Genetic Characterization of Piroplasms in Donkeys and Horses from Nigeria
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Sample Collection
2.2. Identification of Equine Piroplasmosis Parasites Using Polymerase Chain Reaction (PCR)
2.3. DNA Sequencing and Analysis
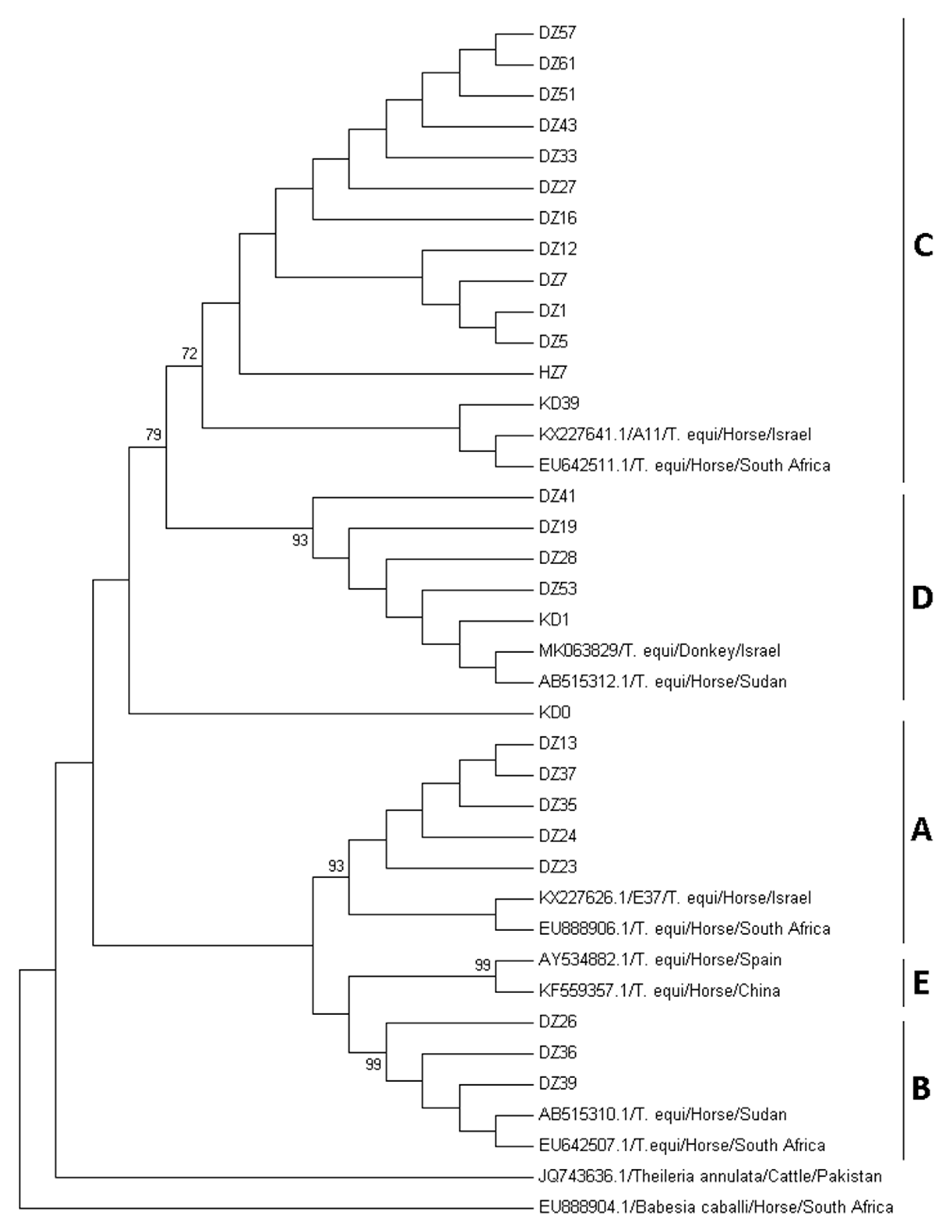
2.4. Phylogenetic Analysis and Genotyping of T. equi Sequences
2.5. Detection of anti-T. equi and anti-B. caballi Antibodies Using Immunofluorescence Antibody Test (IFAT)
3. Results
3.1. Study Population
3.2. Equine Piroplasmosis Infection in Donkeys
3.3. Equine Piroplasmosis Infection in Horses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hunfeld, K.P.; Hildebrandt, A.; Gray, J. Babesiosis: Recent insights into an ancient disease. Int. J. Parasitol. 2008, 38, 1219–1237. [Google Scholar] [CrossRef] [PubMed]
- Onyiche, T.E.; Suganuma, K.; Igarashi, I.; Yokoyama, N.; Xuan, X.; Thekisoe, O. A Review on Equine Piroplasmosis: Epidemiology, Vector Ecology, Risk Factors, Host Immunity, Diagnosis and Control. Int. J. Environ. Res. Public Health 2019, 16, 1736. [Google Scholar] [CrossRef] [PubMed]
- Rothschild, C.M. Equine piroplasmosis. J. Equine Vet. Sci. 2013, 33, 497–508. [Google Scholar] [CrossRef]
- Wise, L.N.; Kappmeyer, L.S.; Mealey, R.H.; Knowles, D.P. Review of equine piroplasmosis. J. Vet. Intern. Med. 2013, 27, 1334–1346. [Google Scholar] [CrossRef]
- Margalit Levi, M.; Tirosh-Levy, S.; Dahan, R.; Berlin, D.; Steinman, A.; Edery, N.; Savitski, I.; Lebovich, B.; Knowles, D.; Suarez, C.E.; et al. First Detection of Diffuse and Cerebral Theileria equi Infection in Neonatal Filly. J. Equine Vet. Sci. 2018, 60, 23–28. [Google Scholar] [CrossRef]
- Scoles, G.A.; Ueti, M.W. Vector ecology of equine piroplasmosis. Annu. Rev. Entomol. 2015, 60, 561–580. [Google Scholar] [CrossRef]
- Oguntomole, O.; Nwaeze, U.; Eremeeva, M.E. Tick-, flea-, and louse-borne diseases of public health and veterinary significance in Nigeria. Trop. Med. Infect. Dis. 2018, 3, 3. [Google Scholar] [CrossRef]
- Biu, A.; Ahmed, M.; Yunusa, A. Prevalence of equine babesiosis in Maiduguri, Nigeria. Int. J. Biomed. Hlth. Sci. 2006, 2, 81–83. [Google Scholar]
- Ehizibolo, D.O.; Kamani, J.; Ehizibolo, P.O.; Egwu, K.O.; Dogo, G.I.; Salami-Shinaba, J.O. Prevalence and significance of parasites of horses in some states of northern Nigeria. J. Equine Sci. 2012, 23, 1–4. [Google Scholar] [CrossRef]
- Garba, U.; Sackey, A.; Tekdek, L.; Agbede, R.; Bisalla, M. Clinical manifestations and prevalence of piroplasmosis in Nigerian royal horses. J. Vet. Adv. 2011, 1, 11–15. [Google Scholar]
- Mshelia, W.; Sambo, K.; Adamu, S.; Edeh, E.; Onoja, I. Persistence of equine piroplasmosis in horses in Nigeria. J. Equine Vet. Sci. 2016, 39, S104–S105. [Google Scholar] [CrossRef]
- Sanusi, M.; Ahmed, I.A.; Tahir, I.; Mai, H.M.; Kalla, D.J.U.; Shuaibu, I. Survey of equine piroplasmosis in the Savanna areas, Bauchi state, North-eastern Nigeria. Ippologia. Anno 2014, 25, 3–8. [Google Scholar]
- Turaki, U.; Kumsha, H.; Biu, A.; Bokko, P. Prevalence of Piroplasmosis amongst local horses in Northeastern Nigeria. IOSR J. Agri. Vet. Sci. 2014, 4, 27. [Google Scholar]
- Nkala, O. The donkey slaughter of West Africa. Oxpeckers Investigative Environmental Journalism. 2019. Available online: https://oxpeckers.org/2019/05/donkey-slaughter-capital-of-west-africa/ (accessed on 18 October 2019).
- Alhassan, A.; Govind, Y.; Tam, N.T.; Thekisoe, O.M.; Yokoyama, N.; Inoue, N.; Igarashi, I. Comparative evaluation of the sensitivity of LAMP, PCR and in vitro culture methods for the diagnosis of equine piroplasmosis. Parasitol. Res. 2007, 100, 1165–1168. [Google Scholar] [CrossRef]
- Munkhjargal, T.; Sivakumar, T.; Battsetseg, B.; Nyamjargal, T.; Aboulaila, M.; Purevtseren, B.; Bayarsaikhan, D.; Byambaa, B.; Terkawi, M.A.; Yokoyama, N.; et al. Prevalence and genetic diversity of equine piroplasms in Tov province, Mongolia. Infect. Genet. Evol. 2013, 16, 178–185. [Google Scholar] [CrossRef]
- Bhoora, R.; Franssen, L.; Oosthuizen, M.C.; Guthrie, A.J.; Zweygarth, E.; Penzhorn, B.L.; Jongejan, F.; Collins, N.E. Sequence heterogeneity in the 18S rRNA gene within Theileria equi and Babesia caballi from horses in South Africa. Vet. Parasitol. 2009, 159, 112–120. [Google Scholar] [CrossRef]
- Mahmoud, M.S.; El-Ezz, N.T.; Abdel-Shafy, S.; Nassar, S.A.; El Namaky, A.H.; Khalil, W.K.; Knowles, D.; Kappmeyer, L.; Silva, M.G.; Suarez, C.E. Assessment of Theileria equi and Babesia caballi infections in equine populations in Egypt by molecular, serological and hematological approaches. Parasit. Vectors 2016, 9, 260. [Google Scholar] [CrossRef]
- Rapoport, A.; Aharonson-Raz, K.; Berlin, D.; Tal, S.; Gottlieb, Y.; Klement, E.; Steinman, A. Molecular characterization of the Babesia caballi rap-1 gene and epidemiological survey in horses in Israel. Infect. Genet. Evol. 2014, 23, 115–120. [Google Scholar] [CrossRef]
- Kappmeyer, L.S.; Perryman, L.E.; Hines, S.A.; Baszler, T.V.; Katz, J.B.; Hennager, S.G.; Knowles, D.P. Detection of equine antibodies to Babesia caballi by recombinant B. caballi rhoptry-associated protein 1 in a competitive-inhibition enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1999, 37, 2285–2290. [Google Scholar] [CrossRef]
- Alhassan, A.; Pumidonming, W.; Okamura, M.; Hirata, H.; Battsetseg, B.; Fujisaki, K.; Yokoyama, N.; Igarashi, I. Development of a single-round and multiplex PCR method for the simultaneous detection of Babesia caballi and Babesia equi in horse blood. Vet. Parasitol. 2005, 129, 43–49. [Google Scholar] [CrossRef]
- Bhoora, R.; Quan, M.; Zweygarth, E.; Guthrie, A.J.; Prinsloo, S.A.; Collins, N.E. Sequence heterogeneity in the gene encoding the rhoptry-associated protein-1 (RAP-1) of Babesia caballi isolates from South Africa. Vet. Parasitol. 2010, 169, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Ketter-Ratzon, D.; Tirosh-Levy, S.; Nachum-Biala, Y.; Saar, T.; Qura’n, L.; Zivotofsky, D.; Abdeen, Z.; Baneth, G.; Steinman, A. Characterization of Theileria equi genotypes in horses in Israel, the Palestinian Authority and Jordan. Ticks Tick Borne Dis. 2017, 8, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Steinman, A.; Zimmerman, T.; Klement, E.; Lensky, I.M.; Berlin, D.; Gottlieb, Y.; Baneth, G. Demographic and environmental risk factors for infection by Theileria equi in 590 horses in Israel. Vet. Parasitol. 2012, 187, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, R.; Goyal, L.; Gupta, A.K. Theileria equi equine merozoite antigen-2 (EMA-2) gene (Indian strain) sequence from a Karnal isolate, NCBI GenBank Acc. KC347578.1. 2018. Available online: https://www.ncbi.nlm.nih.gov/nuccore/KC347578.1 (accessed on 18 October 2019).
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Dahmana, H.; Amanzougaghene, N.; Davoust, B.; Normand, T.; Carette, O.; Demoncheaux, J.-P.; Mulot, B.; Fabrizy, B.; Scandola, P.; Chik, M. Great diversity of Piroplasmida in Equidae in Africa and Europe, including potential new species. Vet. Parasitol. Reg. Stud. Rep. 2019, 18, 100332. [Google Scholar] [CrossRef]
- Ros-Garcia, A.; M’Ghirbi, Y.; Hurtado, A.; Bouattour, A. Prevalence and genetic diversity of piroplasm species in horses and ticks from Tunisia. Infect. Genet. Evol. 2013, 17, 33–37. [Google Scholar] [CrossRef]
- Salim, B.; Bakheit, M.A.; Kamau, J.; Nakamura, I.; Sugimoto, C. Nucleotide sequence heterogeneity in the small subunit ribosomal RNA gene within Theileria equi from horses in Sudan. Parasitol. Res. 2010, 106, 493–498. [Google Scholar] [CrossRef]
- Knowles, D.P.; Kappmeyer, L.S.; Haney, D.; Herndon, D.R.; Fry, L.M.; Munro, J.B.; Sears, K.; Ueti, M.W.; Wise, L.N.; Silva, M.; et al. Discovery of a novel species, Theileria haneyi n. sp., infective to equids, highlights exceptional genomic diversity within the genus Theileria: Implications for apicomplexan parasite surveillance. Int. J. Parasitol. 2018, 48, 679–690. [Google Scholar] [CrossRef]
- Wise, L.N.; Kappmeyer, L.S.; Knowles, D.P.; White, S.N. Evolution and diversity of the EMA families of the divergent equid parasites, Theileria equi and T. haneyi. Infect. Genet. Evol. 2019, 68, 153–160. [Google Scholar] [CrossRef]
- Bhoora, R.; Quan, M.; Matjila, P.T.; Zweygarth, E.; Guthrie, A.J.; Collins, N.E. Sequence heterogeneity in the equi merozoite antigen gene (ema-1) of Theileria equi and development of an ema-1-specific TaqMan MGB assay for the detection of T. equi. Vet. Parasitol. 2010, 172, 33–45. [Google Scholar] [CrossRef]
- Manna, G.; Cersini, A.; Nardini, R.; Bartolome Del Pino, L.E.; Antognetti, V.; Zini, M.; Conti, R.; Lorenzetti, R.; Veneziano, V.; Autorino, G.L.; et al. Genetic diversity of Theileria equi and Babesia caballi infecting horses of Central-Southern Italy and preliminary results of its correlation with clinical and serological status. Ticks Tick Borne Dis. 2018, 9, 1212–1220. [Google Scholar] [CrossRef]
- Knowles, D.P., Jr.; Kappmeyer, L.S.; Stiller, D.; Hennager, S.G.; Perryman, L.E. Antibody to a recombinant merozoite protein epitope identifies horses infected with Babesia equi. J. Clin. Microbiol. 1992, 30, 3122–3126. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, R.; Gupta, A.K.; Yadav, S.C.; Goyal, S.K.; Khurana, S.K.; Singh, R.K. Development of EMA-2 recombinant antigen-based enzyme-linked immunosorbent assay for seroprevalence studies of Theileria equi infection in Indian equine population. Vet. Parasitol. 2013, 198, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, R.; Sugimoto, C. A perspective on Theileria equi infections in donkeys. Japan. J. Vet. Res. 2009, 56, 171–180. [Google Scholar]
| Primer | Sequence 5′-3′ | Target Gene | Amplicon Size (bp) | Reference |
|---|---|---|---|---|
| Bec-UF2 | TCGAAGACGATCAGATACCGTCG | T. equi/B. caballi 18S rRNA | 400 | [21] |
| Equi-R | TGCCTTAAACTTCCTTGCGAT | |||
| Bc9_RAP2F | ACTAGCGACCCCAACGCTACTGAC | B. caballi rap-1 | 400 | [22] |
| Bc9_RAP2R | TTGGAGCATGAAGTCCTTCAGC | |||
| EMA-1F | GCATCCATTGCCATTTCGAG | T. equi ema-1 | 750 | [21] |
| EMA-1R | TGCGCCATAGACGGAGAAGC | |||
| EMA-2F | AATGTTGAGCAAGTCCTTCG | T. equi ema-2 | 800 | [25] |
| EMA-2R | TTAGTAGAACAAAGCAACGGC |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunday Idoko, I.; Tirosh-Levy, S.; Leszkowicz Mazuz, M.; Mohammed Adam, B.; Sikiti Garba, B.; Wesley Nafarnda, D.; Steinman, A. Genetic Characterization of Piroplasms in Donkeys and Horses from Nigeria. Animals 2020, 10, 324. https://doi.org/10.3390/ani10020324
Sunday Idoko I, Tirosh-Levy S, Leszkowicz Mazuz M, Mohammed Adam B, Sikiti Garba B, Wesley Nafarnda D, Steinman A. Genetic Characterization of Piroplasms in Donkeys and Horses from Nigeria. Animals. 2020; 10(2):324. https://doi.org/10.3390/ani10020324
Chicago/Turabian StyleSunday Idoko, Idoko, Sharon Tirosh-Levy, Monica Leszkowicz Mazuz, Babagana Mohammed Adam, Bello Sikiti Garba, Daniel Wesley Nafarnda, and Amir Steinman. 2020. "Genetic Characterization of Piroplasms in Donkeys and Horses from Nigeria" Animals 10, no. 2: 324. https://doi.org/10.3390/ani10020324
APA StyleSunday Idoko, I., Tirosh-Levy, S., Leszkowicz Mazuz, M., Mohammed Adam, B., Sikiti Garba, B., Wesley Nafarnda, D., & Steinman, A. (2020). Genetic Characterization of Piroplasms in Donkeys and Horses from Nigeria. Animals, 10(2), 324. https://doi.org/10.3390/ani10020324






