1. Introduction
There is ongoing loss of biological diversity worldwide (Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services, 2019). A major reason for this loss in Sweden is abandonment of grasslands and forest encroachment induced by a lack of large herbivores [
1]. This study was part of a 2.5-year project studying the effect of year-round grazing, without supplementary feeding, by horses on biological diversity and horse growth, health, and welfare. A recent publication from this project reports that horse grazing increases plant diversity and the presence of pollinators [
2]. Year-round horse grazing, as tested in the project, can therefore be recommended from a biological diversity perspective. However, to our knowledge, there have been no long-term studies on the occurrence of endo- and ectoparasites in such a system. A strategy to control endo- and ectoparasites is needed to keep grazing horses healthy and in functioning body condition, so that challenging weather and periodic feed shortages do not affect their health and welfare.
All grazing horses are infected with parasites. The most important parasites to control in Swedish horses are cyathostomins (small strongyles),
Strongylus vulgaris (large strongyle),
Anoplocephala perfoliata (tapeworm), and
Parascaris spp. (roundworm) [
3]. All these parasites are gastrointestinal nematodes transmitted by grazing infected pasture. The infection builds up on pasture when adult worms shed their eggs in horse feces. Grazing horses are then infected by ingestion of the infective stage of the parasite. Parasite infection can have a negative impact on horse health, e.g.,
S. vulgaris and
A. perfoliata are associated with an increased risk of colic [
4,
5], while a high burden of
Parascaris spp. worms can lead to rupture of the small intestine [
6]. Cyathostomins can cause larval cyathostominosis, which is associated with the sudden development of large numbers of cyathostome larvae in the wall of the large intestine [
7]. In addition to the above-mentioned symptoms, parasite infection can cause weight loss and reduced growth in foals [
3]. Apart from pasture-borne parasites, horses can also be infested with
Oxyuris equi (pinworm),
Gastrophilus spp. (bot fly), and the ectoparasite
Bovicola equi (chewing louse). Because of the obvious negative effects of many parasites, parasite monitoring is generally recommended, especially in foals and young horses (less than three years of age) as they are more susceptible to parasite infections.
The cornerstone in controlling gastrointestinal parasites has long been regular treatment with anthelmintic drugs, which belong to three major drug classes: (i) macrocyclic lactones, (ii) benzimidazoles, and (iii) tetrahydropyrimidines. However, overuse of these drugs has resulted in problems with widespread resistance to benzimidazoles among cyathostomins and more recently also resistance to pyrantel [
8]. However, in Sweden there is only suspected resistance to pyrantel among cyathostomins, with treatment efficacy 92% (confidence interval 80–96%) [
9,
10]. The problem is equally difficult with
Parascaris spp., with widespread resistance to macrocyclic lactones and emerging resistance to pyrantel [
8,
11]. Infestations of
B. equi are treated with a wide range of systemic or topical insecticides, including pyrethroids, organophosphates, and macrocyclic lactones [
3].
From a biological preservation perspective, the use of anthelmintics in grazing animals should be low or ideally zero. The ecotoxic effects of anthelmintic drugs are mainly caused by the macrocyclic lactones. These have a wide spectrum of activity against endo- and ectoparasites, which increases the potential for impacts on non-target organisms. Drugs belonging to the macrocyclic lactones are only partly metabolized by the animal, and the main route of excretion is via feces [
12]. Concentrations of macrocyclic lactones in feces from treated animals are lethally toxic for dung fauna and for freshwater invertebrates [
12,
13]. The two other drug classes, benzimidazoles and tetrahydropyrimidines, show little ecotoxic impact on dung or soil fauna [
12].
The risk of an individual animal being heavily infected with parasites is dependent on several individual factors, among which age [
14], immune response [
14], and grazing behavior [
15] may be the most important. To our knowledge, individual variations in parasite egg counts in young horses kept in a year-round grazing system without supplementary feeding have not been studied previously. In addition, little is known about how pasture area (enclosure) affects the variation in parasite egg excretion. If pasture area is important, such knowledge could be used to identify high- and low-risk pastures.
The present study investigated the occurrence of gastrointestinal parasites in horses kept for 2.5-years in three enclosures in a year-round grazing system without supplementary feeding. Specific objectives of the work were to (1) Evaluate the possibility of using only pyrantel (tetrahydropyrimidine) to reduce cyathostomin egg excretion by >85% at the following monthly sample, and (2) document the effect of individual animal, enclosure, and season on parasite egg counts. The relationship between parasite egg counts and body condition and the behavioral response of horses to an outbreak of B. equi were also studied. The hypotheses tested were that: (i) pyrantel can efficiently control parasites in a year-round grazing system and (ii) individual animal, enclosure, and season have effects that are relevant to consider in future free-range grazing systems.
2. Materials and Methods
The study was approved by Uppsala animal welfare ethics committee (license number: C28/14).
2.1. Project Outline
The study was carried out between May 2014 and September 2016 in Krusenberg, Uppsala, Sweden (59°44′8″ N, 17°38′58″ E). Three enclosures (13, 11, and 10 ha), consisting of approximately one-third grassland and two-thirds forest, were established. Based on prior estimates of grass production, the grassland area provided was expected to meet the energy requirements of three or four 250-kg horses per enclosure. The land had not been grazed by horses in the years before the study, but enclosures 1 and 2 had been grazed by cattle, and enclosure 3 had been used for forage production.
An ancient, endangered native horse breed, the Gotlandsruss, was used in the study because it is known as an ‘easy keeper’ and was expected to cope with a year-round free-range system without supplemental feeding at the latitude of the study site. Twelve one-year old Gotlandsruss stallions (mean body weight 185 ± 21 kg at the start) were purchased from six breeders and all breeders delivered two or three horses, with one exception (only one horse). The horses were divided into groups of four so that horses from the same breeder and same sire were spread in different groups (horses 1–4, 5–8, and 9–12 in groups A, B, and C, respectively). The study was performed according to a Latin square design, with three periods, three enclosures, and three groups of horses (
Table 1). The groups were randomly (by drawing) released into the enclosures in May 2014. The groups were rotated between the enclosures in May 2015 and May 2016, i.e., each group grazed each enclosure for one growing season. One horse had to be removed after 20 months due to an injury.
Each enclosure contained a shelter of 16 m2 and water was offered in automatic water troughs, located in the forest, during spring, summer, and autumn. When the temperature was below 0 °C in winter, water was offered once/day in plastic troughs. In all enclosures, even during wintertime, water was also available from streams in the forest. A salt block with trace minerals (May 2014–August 2014: Ab Hansson & Möhring, Halmstad, Sweden, content (mg/kg): zinc 300, manganese 200, copper 80, iodine 50, selenium 20 and cobalt 12. August 2014–September 2016: Standard, KNC, Netherlands, content (mg/kg): zinc 810, copper 220, iodine 100, selenium 20) was provided in all enclosures.
Horse body condition was scored weekly (scale 1–9) according to Henneke et al. [
16]. If a horse scored <4, it was moved temporarily from the study enclosure to a nearby ungrazed enclosure and kept there until it scored >4 and conditions in the original enclosure were considered good enough for maintenance of body condition. In total, four horses were removed during the first winter, for approximately three weeks each.
All horses were weighed on a scale every other week. Horses were checked daily for health issues (such as wounds, lameness, coughing, nose flow), thermoregulatory responses (such as shivering and sweating), and abnormal behavior (such as repeated scratching, looking at the belly, unwillingness to move, or a body position indicating depression).
2.2. Parasite Analysis
Fresh feces samples were collected on a monthly basis. For collection of individual samples, the horses were tracked until a minimum of two horses/enclosure defecated. Egg counts for strongyles and
Parascaris spp. were carried out for each horse by a modified centrifugation-enhanced McMaster technique using saturated NaCl solution with density 1.18 g/cm
3, with a theoretical sensitivity of 50 eggs per gram (EPG) [
17].
Individual larval cultures for detection of
S. vulgaris were performed on 50 g feces mixed with an equal volume of vermiculite (Weibulls, Sweden) according to Bellaw and Nielsen [
18]. Third-stage (L3) larvae were harvested after sedimentation for 12–16 h at +20 °C by the inverted Petri dish method [
19]. In brief, approximately 20 mL of the fluid were collected in a 50-mL Falcon tube and centrifuged at 248×
g for 3 min. Larvae were examined and identified under a microscope using morphological criteria, counting 100 larvae/culture when possible [
20].
Eggs of
A. perfoliata were isolated from 30 g feces using the modified flotation technique described by Beroza et al. [
21]. The feces were mixed with 60 mL tap water, strained into four 15-mL test-tubes, and centrifuged at 1000×
g for 10 min. The supernatant was removed and the pellet was dissolved in sugar salt solution (saturated sodium chloride solution with 50% glucose with density 1.280 g/cm
3) to form a convex meniscus. A cover glass (18 mm × 18 mm) was placed on top and the tubes were centrifuged for 5 min at 214×
g in a swing-out centrifuge. The samples were left for 5 min after centrifugation, and then transferred to microscope slides and microscopically examined at 40–100 × magnification.
Infection with O. equi was diagnosed by applying one piece of adhesive tape (7 cm × 2 cm) to the peri-anal region of each horse and then placing the tape on a glass slide and examining it under optical microscope (40 × magnification).
Occurrence (yes or no) of Gasterophilus spp. (botfly) eggs or egg shells in the horse coat was recorded on a weekly basis.
2.3. Bovicola Equi
In April 2015 (sunny daytime conditions), the number of B. equi adults and eggs in a circular area (diameter 22 mm) in the center of the forelock and mane was recorded for all horses. The location and total area of hair loss were subjectively estimated by one person for all individuals. Coat length was measured at the withers and rump, using a measuring tape, and a mean of these was calculated.
In addition, a behavioral study was performed where the numbers of scratching and rubbing sessions performed within 30 min were recorded for all horses in the study and also for a control group of horses (n = 7) known not to carry B. equi. These horses (age 2 years n = 3, 3 years n = 2, >6 years n = 2, two Gotlandruss and five Standardbred horses, all stallions but one) were kept in a free-range system with access to shelter on two farms nearby (within 20 km) and were changing from winter to summer coat, as were the experimental horses. These horses were checked daily (general health and behavior), as were the experimental horses. A scratching session was defined as a single or repeated scratching/rubbing movement. Three random recordings (the first horse that scratched after the 30 min session) were made, on three different horses, of the time spent on one scratching session, which was found to range from 2 to 8 s.
2.4. Deworming Strategies
All drug classes used were in oral formulations and approved for use in horses in Sweden [
22]. The drug of choice in this study was the tetrahydropyrimidine pyrantel embonate paste (Banminth
® Pharmaxim), due to the resistance of cyathostomins to fenbendazole and the documented ecotoxic effect of macrocyclic lactones. The intention was to deworm all horses when mean EPG of the study population (all 12 horses) was >200, regardless of horse group. Infections with cyathostomins and
O. equi were treated with pyrantel embonate paste at a dosage of 19 mg/kg bodyweight (6.6 mg of pyrantel base), infections with
P. equorum with fenbendazole (Axilur
® Intervet) at a dosage of 7.5 mg/kg bodyweight, and infections with
A. perfoliata with a double dose of Banminth
® Pharmaxim (38 mg/kg bodyweight) according to the manufacturers’ recommendations. The reduction in cyathostomin egg excretion was calculated at the next monthly sampling occasion. One of the other drugs used for deworming during the study was ivermectin (Normectin
® N-vet) at 0.2 mg/kg bodyweight prior to onset of the study. Moxidectin (Cydectin
® Orion Pharma Animal Health) at 0.4 mg/kg bodyweight was used when the reduction in cyathostomin eggs was ˂85% at the following monthly sample.
2.5. Statistical Analyses
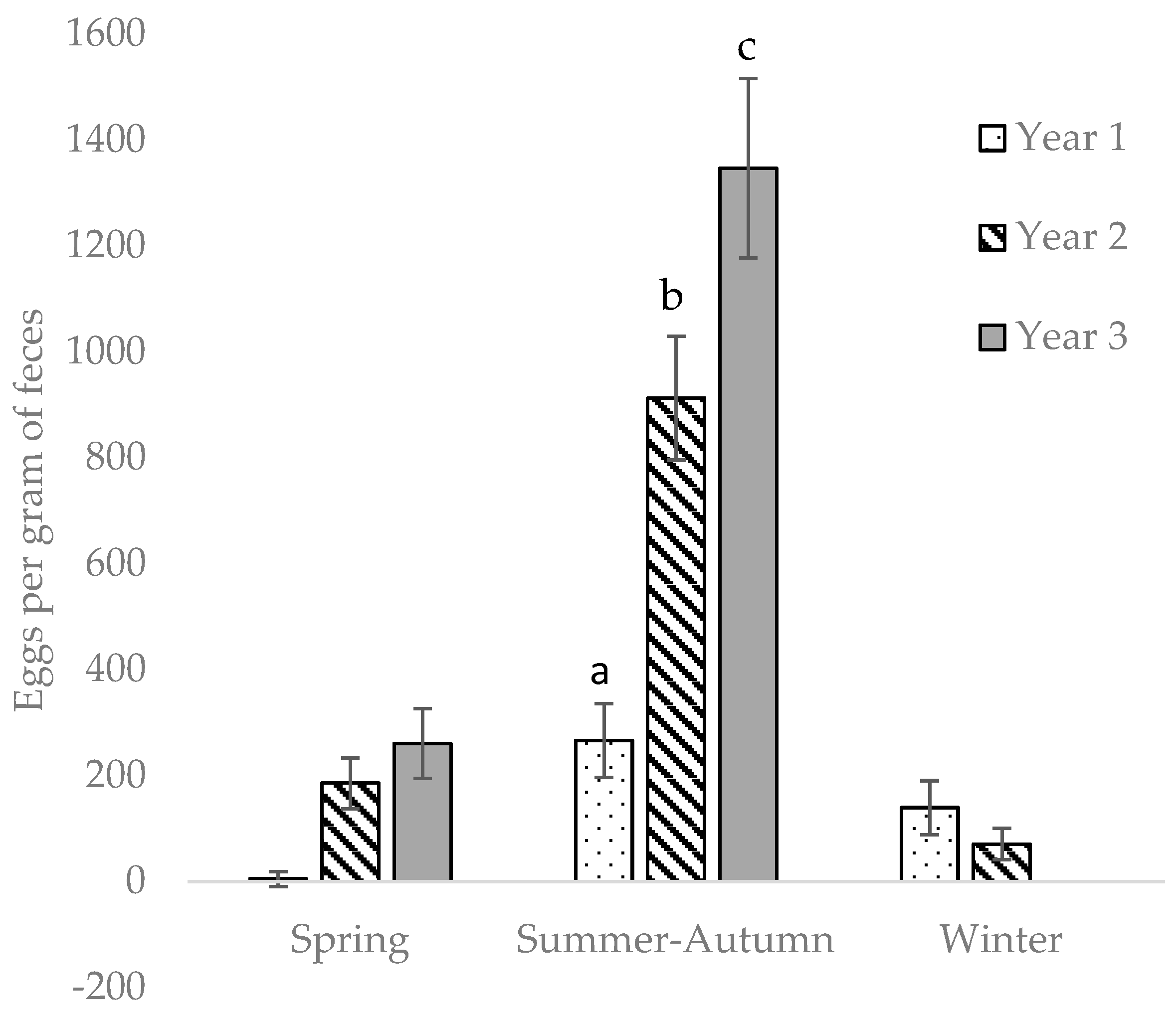
All statistical analyses were performed in Statistical Analysis Systems package 9.4 (SAS Institute Inc., Cary, NC, USA). Differences were considered significant at p < 0.05. The seasons were defined thus: November–February = winter, March–June = spring, July–October = summer–autumn. Each year was defined as running from March–February, i.e., the start of spring season until the end of winter season, as the experiment started in spring. To estimate differences in EPG between enclosures, seasons, and years, Poisson regression was used (procedure GLIMMIX in SAS), including the default log-link for this model, which means that the expected count of eggs in the model was log-transformed. To account for overdispersion, the residual variance was estimated separately using the random residual statement. The model included fixed effects of individual horse, year, and season, with enclosure as random effect. To analyze the effect of horse group on EPG, the same model, but without the effect of individual horse, was used. The effect of interaction between season and year was also tested.
For analysis of possible differences in EPG counts between horses temporarily removed during the first winter due to low body condition and those that remained in the project, the same model as described above was applied, using EPG counts from the first year of the study. This included fixed effects of month and body condition class (removed or retained in study enclosure), with enclosure as random effect. Values presented are least square means (LSmeans) and standard error of the mean (SEM), unless otherwise stated.
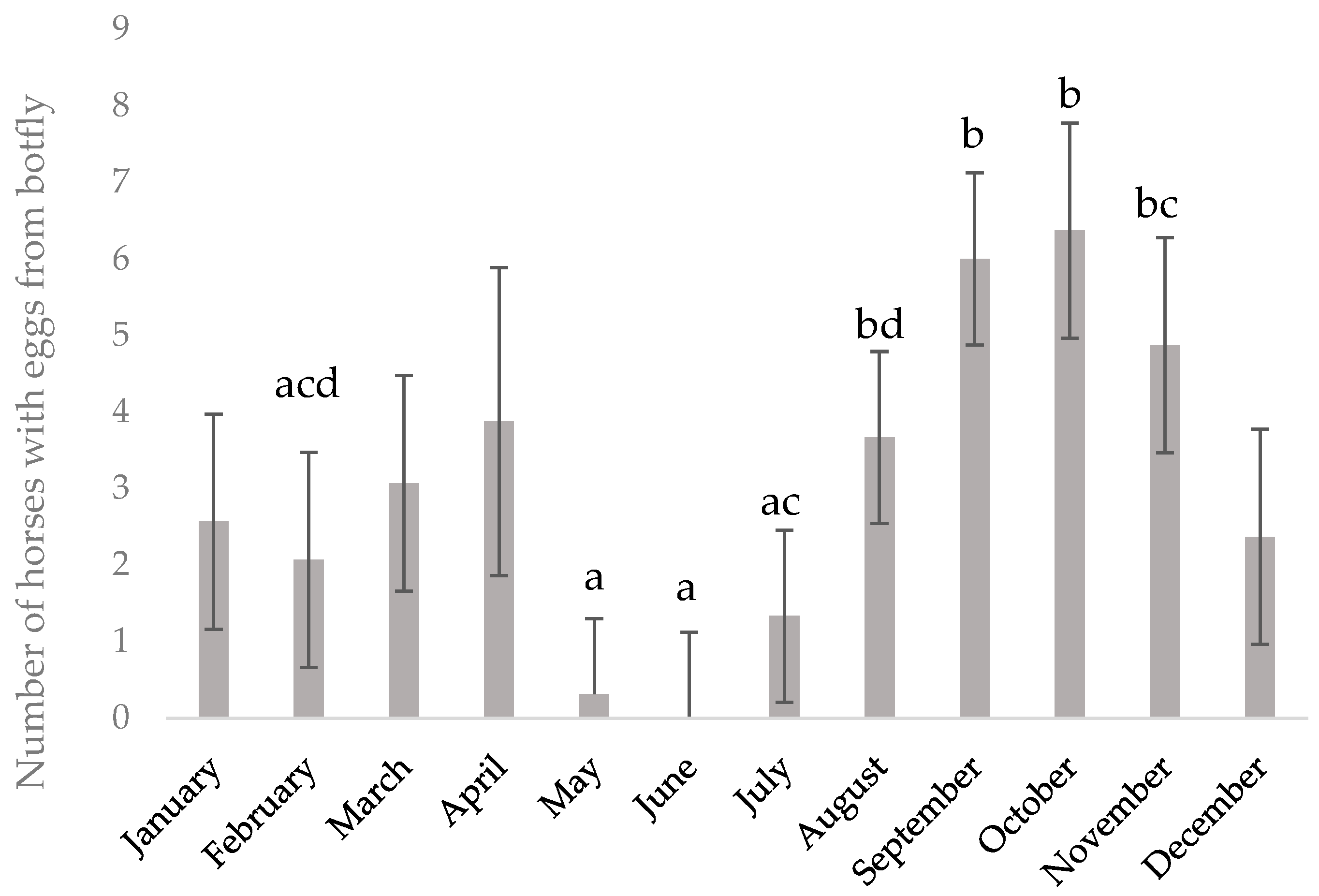
The numbers of horses on which Gastrophilus spp. eggs were found in the coat were summed up per month and analyzed for differences between months using the GLM procedure, including the effect of year. Results are presented as LSmeans ± SE.
For analysis of differences between the experimental horses and control horses in terms of number of scratching sessions, a two-sample t-test was used. A two-sample t-test was also used to compare coat, mane, and tail length between experimental horses on which B. equi eggs and adults were found (n = 6) and those on which no B. equi eggs or adults were found (n = 6). Results are presented as means ± SD.
4. Discussion
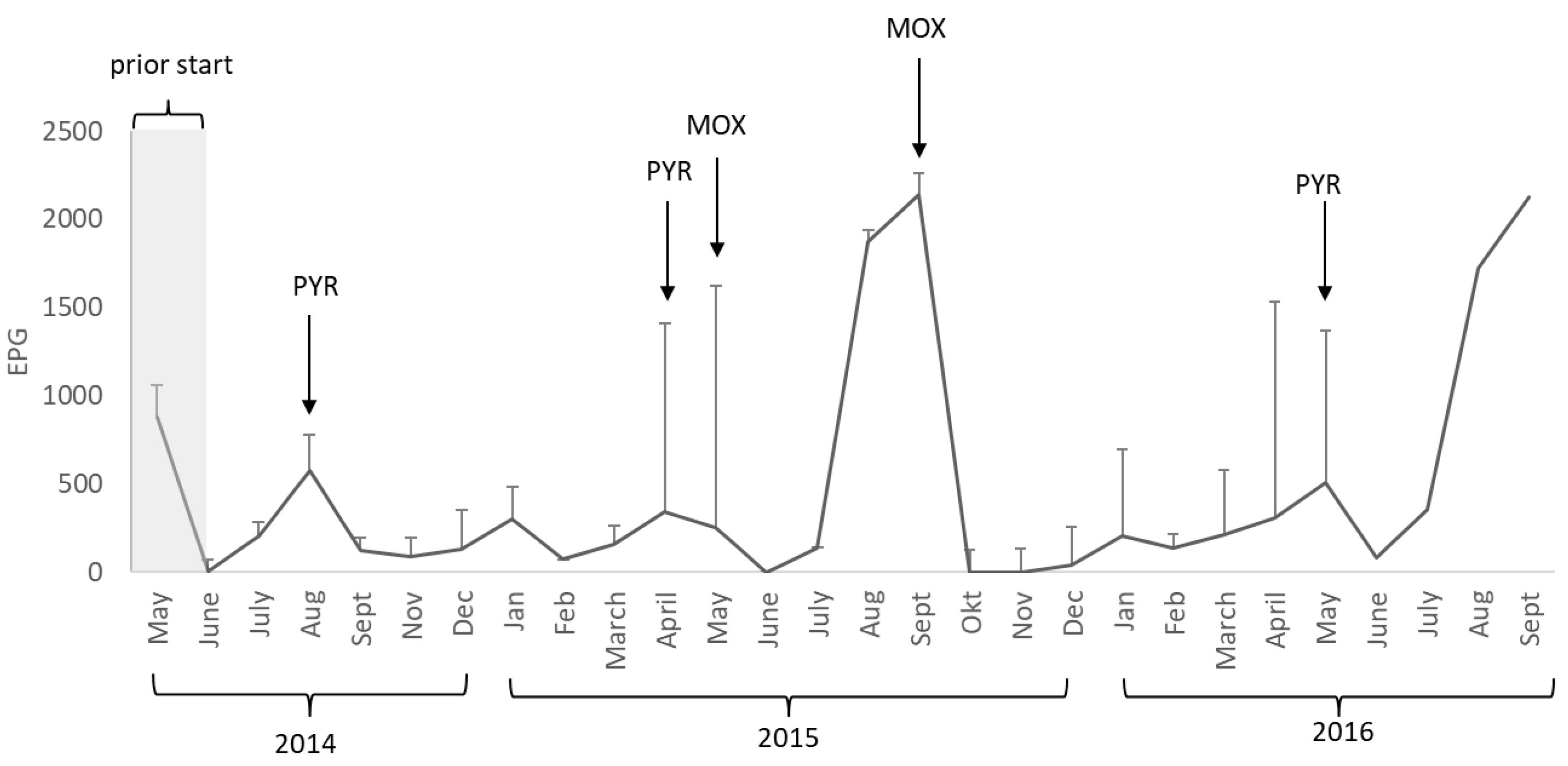
In this study, the horses started grazing clean pastures and were pre-treated with ivermectin. However, after only three months of grazing, the mean EPG for cyathostomins in the study population exceeded the threshold of 200 EPG. In addition, the horses were found to be infested with Parascaris spp., A. perfoliata, O. equi, B. equi, and Gastrophilus spp. (at least eggs or shells attached to the hair coat) throughout the study, but not with S. vulgaris. This proves the importance of monitoring parasite occurrence in young horses, even if a parasite-free pasture is used.
It is known that encysted cyathostomins undergo arrested development in the northern temperate climate zone [
23,
24], and that these encysted mucosal stages are generally not susceptible to single-dose anthelmintics (except moxidectin) [
25]. Parasites in this stage were not affected by the ivermectin treatment performed prior to onset of our study and were the source of contamination of the pastures.
The objective in this study was to use pyrantel for treatment of endoparasites, due to the widespread resistance to benzimidazoles reported in cyathostomins [
8] and the documented ecotoxic effect of macrocyclic lactones [
13]. Pyrantel-only treatment was not successful in reducing cyathostomin egg excretion by 85%. After two treatments with pyrantel (in August 2014 and April 2015), the observed reduction in egg excretion was only 24% at the next monthly sample. This required deworming with moxidectin in May 2015, with a reduction in egg excretion of 100% at the next monthly sample, but cyathostomin eggs re-occurred after only eight weeks, indicating a shorter egg reappearance period (ERP) than expected [
5]. As the mean EPG in the study population was escalating in August (1875 ± 1070) and September (2143 ± 1370) 2015, a second treatment with moxidectin was performed in September 2015. The final deworming in the study was performed in May 2016 using pyrantel, with an observed reduction in cyathostomin eggs of 84% at the next monthly sample. The intention of this study was not to evaluate treatment efficacy, but to monitor egg counts on a monthly basis. Thus the reduction in cyathostomin eggs reported here is based on a second sample taken approximately 21–28 days after treatment, and not 10–14 days after treatment as recommended by the guidelines on measuring clinical efficacy of the drug [
5]. However, the reduction in cyathostomin eggs and the ERP is not satisfactory according to the latest study performed in Sweden, with an ERP of 5–6 weeks [
9]. In summary, the horses were dewormed five times during the study, three times with pyrantel and twice with moxidectin, and the moxidectin treatments were more effective in reducing egg output than pyrantel.
During the study period, the weather conditions were within the expected range in the region [
26,
27], so the parasite survival observed can be assumed to be representative of the local circumstances. Temperature and moisture are important factors for the development from eggs to infective larvae (stage L3). An experimental study has shown that the development of cyathostomin eggs to L3 occurs at temperatures from +8 to +35 °C in laboratory conditions and takes between 3 and 24 days [
28]. A field study in Ukraine (similar winter climate to Sweden) found that strongyle eggs did not develop into L3 when the temperature was below +3 °C, but that L3 which had already developed could survive within feces deposits and act as a reservoir for re-infection of horses at the beginning of the grazing season [
29].
In our year-round grazing system, the pastures were continually contaminated with parasite eggs for three grazing seasons. There were two peaks of EPG per year, in spring and summer–autumn. We observed a gradual increase in cyathostomin EPG in the horses with every passing year, most likely due to increased numbers of L3 on the pasture, and the horses were re-infected soon after anthelmintic treatment.
Selective parasite treatment in horses is generally based on individual monitoring of fecal egg counts and treating only individuals that are shedding moderate to high numbers of eggs (>200 EPG) [
5]. The aim with this strategy is to reduce total egg contamination of pastures and lower the risk of grazing horses being infected with new parasites. However, it was not possible to determine individual fecal egg counts for all horses in this study, so a modified selective treatment strategy was applied to monitor parasite occurrence. A possible limitation of this approach is that not all horses were sampled on all occasions and therefore the results may be biased by some individuals to a higher extent than others. However, individual horses were sampled at least 14 times during the 2.5-year study, which can be considered quite frequent. To account for this, LSmeans (where missing values are taken into account) were used in presentation of results.
In addition to infection with cyathostomins, the horses in this study were infected with other gastrointestinal parasites, such as
Parascaris spp.,
A. perfoliata, and
O. equi, during the first season (2014/2015).
Parascaris spp. is considered to be most important parasite of foals, with most individuals developing age-dependent immunity at around one year of age [
6]. The horses in our study were 1.5 years of age in winter 2014/2015 and the number of eggs excreted was low (50–150 EPG), most likely due to emerging immunity. After one deworming with fenbendazole, the infection was successfully eradicated. In January–April 2015, infection with
O. equi spread among the horses, with 10 out of the 12 horses infected. Like
Parascaris spp., patent
O. equi infections are reported to be more common in weanlings and young adults [
30]. The
O. equi infection in our horses was effectively treated with fenbendazole in combination with manual cleansing of the perianal area on a weekly basis to decrease transmission of eggs to the surroundings. The
O. equi infection was the only infection that appeared to cause some discomfort in the horses, as they were observed scratching their tails now and then during this period. One horse also showed mucus excretion from the anus, as described earlier [
31]. Eggs of
A. perfoliata were detected in six out of the 12 horses in May–June 2015. This parasite has an indirect life cycle and is dependent on oribatid mites to complete the cycle [
32]. The horses were successfully treated with pyrantel and no re-infection occurred. This suggests that the number of oribatid mites, the intermediate host, was low on the pastures in all enclosures.
Horses with
B. equi infestation were found in all groups (A–C), and in total six out of the 12 horses were infested. A large variation in louse counts between individuals housed together has been described in horses [
33]. Studies on factors that render individual horses susceptible to lice infestation are scarce. Studies in which sheep were subjected to an equal challenge with
Bovicula ovis (sheep chewing louse) found large variations in susceptibility between individuals [
34]. Interestingly, in that study one-third of the ewes never became infested despite having lice applied on five separate occasions and also being housed together with infested animals. A number of factors, such as season, genotype, age, and body condition, have been suggested to influence susceptibility to lice infestation in sheep [
35], with individuals with slow growth and loss of body weight possibly being more susceptible [
34,
35]. However, in the present study there was no difference in body condition between infested and non-infested horses. The behavioral study showed that scratching behaviors were not more common in infested individuals than in individuals found to be lice-free. In addition, no effects that could be linked to the partial hair loss associated with
B. equi infestation were observed (e.g., horses were checked daily for shivering). This indicates that an infestation of 5–9
B. equi eggs and adults per 4 cm
2, as found in the present study, induces little or no challenge in affected horses and is therefore probably not a significant health and welfare problem. This knowledge is of relevance for management systems expected to support biological diversity, i.e., minimizing the use of insecticides, in this case anthelmintics.
As expected, the number of individuals with
Gastrophilus spp. eggs attached to the hair coat increased in August, but eggs, or at least egg shells, were found until April, i.e., as long as horses still had their winter coat. The possible impact of
Gastrophilus spp. on the health of the horses in this study is unknown, as the gastric mucosa was not examined. However, while some horses were likely infected with
Gastrophilus spp., this parasite is generally not associated with any serious health issues [
36].
The horses that were taken out of the study due to low body condition did not shed more strongyle eggs than the remaining horses. In wild horses that die from starvation, heavy parasite burdens have been observed [
37]. High intestinal parasite loads have also been linked to decreased gut microbial diversity and increased fecal excretion of metabolites [
38]. Body condition is reported to be negatively correlated with parasite burden in feral horses [
39]. The lower limit of body condition score set for horses to remain in the present study was 4 (scale 1–9), which is still ‘healthy’ condition (corresponding to the fat content of equine athletes [
40,
41]). The results indicate that at this body condition, horses may still invest energy in resistance to parasites, which may not be the case in horses in poorer body condition [
42,
43,
44]. The level of 4 was chosen because below this horses most likely face challenges in thermoregulation during cold, rainy weather. However, the link between body condition and strongyle egg counts in growing horses needs further investigation.
It is commonly argued that animal density/pasture availability has a strong effect on parasite occurrence [
15], indicating that increasing the pasture area would be one possible strategy to limit parasite occurrence. However, in the present study, pasture area and availability did not explain the variation in EPG, with the largest enclosure (2) showing the same EPG counts as the other enclosures. The area of grassland in enclosure 2 was 0.6 ha greater than in the other two enclosures, and it had a significantly greater amount of pasture (1393 ± 114, 957 ± 114, and 741 ± 114 kg dry matter/ha in enclosures 2, 1, and 3, respectively) and significantly greater energy availability (11204 ± 928, 8494 ± 895, and 7351 ± 895 MJ metabolisable energy/ha, respectively) [
26]). This implies that an increase of 0.15 ha/horse, or around 125 kg dry matter pasture per horse and month, is not enough to decrease cyathostomin egg excretion and thereby the need for anthelmintic treatment.
Sweden has been identified as an area where climate conditions may allow free ranging horses [
45]. The horse breed used in this study, the Gotlandsruss, was chosen due to its hardy qualities, as it is an ancient breed expected to have the traits required for extensive management. These include low energy requirements and a thick winter coat. Ability to withstand parasite infections may be another desirable trait in free-ranging horses. In this study, cyathostomins were the only endoparasite found in three individual horses, two of which were by the same sire and from the same breeder. There was also a large variation in cyathostomin EPG count between individuals. In addition, only half the horses were infested with chewing louse. Such differences should be taken into account both when selecting animals for year-round grazing systems and in breeding, as immunity to cyathostomins is a heritable trait [
42]. If year-round grazing is to be applied on a large scale to increase biodiversity in the cultivated landscape, individual feces sampling and selective treatment may not be feasible. Therefore, animals that excrete low amounts of parasite eggs should be used in these systems, as the contamination of pasture will be reduced. We also made the interesting observation that among the horses in the study, which included five chestnuts and seven bay horses, only bay horses were infested with lice. However, whether
B. equi has a preference for coat color or linked characteristics needs to be confirmed using a much larger dataset. Interestingly, it has been suggested previously that there could be an association between coat color and tolerance to
Haemonchus contortus infection in sheep [
46].