Simple Summary
In mammals, KISS-1 metastasis suppressor (KISS1) has emerged to stimulate the secretion of gonadotropin-releasing hormone (GnRH) to initiate the first estrus in the hypothalamus. However, KISS1 was recently demonstrated to be widely expressed in various ovarian compartments, including oocytes, granulosa cells (GCs) and theca cells. But the biological functionalities of KISS1 have not been explored in GCs. In this study, the overexpression plasmid of pcDNA3.1-KISS1 was built to explore the biological effects of KISS1 on the phosphoinositide 3-kinases (PI3K) signaling pathway, estrogen signaling pathway, cell apoptosis, cell cycle, and estradiol-17β (E2) secretion in porcine GCs. We found that overexpression of KISS1 could affect the PI3K signaling pathway, significantly decrease the apoptosis of GCs, and suppress GCs at G0/G1 phase of the cell cycle. Furthermore, overexpression of KISS1 could activate the estrogen synthesis signaling pathway and significantly increase the concentration of E2 in the supernatant and the mRNA expression levels of ESR1 and ESR2. These findings were highly accorded with the supposed role of KISS1 to promote the follicular development. This study would be of great interest for exploring the biological functionalities of KISS1 in regulating the maturation of follicles in mammals.
Abstract
Previous studies have strongly recommended that KISS-1 metastasis suppressor (KISS1) plays an essential gatekeeper of the initiation of reproductive maturation in mammals. However, KISS1 has been recently reported to highly express in ovarian granulosa cells (GCs). But the biological functionalities of KISS1 on cell apoptosis, cell cycle, and synthesis of estradiol-17β (E2) have not been explored in GCs. In this study, using porcine GCs as a cellular model, the overexpression plasmid of KISS1 was built to explore the biological effects of KISS1 on the PI3K signaling pathway, estrogen signaling pathway, cell apoptosis, cell cycle, and E2 secretion. We found that mRNA of KISS1 highly expressed in the ovary and significantly increased from immature to mature follicles in gilts. Overexpression of KISS1 could significantly increase the mRNA expression of PIK3CG, PIK3C1, and PDK1, and significantly decreased the mRNA levels of FOXO3, TSC2, and BAD of PI3K signaling pathway. Furthermore, results of the flow cytometry showed that overexpression of KISS1 significantly inhibited the apoptosis of GCs and decreased the percentage of GCs at G0/G1 phase of the cell cycle. Additionally, overexpression of KISS1 could increase the mRNA levels of Star, CYP17, 3B-HSD, 17B-HSD of estrogen synthesis signaling pathway, significantly increase the concentration of E2 in the supernatant of the cultured GCs, and up-regulate the mRNA expression levels of ESR1 and ESR2. These results suggested that KISS1 might suppress cell apoptosis through activating the PI3K signaling pathway and stimulate synthesis of E2 via boosting the estrogen synthesis signaling pathway. This study would be of great interests for exploring the biological functionalities of KISS1 in the folliculogenesis and sex steroid production of the ovaries in mammals.
1. Introduction
In many mammalian species, the initiation of first estrus indicating the sexual and reproductive maturation is activated as a result of an increase in the gonadotropin releasing hormone (GnRH) secretion [1,2]. Kisspeptins, the products of KISS1 gene, have recently emerged as an essential gatekeeper for the onset of first estrus via directing the stimulation of GnRH secretion at the hypothalamic level [3,4,5]. Recent studies have shown that KISS1 are widely expressed in the ovarian tissues [6,7], indicating its additional local function in reproduction at the extra-hypothalamic level. In gene knockout models, KISS1−/− [8,9] or KISS1 receptor (KISS1R)−/− [10,11] mice show small ovarian size and weight, compared to the wild-type counterparts. In cats, KISS1 and KISS1R are expressed in various ovarian compartments, including oocytes, granulosa cells (GCs) and theca cells [6,12]. Compared with the theca cells and other ovarian cells, previous studies report that KISS1 mRNA expression is significantly higher in the GCs and suggest that GC is the major site for kisspeptin synthesis in ovaries of mammals [6,13]. These results suggest the proposal and essential role of KISS1 in ovaries of mammals.
In mammals, it is widely known that GCs play a vital regulatory role in deciding the fate of follicles and the follicular maturation [14,15,16]. Furthermore, estrogens produced in GCs are suggested to support the survival and proliferation of GCs [17,18] and facilitate the maturation of follicles [19,20], which are mainly regulated and controlled by phosphatidylinositol 3-OH-kinase (PI3K) signaling pathway [21,22,23]. Recently, the mRNA level of KISS1 has been found to be significantly upregulated in human granulosa lutein cells obtained from women with polycystic ovary syndrome [24,25], and KISS1 has been suggested to stimulate progesterone in GCs of pigs [26]. However, the biological functions of KISS1 on cell survival and steroidogenesis were still unknown in mammalian GCs.
In this study, the expression changes of KISS1 mRNA were first detected between immature to mature follicles in pigs. Using porcine GCs as a cellular model, the overexpression plasmid of KISS1 was then built to explore the biological effects of KISS1 on the PI3K signaling pathway, estrogen signaling pathway, cell apoptosis, cell cycle, and estradiol-17β (E2) secretion. To the best of our knowledge, this study is the first time to explore the biological molecular functionalities of KISS1 on cell survival and steroidogenesis in mammalian GCs.
2. Methods and Materials
2.1. Ethics Approval
All experiments in the present study were performed in accordance with the guidelines of the Animal Care and Use Committee of South China Agricultural University Guangzhou, China (Approval Number: SCAU#2013-10).
2.2. Animals and Sample Preparation
In female pigs, the follicular maturation could be handily identified by the first standing reflex with the back-pressure test and boar contact [27]. Three Landrace × Yorkshire crossbred gilts at the day that they exhibited the first estrus and standing reflex were selected. The signs of first estrus were checked and recorded twice daily at 09:00 and 15:30 by inspection of the vulva and assessment of the standing reflex according to other studies [28,29]. The tissues of heart, liver, spleen, lung, cerebrum, cerebellum, pituitary, and ovary were collected from these pigs. At least three of the largest follicles (8–10 mm) of the unilateral ovaries from these gilts were collected as the mature follicles, and as least three of follicles within the length of 5–7 mm was collected as the immature follicles. Pigs were fed the same diet daily and reared in the same conditions and environments. The collected follicles were frozen quickly in liquid nitrogen and then stored at −80 °C for further use.
2.3. Culture of Porcine GCs In Vitro
The porcine ovarian GCs were cultured as previously described in our studies [30,31]. Briefly, ovaries were collected from a local slaughterhouse for pigs in Guangzhou and transferred to our laboratory in phosphate-buffered saline (PBS) containing penicillin (100 IU/mL) and streptomycin (100 μg/ mL) (Invitrogen, Shanghai, China) at a storage temperature of >30 °C. Subsequently, 5–7 mm follicles were punctured for GC collection using a 1-mL syringe, and the isolated GCs were washed twice with PBS preheated to 37 °C. The cells were seeded into 25-cm2 flasks and cultured at 37 °C under 5% CO2 in DMEM (Hyclone, Logan, UT, USA) containing 10% fetal bovine serum (Hyclone), 100 IU/mL penicillin, and 100 μg/mL streptomycin. When cells reached 80% coverage of the flask, cells were seeded into 24-well plates for further experiments.
2.4. Real-Time Quantitative PCR Analysis
When the cells reached 30–50% coverage of one well, pcDNA3.1-KISS1 and pcDNA3.1-Basic were transfected into the cells at 200 ng for 48 h, respectively. At least 3 wells per group was collected for extraction of total RNA. Total RNA was extracted using TRIzol reagent (TaKaRa, Tokyo, Japan) and then reverse-transcribed using a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, MA, USA) for mRNAs. The relative expression levels of mRNAs were quantified using Maxima SYBR Green qRT-PCR Master Mix (2×) (Thermo Scientific) -in a LightCycler Real-Time PCR system. The expression levels of GAPDH mRNAs were used as endogenous controls, and the fold changes were calculated using the 2−ΔΔct method. The primer sequences are listed in Table 1.

Table 1.
Primers used in the present study.
2.5. Cell Apoptosis Assay
According to the NCBI database, the coding sequences of KISS1 (Gene ID: 100145896, Accession Number: NM_001134964.1) were cloned into pcDNA3.1 (+) (ThermoFisher, Guangzhou, China) with the restrictive enzymes of EcoRI and NotI. The pcDNA3.1-KISS1 and pcDNA3.1-Basic were transfected in GCs by using LipofectamineTM 3000 Transfection Reagent (ThermoFisher).
Analysis and detection of cell apoptosis were referred to in one of our previous studies [32]. Cell apoptosis assays were performed using an Annexin V-FITC Apoptosis Detection Kit (BioVision, Milpitas, CA, USA) according to the manufacturer’s instructions. Briefly, GCs (1–5 × 105 cells/well) were cultured in triplicate in 6-well plates at one day prior to transfection. When the cells reached 30–50% coverage of one well, pcDNA3.1-KISS1 and pcDNA3.1-Basic were transfected into the cells at 200 ng for 48 h, respectively. The cells were then harvested, washed twice with ice-cold PBS, and resuspended in 500 μL of binding buffer. Next, 1.25 μL of Annexin V-FITC was added in the dark for 15 min at room temperature, then 1000× g centrifugation for 5 min at room temperature to remove the supernatant. The cells were gently resuspended with 0.5 mL precooling 1× solution, and 10 μL of PI (propidium iodide; 50 μg/mL, BD, New York, NY, USA) were added. Last, the cells were analyzed in a flow cytometer (Becton Dickinson Co., San Jose, CA, USA) using the FITC signal detector (FL1) and phycoerythrin emission signal detector (FL2). All experiments were performed at least three times. Cells in the lower right quadrant are annexin-positive/PI-negative early apoptotic cells. The cells in the upper right quadrant are annexin-positive/PI-positive late apoptotic cells. Cells undergoing early and late apoptosis were identified as the apoptotic cells.
2.6. Cell Cycle Analysis
When the cells reached 30–50% coverage of one well, pcDNA3.1-KISS1 and pcDNA3.1-Basic were transfected into the cells at 200 ng for 48 h, respectively. The cells were then harvested, washed twice with ice-cold PBS, and resuspended with a propidium iodide/RNase A solution at 37 °C in a dark for 30 min. Then cells were analyzed by flow cytometry (Becton Dickinson Co., San Jose, CA, USA). For each analysis, a minimum of 20,000 cells were analyzed.
2.7. ELISA for Measurements of Steroid Hormones
After transduction with pcDNA3.1-KISS1 and pcDNA3.1-Basic for 48 h, the concentrations of E2 in the culture supernatants were measured with ELISA kits (Beijing north institute of biological technology, Beijing, China) according to the manufacturer’s instructions.
2.8. Data Analysis
All cell experiments were repeated at least three times independently. For cell apoptosis, the fold change of cell apoptosis rate was presented, compared to the blank group. Compared to the blank group, the fold change of the concentrations of E2 was exhibited. Data were expressed as means ± SD of repeated experiments. Statistical analyses were carried out using R software (version-3.4.3, https://www.r-project.org/). Tukey’s test was used to test whether the variances among the mRNA relative expression of KISS1 in different tissues were significant. Student’s t-test (two-tailed) was used to analyze the significance of differences of two groups in data. * indicates p < 0.05; ** indicates p < 0.01.
3. Results
3.1. Expression of KISS1 during Follicular Maturation in Pigs
The mRNA expression pattern of KISS1 was first explored for tissues from gilts with the first standing reflex (see Methods and Materials, Section 2.2. Animals and Sample Preparation). Among the tissues of heart, liver, spleen, lung, cerebellum, pituitarium, and ovary, we found that mRNA of KISS1 expressed the highest in the ovary (Figure 1A). To further investigate the biological role of KISS1 during the follicular maturation, the mRNA expression levels of KISS1 were examined in porcine follicles from immature to mature (see Methods and Materials, Section 2.2. Animals and Sample Preparation) (Figure 1B), and mRNA expression levels of KISS1 in mature follicles were observed to be significantly higher than immature follicles (Figure 1C). These observations indicated that KISS1 might be involved in the processes of follicular mature in pigs.
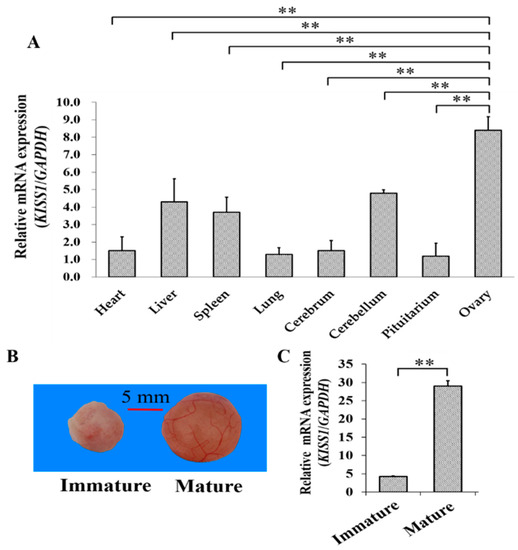
Figure 1.
Relative mRNA Expression of KISS1 during follicular maturation in pigs. (A) Tissue expression profile of KISS1 mRNA in gilts with the first standing reflex. (B) Representation of immature to mature follicles. (C) Changes of KISS1 mRNA expression from immature to mature follicles. ** indicates p < 0.01.
3.2. Biological Effects of KISS1 on Cell Apoptosis and Cell Cycle of GCs
The overexpression plasmid of KISS1 were then built to explore the effects of KISS1 on PI3K signaling pathway, apoptosis and cycle in GCs (Figure 2). We found that the mRNA expression of KISS1 was the highest at the 200 ng of pcDNA3.1-KISS1 (Figure 2A), and the 200 ng of pcDNA3.1-KISS1 plasmid was selected and used for further analysis. Compared to the control group, we found pcDNA3.1-KISS1 could significantly increase the mRNA expression levels of PIK3CG, PIK3C1 and PDK1 (Figure 2B), but pcDNA3.1-KISS1 significantly decreased the mRNA expression levels of FOXO3, TSC2 and BAD (Figure 2C).
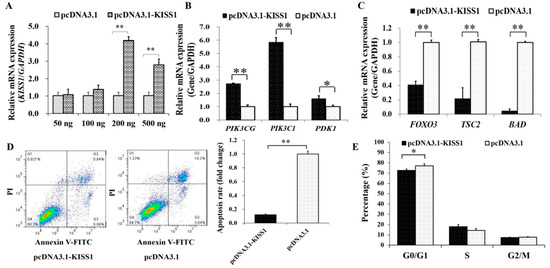
Figure 2.
Biological Effects of KISS1 on cell apoptosis and cell cycle of GCs. (A) Relative mRNA expressions of KISS1 against the different concentrations of pcDNA3.1-KISS1 plasmid. (B) Relative mRNA expressions of PIK3CG, PIK3C1, and PDK1 were stimulated by pcDNA3.1-KISS1. (C) Relative mRNA expressions of FOXO3, TSC2, and BAD were suppressed by pcDNA3.1-KISS1. (D) PcDNA3.1-KISS1 decreased cell apoptosis. (E) Effects of pcDNA3.1-KISS1 on the percentage of different cell cycle stage. * indicates p < 0.01. ** indicates p < 0.01. Data were represented as means ± SD.
Furthermore, the annexin V-FITC flow cytometry was used to analysis the cell apoptosis, and the apoptosis rate of GCs in pcDNA3.1-KISS1 group was observed to be significantly lower than the control group (Figure 2D). Analysis of cell cycle showed that pcDNA3.1-KISS1 significantly decreased the percentage of cells in G0/G1 (Figure 2E).
3.3. Biological Effects of KISS1 on Synthesis of Estrogen in GCs
To further validate the biological functions of KISS1 on the synthesis of E2 in GCs, pcDNA3.1-KISS1 was transfected into porcine GCs. The mRNA expression of Star, CYP17, 3B-HSD, 17BHSD and CYP19A from the estrogen signaling pathway were first detected (Figure 3A). Compared to the control group, overexpression of KISS1 could significantly increase mRNA expression levels of CYP19A but significantly decrease the mRNA expression levels of 3B-HSD (Figure 3A). Moreover, overexpression of KISS1 could significantly increase the concentrations of E2 in the culture supernatants (Figure 3B). Additionally, the mRNA expression changes of ESRs were also detected after the administration of pcDNA3.1-KISS1, and pcDNA3.1-KISS1 was observed to significantly increase the mRNA expression levels of ESR1 and ESR2 (Figure 3C).
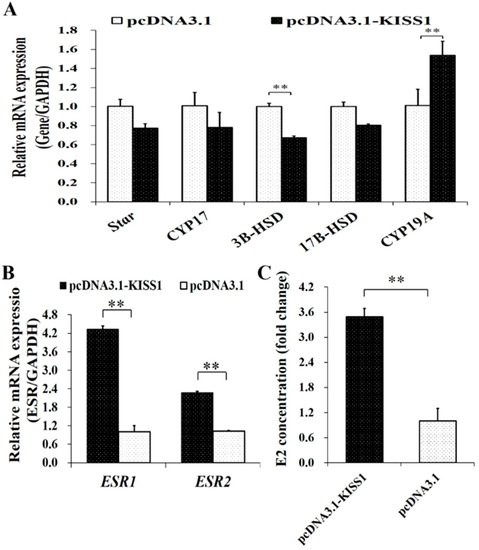
Figure 3.
Biological Effects of KISS1 on synthesis of estrogen in GCs. (A) Effects of pcDNA3.1-KISS1 on the relative mRNA levels of Star, CYP17, 3B-HSD, 17B-HSD, and CYP19A. (B) Concentrations of E2 was stimulated by pcDNA3.1-KISS1. (C) Effects of pcDNA3.1-KISS1 on the relative mRNA levels of ESR1 and ESR2. ** indicates p < 0.01. Data were represented as means ± SD.
4. Discussion
Recently, although new insight on KISS1 in ovaries has been described in several mammals [6,33,34], very little is known regarding the biological functions of KISS1 in the ovarian tissues. KISS1 has been suggested to highly express in GCs [6,13] and might play a significantly regulatory role in regulating follicular maturation in mammals [6,7,35]. In this study, we found that mRNA level of KISS1 expressed relatively highly in the ovary (Figure 1A) and significantly increased from immature to mature follicles (Figure 1B,C).
Most importantly, the relative mRNA level of KISS1 is significantly higher in the follicular stage than in the luteal stage [6]. In rats, KISS1 mRNA expression is changed in a cyclic-dependent manner in the ovary, with a rise during the preovulatory period [13,36]. In cows, compared to control group, administration of kisspeptin, the product of KISS1, can accelerate the follicular growth and increase the follicular sizes of the dominant follicles [34]. In mice, KISS1 has been identified as one of the differentially expressed genes of ESR2-mutant young adult females with the failure of follicular maturation [37]. In humans, compared to the normal women, the infertile women with polycystic ovary syndrome have increased serum kisspeptin levels [38], and serum kisspeptin has a positive correlation with the serum levels of LH and leptin [39]. These observations suggest that ovarian KISS1 may play a critical role in regulating the follicle maturation.
The PI3K signaling pathway is widely reported to be crucial for follicle growth [40] and promotes cell survival and suppress apoptosis in mammalian GCs [21,23]. Most importantly, the growth and proliferation of GCs play critical roles in the biological processes of recruitment, selection and maturation of follicles [20,41]. In this study, we found that pcDNA3.1-KISS1 could significantly increase the mRNA expression of PIK3CG, PIK3C1, PDK1 (Figure 2B), which are the important upstream genes of the PI3K signaling pathway. But pcDNA3.1-KISS1 significantly decreased the mRNA levels of FOXO3, TSC2, and BAD (Figure 2C), which are the important downstream genes of the PI3K signaling pathway. Previous studies recommend that PIK3CG exhibits significant DNA copy number gains in ovarian cancer, compared to normal ovary in humans [42], PIK3C1 promotes the activation of primordial follicles [43], and moreover, in PDK1 depletion in oocytes depletes the majority of primordial follicles around the onset of sexual maturity and causes premature ovarian failure in mice [44]. In addition, FOXO3 depletion in GCs disrupts the normal ovarian follicular growth in mice [45], and a lower FOXO3 mRNA expression in GCs may lead to infertility in women [46]. BAD can reduce progesterone levels by promoting ovarian GC apoptosis in sheep [47]. Being TSC2-deficient in oocytes results in the depletion of follicles in early adulthood [48]. Furthermore, results of Annexin V-FITC flow cytometry further identified that pcDNA3.1-KISS1 inhibited the apoptosis of GCs (Figure 2D). Moreover, pcDNA3.1-KISS1 was found to decrease the percentage of GCs at G0/G1 phase of the cell cycle (Figure 2E). These observations demonstrated that KISS1 was likely to be involved in the PI3K signaling pathway, suppress cell apoptosis, and regulate cell cycle entry.
In mammalian ovaries, E2 was mainly synthetized and produced in GCs [49,50] and then to stimulate the differentiation of GCs [17,18] and facilitate the maturation of follicles [19]. In this study, we found that pcDNA3.1-KISS1 could significantly increase the mRNA levels of 3B-HSD and CYP19A of estrogen synthesis signaling pathway (Figure 3A) and increase the concentration of E2 in the supernatant of the cultured GCs (Figure 3B). Moreover, pcDNA3.1-KISS1 could increase the mRNA expression levels of ESR1 and ESR2 (Figure 3C). These results suggested that KISS1 might stimulate the synthesis of E2 in porcine GCs.
5. Conclusions
Collectively, KISS1 was observed to involve in PI3K signaling pathway, suppress cell apoptosis, and stimulate synthesis of E2 via boosting the estrogen synthesis signaling pathway. Moreover, these findings were highly accorded with the supposed role of KISS1 promoting the follicular mature. These results would be of great interest for exploring the biologically molecular functionalities of KISS1 on cell survival and steroidogenesis in mammalian GCs.
Author Contributions
Conceived and designed the experiments: Z.Z., X.X. and J.L. Prepared biological samples: X.X., Q.L., J.W., Z.L. and Y.Z. Wrote the paper: X.Y., X.X. and Z.Z. Revised the paper: J.L., Z.Z., and H.Z. All authors reviewed and approved the final manuscript.
Funding
This work was supported by the earmarked fund for China Agriculture Research System (CARS-35), the Basic Work of Science and Technology Project (2014FY120800) and Guangdong Sailing Program (2014YT02H042).
Conflicts of Interest
The authors declare no competing interests.
References
- Plant, T.M. Neuroendocrine control of the onset of puberty. Front. Neuroendocr. 2015, 38, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Yoo, H.W. Control of puberty: Genetics, endocrinology, and environment. Curr. Opin. Endocrinol. Diabetes Obes. 2013, 20, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Leka-Emiri, S.; Chrousos, G.P.; Kanaka-Gantenbein, C. The mystery of puberty initiation: Genetics and epigenetics of idiopathic central precocious puberty (ICPP). J. Endocrinol. Investig. 2017, 40, 789–802. [Google Scholar] [CrossRef] [PubMed]
- Adekunbi, D.A.; Li, X.F.; Li, S.; Adegoke, O.A.; Iranloye, B.O.; Morakinyo, A.O.; Lightman, S.L.; Taylor, P.D.; Poston, L.; O’Byrne, K.T. Role of amygdala kisspeptin in pubertal timing in female rats. PLoS ONE 2017, 12, e0183596. [Google Scholar] [CrossRef] [PubMed]
- Toro, C.A.; Aylwin, C.F.; Lomniczi, A. Hypothalamic Epigenetics Driving Female Puberty. J. Neuroendocr. 2018, 30. [Google Scholar] [CrossRef] [PubMed]
- Tanyapanyachon, P.; Amelkina, O.; Chatdarong, K. The expression of kisspeptin and its receptor in the domestic cat ovary and uterus in different stages of the ovarian cycle. Theriogenology 2018, 117, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.L.; Zhao, H.; Chang, H.M.; Yu, Y.; Qiao, J. Kisspeptin/Kisspeptin Receptor System in the Ovary. Front. Endocrinol. (Lausanne) 2017, 8, 365. [Google Scholar] [CrossRef]
- Colledge, W.H. Transgenic mouse models to study Gpr54/kisspeptin physiology. Peptides 2009, 30, 34–41. [Google Scholar] [CrossRef]
- Lapatto, R.; Pallais, J.C.; Zhang, D.S.; Chan, Y.M.; Mahan, A.; Cerrato, F.; Le, W.W.; Hoffman, G.E.; Seminara, S.B. Kiss1(−/−) mice exhibit more variable hypogonadism than Gpr54(−/−) mice. Endocrinology 2007, 148, 4927–4936. [Google Scholar] [CrossRef]
- Funes, S.; Hedrick, J.A.; Vassileva, G.; Markowitz, L.; Abbondanzo, S.; Golovko, A.; Yang, S.J.; Monsma, F.J.; Gustafson, E.L. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem. Biopyhs. Res. Commun. 2003, 312, 1357–1363. [Google Scholar] [CrossRef]
- Seminara, S.B.; Messager, S.; Chatzidaki, E.E.; Thresher, R.R.; Acierno, J.S.; Shagoury, J.K.; Bo-Abbas, Y.; Kuohung, W.; Schwinof, K.M.; Hendrick, A.G.; et al. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 2003, 349, 1614–1627. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthi, V.P.; Khristi, V.; Ghosh, S.; Yerrathota, S.; Dai, E.; Roby, K.F.; Wolfe, M.W.; Rumi, M.A.K. ESR2 Is Essential for Gonadotropin-Induced Kiss1 Expression in Granulosa Cells. Endocrinology 2018, 159, 3860–3873. [Google Scholar] [CrossRef] [PubMed]
- Ricu, M.A.; Ramirez, V.D.; Paredes, A.H.; Lara, H.E. Evidence for a celiac ganglion-ovarian kisspeptin neural network in the rat: Intraovarian anti-kisspeptin delays vaginal opening and alters estrous cyclicity. Endocrinology 2012, 153, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Saatcioglu, H.D.; Cuevas, I.; Castrillon, D.H. Control of Oocyte Reawakening by Kit. PLoS Genet. 2016, 12, e1006215. [Google Scholar] [CrossRef] [PubMed]
- Douville, G.; Sirard, M.A. Changes in granulosa cells gene expression associated with growth, plateau and atretic phases in medium bovine follicles. J. Ovarian Res. 2014, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.I.; Dias, F.C.; Dufort, I.; Misra, V.; Sirard, M.A.; Singh, J. Stable reference genes in granulosa cells of bovine dominant follicles during follicular growth, FSH stimulation and maternal aging. Reprod. Fertil. Dev. 2016, 28, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef]
- Valdez, K.E.; Turzillo, A.M. Regulation of nuclear factor-kappaB (NF-kappaB) activity and apoptosis by estradiol in bovine granulosa cells. Mol. Cell Endocrinol. 2005, 243, 66–73. [Google Scholar] [CrossRef]
- Chou, C.H.; Chen, M.J. The Effect of Steroid Hormones on Ovarian Follicle Development. Vitam. Horm. 2018, 107, 155–175. [Google Scholar]
- McGee, E.A.; Hsueh, A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [CrossRef]
- Zheng, W.; Nagaraju, G.; Liu, Z.; Liu, K. Functional roles of the phosphatidylinositol 3-kinases (PI3Ks) signaling in the mammalian ovary. Mol. Cell Endocrinol. 2012, 356, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Makker, A.; Goel, M.M.; Mahdi, A.A. PI3K/PTEN/Akt and TSC/mTOR signaling pathways, ovarian dysfunction, and infertility: An update. J. Mol. Endocrinol. 2014, 53, R103–R118. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.L.; Cowan, R.G.; Harman, R.M.; Quirk, S.M. Cell cycle progression and activation of Akt kinase are required for insulin-like growth factor I-mediated suppression of apoptosis in granulosa cells. Mol. Endocrinol. 2004, 18, 326–338. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.L.; Zhao, H.; Min, Z.; He, Y.; Li, T.; Zhen, X.; Ren, Y.; Chang, H.M.; Yu, Y.; Li, R. Increased Expression of KISS1 and KISS1 Receptor in Human Granulosa Lutein Cells-Potential Pathogenesis of Polycystic Ovary Syndrome. Reprod. Sci. 2018. [Google Scholar] [CrossRef] [PubMed]
- Blasco, V.; Pinto, F.M.; Fernandez-Atucha, A.; Prados, N.; Tena-Sempere, M.; Fernandez-Sanchez, M.; Candenas, L. Altered expression of the kisspeptin/KISS1R and neurokinin B/NK3R systems in mural granulosa and cumulus cells of patients with polycystic ovarian syndrome. J. Assist. Reprod. Genet. 2019, 36, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Basini, G.; Grasselli, F.; Bussolati, S.; Ciccimarra, R.; Maranesi, M.; Bufalari, A.; Parillo, F.; Zerani, M. Presence and function of kisspeptin/KISS1R system in swine ovarian follicles. Theriogenology 2018, 115, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.L.; Willis, H.J.; Kirkwood, R.N.; Foxcroft, G.R. Impact of boar exposure on puberty attainment and breeding outcomes in gilts. Theriogenology 2002, 57, 2015–2025. [Google Scholar] [CrossRef]
- Ieda, N.; Uenoyama, Y.; Tajima, Y.; Nakata, T.; Kano, M.; Naniwa, Y.; Watanabe, Y.; Minabe, S.; Tomikawa, J.; Inoue, N.; et al. KISS1 gene expression in the developing brain of female pigs in pre- and peripubertal periods. J. Reprod. Dev. 2014, 60, 312–316. [Google Scholar] [CrossRef]
- Zhuo, Y.; Zhou, D.; Che, L.; Fang, Z.; Lin, Y.; Wu, D. Feeding prepubescent gilts a high-fat diet induces molecular changes in the hypothalamus-pituitary-gonadal axis and predicts early timing of puberty. Nutrition 2014, 30, 890–896. [Google Scholar] [CrossRef]
- Liu, J.Y.; Du, X.; Zhou, J.L.; Pan, Z.X.; Liu, H.L.; Li, Q.F. MicroRNA-26b Functions as a Proapoptotic Factor in Porcine Follicular Granulosa Cells by Targeting Sma-and Mad-Related Protein 4. Biol. Reprod. 2014, 91, 1–12. [Google Scholar] [CrossRef]
- Yuan, X.; Deng, X.; Zhou, X.; Zhang, A.; Xing, Y.; Zhang, Z.; Zhang, H.; Li, J. MiR-126-3p promotes the cell proliferation and inhibits the cell apoptosis by targeting TSC1 in the porcine granulosa cells. In Vitro Cell. Dev. Biol. Anim. 2018, 54, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Zhou, X.; He, Y.; Zhong, Y.; Zhang, A.; Zhang, Z.; Zhang, H.; Li, J. C/EBPbeta Promotes STAT3 Expression and Affects Cell Apoptosis and Proliferation in Porcine Ovarian Granulosa Cells. Genes 2018, 9, 295. [Google Scholar] [CrossRef] [PubMed]
- Fernandois, D.; Cruz, G.; Na, E.K.; Lara, H.E.; Paredes, A.H. Kisspeptin level in the aging ovary is regulated by the sympathetic nervous system. J. Endocrinol. 2017, 232, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Naniwa, Y.; Nakatsukasa, K.; Setsuda, S.; Oishi, S.; Fujii, N.; Matsuda, F.; Uenoyama, Y.; Tsukamura, H.; Maeda, K.; Ohkura, S. Effects of full-length kisspeptin administration on follicular development in Japanese Black beef cows. J. Reprod. Dev. 2013, 59, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Babwah, A.V. Kisspeptin: Beyond the brain. Endocrinology 2015, 156, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- Castellano, J.M.; Gaytan, M.; Roa, J.; Vigo, E.; Navarro, V.M.; Bellido, C.; Dieguez, C.; Aguilar, E.; Sanchez-Criado, J.E.; Pellicer, A.; et al. Expression of KiSS-1 in rat ovary: Putative local regulator of ovulation? Endocrinology 2006, 147, 4852–4862. [Google Scholar] [CrossRef] [PubMed]
- Khristi, V.; Chakravarthi, V.P.; Singh, P.; Ghosh, S.; Pramanik, A.; Ratri, A.; Borosha, S.; Roby, K.F.; Wolfe, M.W.; Rumi, M.A.K. ESR2 regulates granulosa cell genes essential for follicle maturation and ovulation. Mol. Cell. Endocrinol. 2018. [Google Scholar] [CrossRef]
- Gorkem, U.; Togrul, C.; Arslan, E.; Oruc, A.S.; Duman, N.B. Is there a role for kisspeptin in pathogenesis of polycystic ovary syndrome? Gynecol. Endocrinol. 2018, 34, 157–160. [Google Scholar] [CrossRef]
- Emekci Ozay, O.; Ozay, A.C.; Acar, B.; Cagliyan, E.; Secil, M.; Kume, T. Role of kisspeptin in polycystic ovary syndrome (PCOS). Gynecol. Endocrinol. 2016, 32, 718–722. [Google Scholar] [CrossRef]
- Li, Q.; He, H.; Zhang, Y.L.; Li, X.M.; Guo, X.; Huo, R.; Bi, Y.; Li, J.; Fan, H.Y.; Sha, J. Phosphoinositide 3-kinase p110delta mediates estrogen- and FSH-stimulated ovarian follicle growth. Mol. Endocrinol. 2013, 27, 1468–1482. [Google Scholar] [CrossRef]
- Hunter, M.G. Oocyte maturation and ovum quality in pigs. Rev. Reprod. 2000, 5, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, J.; Yang, N.; Greshock, J.; Liang, S.; Hasegawa, K.; Giannakakis, A.; Poulos, N.; O’Brien-Jenkins, A.; Katsaros, D.; et al. Integrative genomic analysis of phosphatidylinositol 3′-kinase family identifies PIK3R3 as a potential therapeutic target in epithelial ovarian cancer. Clin. Cancer Res. 2007, 13, 5314–5321. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Zhang, J.; Wen, J.; Wang, Y.; Niu, W.; Teng, Z.; Zhao, T.; Dai, Y.; Zhang, Y.; Wang, C.; et al. CDC42 controls the activation of primordial follicles by regulating PI3K signaling in mouse oocytes. BMC Biol. 2018, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Reddy, P.; Adhikari, D.; Zheng, W.; Liang, S.; Hamalainen, T.; Tohonen, V.; Ogawa, W.; Noda, T.; Volarevic, S.; Huhtaniemi, I.; et al. PDK1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum. Mol. Genet. 2009, 18, 2813–2824. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Castrillon, D.H.; Zhou, W.; Richards, J.S. FOXO1/3 depletion in granulosa cells alters follicle growth, death and regulation of pituitary FSH. Mol. Endocrinol. 2013, 27, 238–252. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Yamashita, Y.; Saito, N.; Hayashi, A.; Hayashi, M.; Terai, Y.; Ohmichi, M. Lower FOXO3 mRNA expression in granulosa cells is involved in unexplained infertility. J. Obstet. Gynaecol. Res. 2017, 43, 1021–1028. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.H.; Wang, X.Y.; Lu, L.L.; Li, X.Y.; Di, R.; He, X.Y.; Hu, W.P.; Zeng, X.Y.; Liu, Q.Y.; Chu, M.X. Expression and Functional Analysis of the BCL2-Associated Agonist of Cell Death (BAD) Gene in the Sheep Ovary During the Reproductive Cycle. Front. Endocrinol. 2018, 9. [Google Scholar] [CrossRef]
- Adhikari, D.; Flohr, G.; Gorre, N.; Shen, Y.; Yang, H.R.; Lundin, E.; Lan, Z.J.; Gambello, M.J.; Liu, K. Disruption of Tsc2 in oocytes leads to overactivation of the entire pool of primordial follicles. Mol. Hum. Reprod. 2009, 15, 765–770. [Google Scholar] [CrossRef]
- Schams, D.; Berisha, B. Steroids as local regulators of ovarian activity in domestic animals. Domest. Anim. Endocrin. 2002, 23, 53–65. [Google Scholar] [CrossRef]
- Conley, A.J.; Howard, H.J.; Slanger, W.D.; Ford, J.J. Steroidogenesis in the preovulatory porcine follicle. Biol. Reprod. 1994, 51, 655–661. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).