Uterine Involution and Reproductive Performance in Dairy Cows with Metabolic Diseases
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Animals
2.3. Study Design
2.4. Blood Sampling and Analysis
2.5. Body Condition Score and Milk Yield
2.6. Reproductive Evaluation
2.7. Statistical Analysis
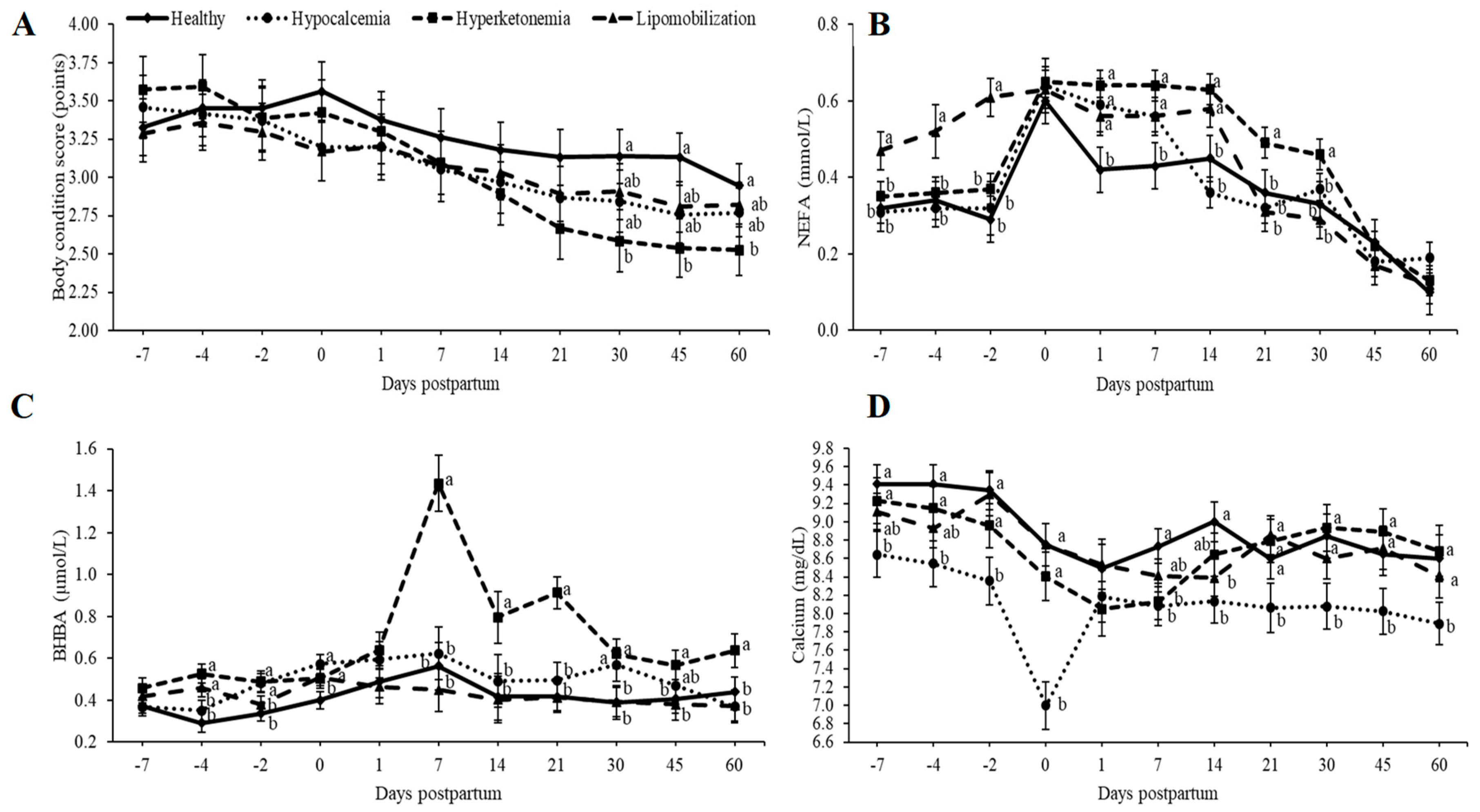
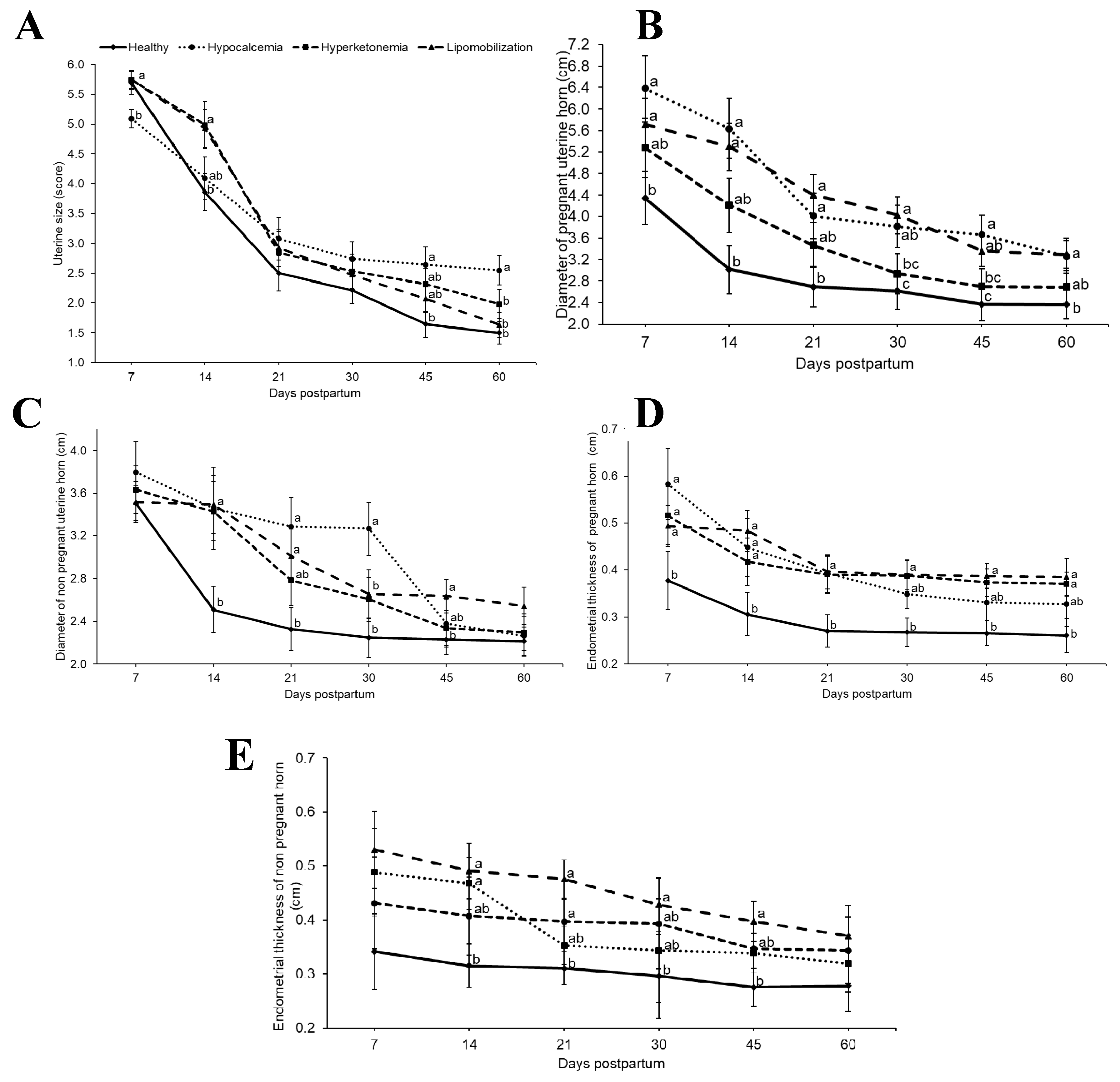
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Grummer, R.R. Impact of changes in organic nutrient metabolism on feeding the transition dairy cow. J. Dairy Sci. 1995, 73, 2820–2833. [Google Scholar] [CrossRef]
- Drackley, J.K. Biology of dairy cows during the transition period: The final frontier? J. Dairy Sci. 1999, 82, 2259–2273. [Google Scholar] [CrossRef]
- Bauman, D.E.; Currie, W.B. Partitioning of nutrients during pregnancy and lactation: A review of mechanisms involving homeostasis and homeorhesis. J. Dairy Sci. 1980, 63, 1514–1529. [Google Scholar] [CrossRef]
- Paiano, R.B.; Lahr, F.C.; Poit, D.A.S.; Costa, A.G.B.V.B.; Birgel, D.B.; Birgel Junior, E.H. Biochemical profile in dairy cows with artificial induction of lactation. Pesqui. Vet. Bras. 2018, 38, 2289–2292. [Google Scholar] [CrossRef]
- Hammon, D.S.; Evjen, I.M.; Dhiman, T.R.; Goff, J.P.; Walters, J.L. Neutrophil function and energy status in Holstein cows with uterine health disorders. Vet. Immunol. Immunopathol. 2006, 113, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Çağdaş, C. Physiological and metabolic changes during the transition period and the use of calcium propionate for prevention or treatment of hypocalcemia and ketosis in periparturient cows. J. Biol. Environ. Sci. 2013, 7, 9–17. [Google Scholar]
- Roche, J.R.; Kay, J.K.; Friggens, N.C.; Loor, J.J.; Berry, D.P. Assessing and managing body condition score for the prevention of metabolic disease in dairy cows. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 387–412. [Google Scholar] [CrossRef]
- Sordillo, L.M.; Raphael, W. Significance of metabolic stress, lipid mobilization, and inflammation on transition cow disorders. Vet. Clin. N. Am. Food Anim. Pract. 2013, 29, 267–278. [Google Scholar] [CrossRef]
- Zhang, G.; Hailemariam, D.; Dervishi, E.; Goldansaz, S.A.; Deng, Q.; Dunn, S.M.; Ametaj, B.N. Dairy cows affected by ketosis show alterations in innate immunity and lipid and carbohydrate metabolism during the dry off period and postpartum. Res. Vet. Sci. 2016, 107, 246–256. [Google Scholar] [CrossRef]
- Bicalho, M.L.S.; Marques, E.C.; Gilbert, R.O.; Bicalho, R.C. The association of plasma glucose, BHBA, and NEFA with postpartum uterine diseases, fertility, and milk production of Holstein dairy cows. Theriogenology 2017, 88, 270–282. [Google Scholar] [CrossRef]
- Borsberry, S.; Dobson, H. Periparturient diseases and their effect on reproductive performance in five dairy herds. Vet. Rec. 1989, 124, 217–219. [Google Scholar] [CrossRef]
- Heuwieser, W.; Tenhagen, B.A.; Tischer, M.; Luhr, J.; Blum, H. Effect of three programs for the treatment of endometritis on the reproductive performance of a dairy herd. Vet. Rec. 2000, 146, 338–341. [Google Scholar] [CrossRef]
- Elkjaer, K.; Labouriau, R.; Ancker, M.L.; Gustafsson, H.; Callesen, H. Short communication: Large-scale study on effects of metritis on reproduction in Danish Holstein cows. J. Dairy Sci. 2013, 96, 372–377. [Google Scholar] [CrossRef]
- Grunert, E.; Birgel, E.H.; Vale, W.G. Patologia e Clínica da Reprodução dos Animais Mamíferos Domésticos, 1st ed.; Varela: São Paulo, Brazil, 2005. [Google Scholar]
- Sheldon, I.M. The postpartum uterus. Vet. Clin. Food Anim. 2004, 20, 569–591. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Williams, E.J.; Miller, A.N.; Nash, D.M.; Herath, S. Uterine diseases in 445 cattle after parturition. Vet. J. 2008, 176, 115–121. [Google Scholar] [CrossRef]
- Bicalho, M.L.S.; Machado, V.S.; Oikonomou, G.; Gilbert, R.O.; Bicalho, R.C. Association between virulence factors of Escherichia coli, Fusobacterium necrophorum, and Aracanobacterium pyogenes and uterine diseases of dairy cows. Vet. Microbiol. 2012, 157, 125–131. [Google Scholar] [CrossRef]
- Mateus, L.; da Costa, L.L.; Bernardo, F.; Silva, J.R. Influence of puerperal uterine infection on uterine involution ovarian activity in dairy cows. Reprod. Domest. Anim. 2002, 37, 31–35. [Google Scholar] [CrossRef]
- Heppelmann, M.; Weinert, M.; Brömmling, A.; Piechotta, M.; Hoedemaker, M.; Bollwein, H. The effect of puerperal uterine disease on uterine involutionin cows assessed by Doppler sonography of the uterine arteries. Anim. Reprod. Sci. 2013, 143, 1–7. [Google Scholar] [CrossRef]
- Chapinal, N.; Carson, M.; Duffield, T.F.; Capel, M.; Godden, S.; Overton, M.; Santos, J.E.P.; LeBlanc, S.J. The association of serum metabolites with clinical disease during the transition period. J. Dairy Sci. 2011, 94, 4897–4903. [Google Scholar] [CrossRef]
- Chapinal, N.; Leblanc, S.J.; Carson, M.E.; Leslie, K.E.; Godden, S.; Capel, M.; Santos, J.E.; Overton, M.W.; Duffield, T.F. Herd-level association of serum metabolites in the transition period with disease, milk production, and early lactation reproductive performance. J. Dairy Sci. 2012, 95, 5676–5682. [Google Scholar] [CrossRef]
- Ribeiro, E.S.; Lima, F.S.; Greco, L.F.; Bisinotto, R.S.; Monteiro, A.P.A.; Favoreto, M.; Ayres, H.; Marsola, R.S.; Martinez, N.; Thatcher, W.W.; et al. Prevalence of periparturient diseases and impacts on fertility of seasonally calving grazing dairy cows supplemented with concentrates. J. Dairy Sci. 2013, 96, 5682–5697. [Google Scholar] [CrossRef]
- McArt, J.A.A.; Nydam, D.V.; Overton, T.R. Hyperketonemia in early lactation dairy cattle: A deterministic estimate of component and total cost per case. J. Dairy Sci. 2015, 98, 2043–2054. [Google Scholar] [CrossRef]
- Ospina, P.; Nydam, D.; Stokol, T.; Overton, T. Evaluation of nonesterified fatty acids and beta-hydroxybutyrate in transition dairy cattle in the northeastern United States: Critical thresholds for prediction of clinical diseases. J. Dairy Sci. 2010, 93, 546–554. [Google Scholar] [CrossRef]
- Goff, J.P. The monitoring, prevention, and treatment of milk fever and subclinical hypocalcemia in dairy cows. Vet. J. 2008, 176, 50–57. [Google Scholar] [CrossRef]
- Oetzel, G.R. Monitoring and testing dairy herds for metabolic disease. Vet. Clin. N. Am. Food Anim. Pract. 2004, 20, 651–674. [Google Scholar] [CrossRef]
- Ferguson, J.D.; Galligan, D.T.; Thomsen, N. Principal descriptors of body condition score in Holstein cows. J. Dairy Sci. 1994, 77, 2695–2703. [Google Scholar] [CrossRef]
- Grunert, E. Female genital system. In Clinical Examination of Cattle, 1st ed.; Rosenberger, G., Dirksen, G., Gründer, H.D., Grunert, E., Krause, D., Stöber, M., Eds.; Paul Parey: Berlin/Hamburg, Germany, 1979; pp. 323–350. [Google Scholar]
- López-Helguera, I.; López-Gatius, F.; Garcia-Ispierto, I. The influence of genital tract status in postpartum period on the subsequent reproductive performance in high producing dairy cows. Theriogenology 2012, 77, 1334–1342. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.; Goetze, L.; Donofrio, G.; Schuberth, H.J. Defining postpartum uterine disease and the mechanisms of infection and immunity in the female reproductive tract in cattle. Biol. Reprod. 2009, 81, 1025–1032. [Google Scholar] [CrossRef]
- Williams, E.J.; Fischer, D.P.; Pfeiffer, D.U.; England, G.C.; Noakes, D.E.; Dobson, H.; Sheldon, I.M. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 2005, 63, 102–117. [Google Scholar] [CrossRef]
- Kimura, K.; Reinhardt, T.; Goff, J. Parturition and hypocalcemia blunts calcium signals in immune cells of dairy cattle. J. Dairy Sci. 2006, 89, 2588–2595. [Google Scholar] [CrossRef]
- Galvão, K.N.; Flaminio, M.J.B.F.; Brittin, S.B.; Sper, R.; Fraga, M.; Caixeta, L.; Ricci, A.; Guard, C.L.; Butler, W.R.; Gilbert, R.O. Association between uterine disease and indicators of neutrophil and systemic energy status in lactating Holstein cows. J. Dairy Sci. 2010, 93, 2926–2937. [Google Scholar] [CrossRef]
- Martinez, N.; Risco, C.A.; Lima, F.S.; Bisinotto, R.S.; Greco, L.F.; Ribeiro, E.S.; Maunsell, F.; Galvão, K.; Santos, J.E.P. Evaluation of peripartal calcium status, energetic profile, and neutrophil function in dairy cows at low or high risk of developing uterine disease. J. Dairy Sci. 2012, 95, 7158–7172. [Google Scholar] [CrossRef]
- Saut, J.P.E.; Oliveira, R.S.B.R.; Martins, C.F.G.; Moura, A.R.F.; Tsuruta, A.S.; Nasciutti, N.R.; Dos Santos, R.M.; Headley, A.S. Clinical Observations of postpartum uterine involution in crossbred dairy cows. Vet. Not. 2011, 17, 16–25. [Google Scholar]
- Dervishi, E.; Zhang, G.; Hailemariam, D.; Dunn, S.M.; Ametaj, B.N. Occurrence of retained placenta is preceded by an inflammatory state and alterations of energy metabolism in transition dairy cows. J. Anim. Sci. Biotechnol. 2016, 7, 26. [Google Scholar] [CrossRef]
- Dervishi, E.; Zhang, G.; Hailemariam, D.; Goldansaz, S.A.; Deng, Q.; Dunn, S.M.; Ametaj, B.N. Alterations in innate immunity reactants and carbohydrate and lipid metabolism precede occurrence of metritis in transition dairy cows. Res. Vet. Sci. 2016, 104, 30–39. [Google Scholar] [CrossRef]
- Zhang, G.; Dervishi, E.; Ametaj, B.N. Milk fever in dairy cows is preceded by activation of innate immunity and alterations in carbohydrate metabolism prior to disease occurrence. Res. Vet. Sci. 2018, 117, 167–177. [Google Scholar] [CrossRef]
- Duffield, T.F.; Lissemore, K.D.; Mcbride, B.W.; Leslie, K.E. Impact of hyperketonemia in early lactation dairy cows on health and production. J. Dairy Sci. 2009, 92, 571–580. [Google Scholar] [CrossRef]
- Shin, E.K.; Jeong, J.K.; Choi, I.S.; Kang, H.G.; Hur, T.Y.; Jung, Y.H.; Kim, I.H. Relationships among ketosis, serum metabolites, body condition, and reproductive outcomes in dairy cows. Theriogenology 2015, 84, 252–260. [Google Scholar] [CrossRef]
- Goff, J.P. Macromineral physiology and application to the feeding of the dairy cow for prevention of milk fever and other periparturient mineral disorders. Anim. Feed Sci. Technol. 2006, 126, 237–257. [Google Scholar] [CrossRef]
- Aleri, J.W.; Hine, B.C.; Pyman, M.F.; Mansell, P.D.; Wales, W.J.; Mallard, B.; Fisher, A.D. Periparturient immunosuppression and strategies to improve dairy cow health during the periparturient period. Res. Vet. Sci. 2016, 108, 8–17. [Google Scholar] [CrossRef]
- Barletta, R.V.; Maturana Filho, M.; Carvalho, P.D.; Del Valle, T.A.; Netto, A.S.; Rennó, F.P.; Mingoti, R.D.; Gandra, J.R.; Mourão, G.B.; Fricke, P.M.; et al. Association of changes among body condition score during the transition period with NEFA and BHBA concentrations, milk production, fertility, and health of Holstein cows. Theriogenology 2017, 104, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Plöntzke, J.; Madoz, L.V.; De la Sota, R.L.; Drillich, M.; Heuwieser, W. Subclinical endometritis and its impact on reproductive performance in grazing dairy cattle in Argentina. Anim. Reprod. Sci. 2010, 122, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Barrio, M.; Vigo, M.; Quintela, L.A.; Becerra, J.J.; García-Herradón, P.J.; Martínez-Bello, D.; Fernandez-Sanchez, F.I.; Prieto, A.; Cainzos, J.; Peña, A.I. Influence of subclinical endometritis on the reproductive performance of dairy cows. Span. J. Agric. Res. 2015, 13, e05SC02. [Google Scholar] [CrossRef]
- Valergakis, G.E.; Oikonomou, G.; Arsenos, G.; Banos, G. Phenotypic association between energy balance indicators and reproductive performance in primiparous Holstein cows. Vet. Rec. 2011, 168, 189. [Google Scholar] [CrossRef]
- Walsh, R.B.; Leslie, K.E.; Leblanc, S.J.; Kelton, D.F.; Walton, J.S.; Duffield, T.F. The effects of subclinical ketosis in early lactation on reproductive performance of postpartum dairy cows. J. Dairy Sci. 2007, 90, 2788–2796. [Google Scholar] [CrossRef] [PubMed]
- McArt, J.A.A.; Nydam, D.V.; Oetzel, G.R.; Overton, T.R.; Ospina, P.A. Elevated non-esterified fatty acids and b-hydroxybutyrate and their association with transition dairy cow performance. Vet. J. 2013, 198, 560–570. [Google Scholar] [CrossRef] [PubMed]
- Walsh, R.B.; Kelton, D.F.; Duffield, T.F.; Leslie, K.E.; Walton, J.S.; Leblanc, S.J. Prevalence and risk factors for postpartum anovulatory condition in dairy cows. J. Dairy Sci. 2006, 90, 315–324. [Google Scholar] [CrossRef]
- Leroy, J.L.M.R.; Vanholder, T.; Van Knegsel, A.T.M.; Garcia-Ispierto, I.; Bols, P.E.J. Nutrient prioritization in dairy cows early postpartum: Mismatch between metabolism and fertility? Reprod. Domest. Anim. 2008, 43, 96–103. [Google Scholar] [CrossRef] [PubMed]
| Item | Cows without Metabolic Diseases | Hypocalcemia | Hyperketonemia | Lipomobilization |
|---|---|---|---|---|
| Milk yield, kg/d ± SD | 29.41 ± 2.10 a | 23.17 ± 3.14 b | 20.16 ± 3.24 b | 20.18 ± 3.63 b |
| Metritis, % (n) | 21.43 (3/14) | 36.36 (4/11) | 27.27 (3/11) | 42.86 (6/14) |
| Endometritis, % (n) | 14.29 (2/14) | 36.36 (4/11) | 27.27 (3/11) | 35.71 (5/14) |
| Service per pregnancy, mean ± SD | 2.14 ± 0.19 b | 3.18 ± 0.22 a | 2.63 ± 0.22 ab | 2.71 ± 0.19 b |
| First ovulation day, mean ± SD | 27.57 ± 3.53 b | 35.18 ± 3.99 ab | 45.45 ± 3.99 a | 42.00 ± 3.53 a |
| Days open, mean ± SD | 124.14 ± 7.67 b | 164.73 ± 8.66 a | 164.82 ± 8.66 a | 160.50 ± 7.67 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braga Paiano, R.; Becker Birgel, D.; Harry Birgel Junior, E. Uterine Involution and Reproductive Performance in Dairy Cows with Metabolic Diseases. Animals 2019, 9, 93. https://doi.org/10.3390/ani9030093
Braga Paiano R, Becker Birgel D, Harry Birgel Junior E. Uterine Involution and Reproductive Performance in Dairy Cows with Metabolic Diseases. Animals. 2019; 9(3):93. https://doi.org/10.3390/ani9030093
Chicago/Turabian StyleBraga Paiano, Renan, Daniela Becker Birgel, and Eduardo Harry Birgel Junior. 2019. "Uterine Involution and Reproductive Performance in Dairy Cows with Metabolic Diseases" Animals 9, no. 3: 93. https://doi.org/10.3390/ani9030093
APA StyleBraga Paiano, R., Becker Birgel, D., & Harry Birgel Junior, E. (2019). Uterine Involution and Reproductive Performance in Dairy Cows with Metabolic Diseases. Animals, 9(3), 93. https://doi.org/10.3390/ani9030093






