 2.44 b | 0.04 | 0.10 |
| P (mmol/l) | 1.39 b | 1.35 b | 1.45 b | 1.69 a | 0.09 | 0.01 |
| Mg (mmol/l) | 1.03 b | 1.16 a | 1.09 a,b | 1.08 b | 0.01 | 0.03 |
| Liver health variables 4 | ||||||
| AST (U/L) | 90.9 b | 105.4 b | 130.8 a | 149.3 a | 9.90 | <0.01 |
| GLDH (U/L) | 17.5 b,c | 23.5 b | 43.7 a,b | 65.8 a | 9.85 | <0.01 |
| GGT (U/L) | 25.4 c | 27.1 c,b | 28.1 b | 30.3 a | 1.60 | <0.01 |
| AP (U/L) | 70.8 b | 75.6 a | 74.8 a | 77.1 a | 19.59 | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kröger, I.; Humer, E.; Neubauer, V.; Reisinger, N.; Zebeli, Q. Feeding Diets Moderate in Physically Effective Fibre Alters Eating and Feed Sorting Patterns without Improving Ruminal pH, but Impaired Liver Health in Dairy Cows. Animals 2019, 9, 128. https://doi.org/10.3390/ani9040128
Kröger I, Humer E, Neubauer V, Reisinger N, Zebeli Q. Feeding Diets Moderate in Physically Effective Fibre Alters Eating and Feed Sorting Patterns without Improving Ruminal pH, but Impaired Liver Health in Dairy Cows. Animals. 2019; 9(4):128. https://doi.org/10.3390/ani9040128
Chicago/Turabian StyleKröger, Iris, Elke Humer, Viktoria Neubauer, Nicole Reisinger, and Qendrim Zebeli. 2019. "Feeding Diets Moderate in Physically Effective Fibre Alters Eating and Feed Sorting Patterns without Improving Ruminal pH, but Impaired Liver Health in Dairy Cows" Animals 9, no. 4: 128. https://doi.org/10.3390/ani9040128
APA StyleKröger, I., Humer, E., Neubauer, V., Reisinger, N., & Zebeli, Q. (2019). Feeding Diets Moderate in Physically Effective Fibre Alters Eating and Feed Sorting Patterns without Improving Ruminal pH, but Impaired Liver Health in Dairy Cows. Animals, 9(4), 128. https://doi.org/10.3390/ani9040128





