Effects of Activated Charcoal-Herb Extractum Complex on Antioxidant Status, Lipid Metabolites and Safety of Excess Supplementation in Weaned Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Charcoal-Herb Extractum Complex (CHC) and Montmorillonite (MMT)
2.2. Animals, Diets and Experimental Design
2.2.1. Experiment 1
2.2.2. Experiment 2
2.3. Chemical Analysis
2.4. Antioxidant Status
2.5. Serum Lipid Metabolites
2.6. Hematological and Serum Biochemical Parameters Analysis
2.7. Histopathology Analysis
2.8. Statistical Analysis
3. Results
3.1. Experiment 1
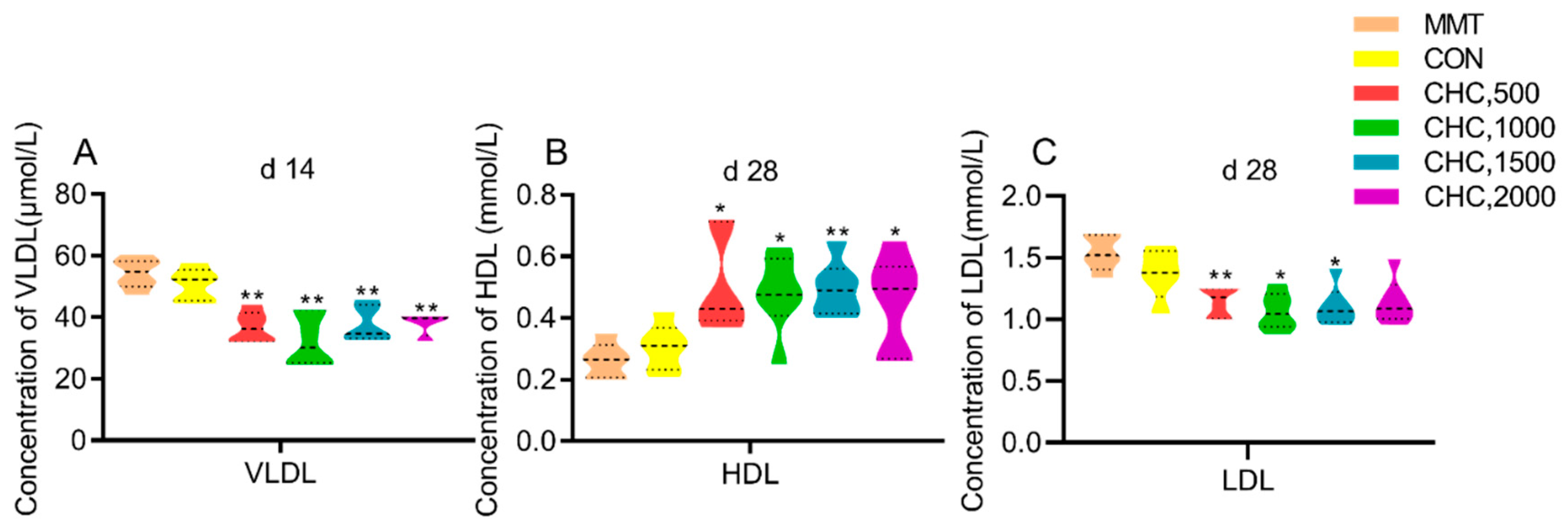
3.1.1. Serum Lipid Metabolites
3.1.2. Antioxidant Status
3.1.3. Serum Biochemical Parameters
3.2. Experiment 2
3.2.1. Growth Performance and Visceral Index
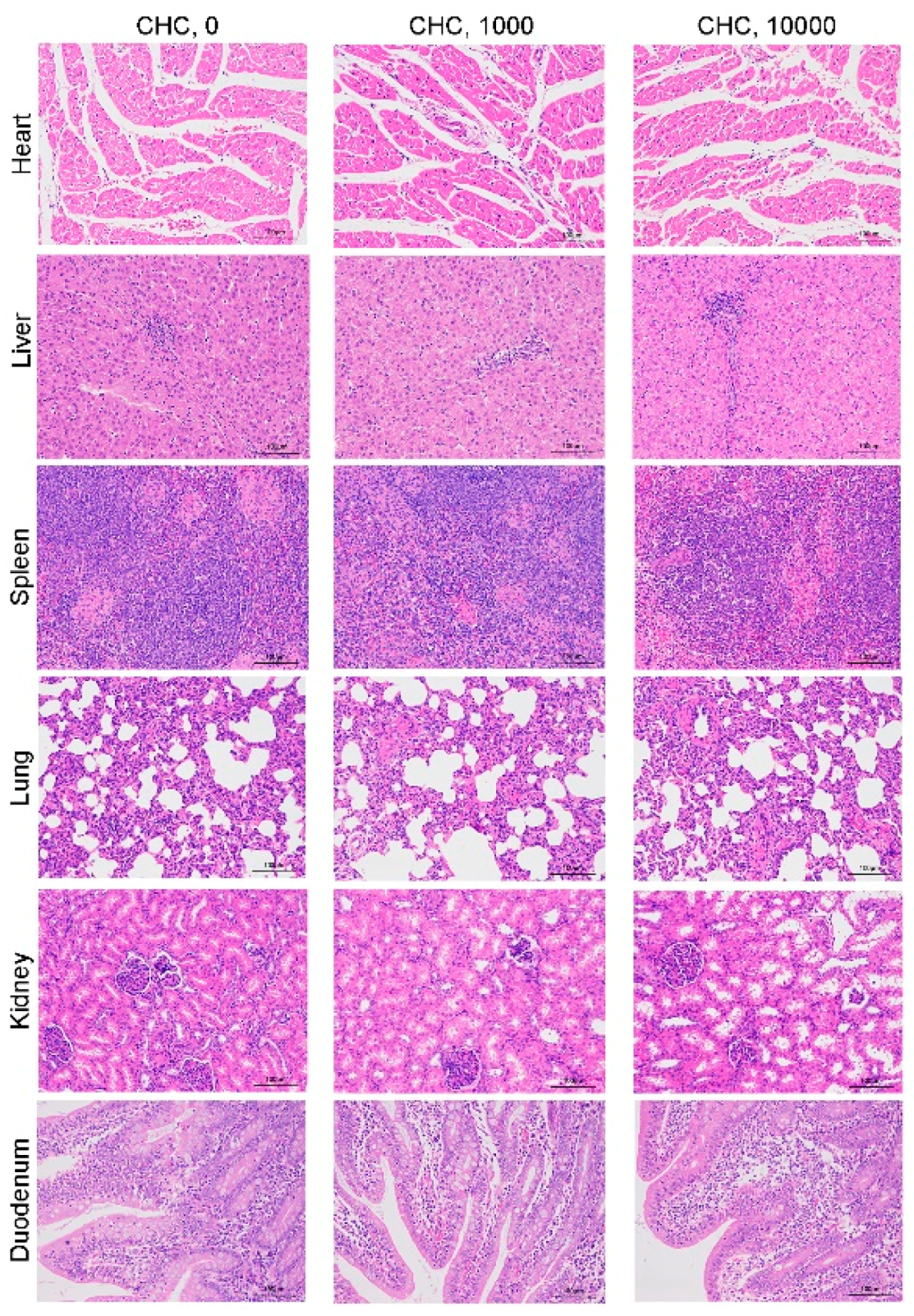
3.2.2. Visceral Organ Histopathology
3.2.3. Hematological and Serum Biochemical Parameters
4. Discussions
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Heo, J.M.; Opapeju, F.O.; Pluske, J.R.; Kim, J.C.; Hampson, D.J.; Nyachoti, C.M. Gastrointestinal health and function in weaned pigs: A review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J. Anim. Physiol. Anim. Nutr. 2013, 97, 207–237. [Google Scholar] [CrossRef] [PubMed]
- Pluske, J.R. Feed- and feed additives-related aspects of gut health and development in weanling pigs. J. Anim. Sci. Biotechnol. 2013, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Ahasan, A.; Invernizzi, G.; Farina, G.; Pilotto, A.; Barbe, F.; Bontempo, V.; Rossi, R.; Bellagamba, F.; Lecchi, C.; Savoini, G.; et al. The effects of superoxide dismutase-rich melon pulp concentrate on inflammation, antioxidant status and growth performance of challenged post-weaning piglets. Animal 2019, 13, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Cabello-Verrugio, C.; Ruiz-Ortega, M.; Mosqueira, M.; Simon, F. Oxidative Stress in Disease and Aging: Mechanisms and Therapies. Oxid. Med. Cell. Longev. 2016, 2016, 8786564. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.P. Cellular defenses against damage from reactive oxygen species. Physiol. Rev. 1994, 74, 139–162. [Google Scholar] [CrossRef] [PubMed]
- Gessner, D.K.; Ringseis, R.; Eder, K. Potential of plant polyphenols to combat oxidative stress and inflammatory processes in farm animals. J. Anim. Physiol. Anim. Nutr. 2017, 101, 605–628. [Google Scholar] [CrossRef]
- Wu, T.; Zhang, Y.; Lv, Y.; Li, P.; Yi, D.; Wang, L.; Zhao, D.; Chen, H.; Gong, J.; Hou, Y. Beneficial Impact and Molecular Mechanism of Bacillus coagulans on Piglets’ Intestine. Int. J. Mol. Sci. 2018, 19, 2084. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Q.; Zhou, Y.; Ahmad, H.; Wang, T. Effects of clinoptilolite on growth performance and antioxidant status in broilers. Biol. Trace Elem. Res. 2013, 155, 228–235. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, L.; Ruan, Z.; Mi, S.; Jiang, M.; Li, X.; Wu, X.; Deng, Z.; Yin, Y. Chlorogenic acid ameliorates intestinal mitochondrial injury by increasing antioxidant effects and activity of respiratory complexes. Biosci. Biotechnol. Biochem. 2016, 80, 962–971. [Google Scholar] [CrossRef]
- Dodson, M.V.; Hausman, G.J.; Guan, L.L.; Du, M.; Rasmussen, T.P.; Poulos, S.P.; Mir, P.; Bergen, W.G.; Fernyhough, M.E.; McFarland, D.C.; et al. Lipid metabolism, adipocyte depot physiology and utilization of meat animals as experimental models for metabolic research. Int. J. Biol. Sci. 2010, 6, 691–699. [Google Scholar] [CrossRef]
- Wang, L.; Gong, L.; Zhu, L.; Peng, C.; Liao, J.; Ke, L.; Dong, B. Effects of activated charcoal-herb extractum complex on the growth performance, immunological indices, intestinal morphology and microflora in weaning piglets. RSC Adv. 2019, 9, 5948–5957. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Mao, X.B.; Yu, B.; He, J.; Zheng, P.; Yu, J.; Luo, J.Q.; Wang, Q.Y.; Chen, D.W. Excess of dietary montmorillonite impairs growth performance, liver function, and antioxidant capacity in starter pigs. J. Anim. Sci. 2017, 95, 2943–2951. [Google Scholar] [CrossRef] [PubMed]
- Association of Official Agricultural Chemists (AOAC). Official Methods of Analysis, 19th ed.; Association of Official Agricultural Chemists: Arlington, VA, USA, 2012. [Google Scholar]
- Cao, J.; Guo, F.; Zhang, L.; Dong, B.; Gong, L. Effects of dietary Selenomethionine supplementation on growth performance, antioxidant status, plasma selenium concentration, and immune function in weaning pigs. J. Anim. Sci. Biotechnol. 2014, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Turpin, D.L.; Langendijk, P.; Plush, K.; Pluske, J.R. Intermittent suckling with or without co-mingling of non-littermate piglets before weaning improves piglet performance in the immediate post-weaning period when compared with conventional weaning. J. Anim. Sci. Biotechnol. 2017, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- De, U.K.; Nandi, S.; Mukherjee, R.; Gaur, G.K.; Verma, M.R. Identification of some plasma biomarkers associated with early weaning stress in crossbred piglets. Comp. Clin. Pathol. 2016, 26, 343–349. [Google Scholar] [CrossRef]
- Xu, P.; Dai, S.; Wang, J.; Zhang, J.; Liu, J.; Wang, F.; Zhai, Y. Preventive obesity agent montmorillonite adsorbs dietary lipids and enhances lipid excretion from the digestive tract. Sci Rep. 2016, 6, 19659. [Google Scholar] [CrossRef]
- Kehrer, J.P.; Klotz, L.O. Free radicals and related reactive species as mediators of tissue injury and disease: Implications for Health. Crit. Rev. Toxicol. 2015, 45, 765–798. [Google Scholar] [CrossRef]
- Granados-Oliveros, G.; Gómez-Vidales, V.; Nieto-Camacho, A.; Morales-Serna, J.A.; Cárdenas, J.; Salmón, M. Photoproduction of H2O2 and hydroxyl radicals catalysed by natural and super acid-modified montmorillonite and its oxidative role in the peroxidation of lipids. RSC Adv. 2013, 3, 937–944. [Google Scholar] [CrossRef]
- Aon, M.A.; Cortassa, S.; Marban, E.; O’Rourke, B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 2003, 278, 44735–44744. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Yu, B.; Chen, D.; Mao, X.; Zheng, P.; Luo, J.; He, J. Dietary chlorogenic acid improves growth performance of weaned pigs through maintaining antioxidant capacity and intestinal digestion and absorption function. J. Anim. Sci. 2018, 96, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.H.; Zhao, K.L.; Chen, X.L.; Xu, J.X. Impact of weaning and an antioxidant blend on intestinal barrier function and antioxidant status in pigs. J. Anim. Sci. 2012, 90, 2581–2589. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.Y.; Piao, X.S. Essential Oil Blend Could Decrease Diarrhea Prevalence by Improving Antioxidative Capability for Weaned Pigs. Animals 2019, 9, 847. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Jiang, F.; Xu, Q.; Chen, D.; Yu, B.; Huang, Z.; Mao, X.; Yu, J.; He, J. New insights into the role of chitosan oligosaccharide in enhancing growth performance, antioxidant capacity, immunity and intestinal development of weaned pigs. RSC Adv. 2017, 7, 9669–9679. [Google Scholar] [CrossRef]
- Chen, J.; Yu, B.; Chen, D.; Huang, Z.; Mao, X.; Zheng, P.; Yu, J.; Luo, J.; He, J. Chlorogenic acid improves intestinal barrier functions by suppressing mucosa inflammation and improving antioxidant capacity in weaned pigs. J. Nutr. Biochem. 2018, 59, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.W.; Li, J.T.; Gong, L.M.; Wu, H.; Zhang, L.Y. Effects of Graded Levels of Montmorillonite on Performance, Hematological Parameters and Bone Mineralization in Weaned Pigs. Asian-Australas. J. Anim. Sci. 2013, 26, 1614–1621. [Google Scholar] [CrossRef]
- Sands, D.C.; McIntyre, J.L.; Walton, G.S. Use of Activated Charcoal for the Removal of Patulin from Cider. Appl. Environ. Microbiol. 1976, 32, 388–391. [Google Scholar]
- Dalvi, R.R.; Ademoyero, A.A. Toxic Effects of Aflatoxin B1 in Chickens Given Feed Contaminated with Aspergillus flavus and Reduction of the Toxicity by Activated Charcoal and Some Chemical Agents. Avian Dis. 1984, 28, 61–69. [Google Scholar] [CrossRef]
- Dalvi, R.R.; McGowan, C. Experimental Induction of Chronic Aflatoxicosis in Chickens by Purified Aflatoxin B1 and Its Reversal by Activated Charcoal, Phenobarbital, and Reduced Glutathione. Poult. Sci. 1984, 63, 485–491. [Google Scholar] [CrossRef]
- Jindal, N.; Mahipal, S.K.; Mahajan, N.K. Toxicity of aflatoxin B1 in broiler chicks and its reduction by activated charcoal. Res. Vet. Sci. 1994, 56, 37–40. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 3, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Erkan, N. Antioxidant activity and phenolic compounds of fractions from Portulaca oleracea L. Food Chem. 2012, 133, 775–781. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Moniem, A.E.A.; Al-Quraishy, S.; Saleh, R.A. Antioxidant effect of purslane (Portulaca oleracea) and its mechanism of action. J. Med. Plants. Res. 2011, 5, 1589–1593. [Google Scholar]
- Lim, Y.Y.; Quah, E.P.L. Antioxidant properties of different cultivars of Portulaca oleracea. Food Chem. 2007, 103, 734–740. [Google Scholar] [CrossRef]
- Yao, D.; Vlessidis, A.G.; Gou, Y.; Zhou, X.; Zhou, Y.; Evmiridis, N.P. Chemiluminescence detection of superoxide anion release and superoxide dismutase activity: Modulation effect of Pulsatilla chinensis. Anal. Bioanal. Chem. 2004, 379, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Sun, Z.D. Study on Antioxidant Activity in vitro and Protection against DNA Oxidative Damage of Flavonoids of Artemisia argyi. Food Sci. 2008, 29, 47–50. [Google Scholar]
- Kuusisto, P.; Manninen, V.; Vapaatalo, H.; Huttunen, J.; Neuvonen, P. Effect of activated charcoal on hypercholesterolaemia. Lancet 1986, 328, 366–367. [Google Scholar] [CrossRef]
- Nafikov, R.A.; Beitz, D.C. Carbohydrate and lipid metabolism in farm animals. J. Nutr. 2007, 137, 702–705. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, L.; Meng, Q.; Wu, W.; Lee, Y.K.; Xie, J.; Zhang, H. Effects of dietary pectin on the profile and transport of intestinal bile acids in young pigs. J. Anim. Sci. 2018, 96, 4743–4754. [Google Scholar] [CrossRef]
- Zidan, Y.; Bouderbala, S.; Djellouli, F.; Lacaille-Dubois, M.A.; Bouchenak, M. Portulaca oleracea reduces triglyceridemia, cholesterolemia, and improves lecithin: Cholesterol acyltransferase activity in rats fed enriched-cholesterol diet. Phytomedicine 2014, 21, 1504–1508. [Google Scholar] [CrossRef]
- Gatreh-Samani, K. Purslane (Portulaca oleracea) effects on serum paraoxanase-1 activity. J. Shahrekord Univ. Med Sci. 2011, 1, 9–15. [Google Scholar]
- Abdel-Tawwab, M.; El-Sayed, G.O.; Shady, S.H. Effect of dietary active charcoal supplementation on growth performance, biochemical and antioxidant responses, and resistance of Nile tilapia, Oreochromis niloticus (L.) to environmental heavy metals exposure. Aquaculture 2017, 479, 17–24. [Google Scholar] [CrossRef]
- Chu, G.M.; Jung, C.K.; Kim, H.Y.; Ha, J.H.; Kim, J.H.; Jung, M.S.; Lee, S.J.; Song, Y.; Ibrahim, R.I.H.; Cho, J.H.; et al. Effects of bamboo charcoal and bamboo vinegar as antibiotic alternatives on growth performance, immune responses and fecal microflora population in fattening pigs. Anim. Sci. J. 2013, 84, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Lee, I.-C.; Kang, S.-S.; Moon, C.-J.; Kim, S.-H.; Shin, D.-H.; Kim, H.-C.; Yoo, J.-C.; Kim, J.-C. Effects of Bamboo Charcoal and Bamboo Leaf Supplementation on Performance and Meat Quality in Chickens. J. Life Sci. 2011, 21, 805–810. [Google Scholar] [CrossRef]
- Ruttanavut, J.; Yamauchi, K.; Goto, H.; Erikawa, T. Effects of Dietary Bamboo Charcoal Powder Including Vinegar Liquid on Growth Performance and Histological Intestinal Change in Aigamo Ducks. Int. J. Poult. Sci. 2009, 3, 229–236. [Google Scholar] [CrossRef]
- Susan, E. Fielder, Serum Biochemical Reference Ranges. Available online: https://www.merckvetmanual.com/special-subjects/reference-guides/serum-biochemical-reference-ranges (accessed on 24 November 2019).

| Items | Phase I | Phase II |
|---|---|---|
| Corn | 59.15 | 60.49 |
| Soybean meal (45% crude protein) | 14.31 | 18.08 |
| Soybean oil | 2.80 | 2.60 |
| Fish meal | 2.40 | 2.20 |
| Soy protein concentrate | 10.10 | 4.80 |
| Whey powder (12% crude protein) | 7.32 | 8.29 |
| Dicalcium phosphate | 1.26 | 1.10 |
| Limestone | 0.72 | 0.60 |
| Salt | 0.24 | 0.24 |
| L-lysine-HCl | 0.51 | 0.50 |
| L-threonine | 0.18 | 0.15 |
| Tryptophan | 0.03 | 0.03 |
| Methionine hydroxy analogue | 0.28 | 0.23 |
| Choline chloride (50%) | 0.20 | 0.20 |
| Vitamine-mineral premix 2 | 0.50 | 0.50 |
| Total | 100.00 | 100.00 |
| Analyzed nutrient levels | ||
| Digestible energy, Mcal/kg | 3.98 | 3.95 |
| Crude protein | 23.50 | 22.99 |
| Ash | 5.55 | 5.50 |
| Ether extract | 6.18 | 6.34 |
| Crude fiber | 3.39 | 3.37 |
| Lysine | 1.72 | 1.56 |
| Methionine | 0.63 | 0.60 |
| Methionine + Cystine | 0.98 | 0.89 |
| Threonine | 1.10 | 1.01 |
| Calcium | 0.85 | 0.83 |
| Total phosphorus | 0.70 | 0.66 |
| Items | MMT, mg kg−1 | CHC, mg kg−1 | SEM | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1000 | 0 | 500 | 1000 | 1500 | 2000 | ANOVA | Linear | Quadratic | ||
| 14 d 2 | ||||||||||
| TC, mmol/L | 1.07 | 1.07 | 1.30 | 1.46 | 1.33 | 1.58 | 0.14 | 0.09 | 0.25 | 0.11 |
| TG, mmol/L | 0.26 | 0.28 | 0.36 | 0.30 | 0.39 | 0.43 | 0.05 | 0.20 | 0.12 | 0.27 |
| 28 d | ||||||||||
| TC, mmol/L | 1.62 | 1.56 | 1.71 | 1.65 | 1.82 | 1.88 | 0.13 | 0.52 | 0.12 | 0.14 |
| TG, mmol/L | 0.26 | 0.30 | 0.32 | 0.27 | 0.30 | 0.29 | 0.04 | 0.88 | 0.68 | 0.66 |
| Items | MMT, mg kg−1 | CHC, mg kg−1 | SEM | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1000 | 0 | 500 | 1000 | 1500 | 2000 | ANOVA | Linear | Quadratic | ||
| Serum 14 d 2 | ||||||||||
| T-AOC, U/mL | 13.54 | 13.39 | 12.36 | 11.03 | 10.18 | 11.78 | 1.56 | 0.62 | 0.81 | 0.20 |
| GSH-Px, U/mL | 797.52 | 776.39 | 760.41 | 788.71 | 761.15 | 787.58 | 14.51 | 0.37 | 0.42 | 0.60 |
| Serum 28 d | ||||||||||
| T-AOC, U/mL | 14.68 | 14.73 | 14.89 | 16.04 | 17.46 | 12.72 | 1.48 | 0.36 | 0.53 | 0.44 |
| GSH-Px, U/mL | 782.19 | 798.42 | 798.04 | 812.50 | 816.36 | 812.78 | 16.89 | 0.71 | 0.99 | 0.35 |
| Kidney | ||||||||||
| T-AOC, U/mg | 2.00 | 2.12 | 2.39 | 2.44 | 2.22 | 2.20 | 0.14 | 0.27 | 0.19 | 0.25 |
| GSH-Px, U/mg | 449.74 | 457.20 | 469.57 | 466.94 | 480.30 | 469.44 | 25.86 | 0.97 | 0.68 | 0.58 |
| Liver | ||||||||||
| T-AOC, U/mg | 2.59 | 2.76 | 2.68 | 3.08 | 2.98 | 3.25 | 0.24 | 0.37 | 0.91 | 0.07 |
| GSH-Px, U/mg | 606.85 | 603.72 | 602.80 | 667.49 | 658.37 | 671.50 | 40.76 | 0.63 | 0.99 | 0.14 |
| Items | MMT, mg kg−1 | CHC, mg kg−1 | SEM | p Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1000 | 0 | 500 | 1000 | 1500 | 2000 | ANOVA | Linear | Quadratic | ||
| 14 d 2 | ||||||||||
| GLU, mmol/L | 4.59 | 4.09 | 4.26 | 4.19 | 4.53 | 4.62 | 0.27 | 0.07 | 0.18 | 0.44 |
| TP, g/L | 33.72 | 32.65 | 38.23 | 38.67 | 40.97 | 46.68 | 1.04 | 0.20 | 0.16 | 0.12 |
| ALB, g/L | 19.88 | 18.28 | 21.95 | 19.72 | 20.83 | 24.58 | 0.16 | 0.42 | 0.07 | 0.48 |
| AST, U/L | 37.07 | 35.92 | 58.52 | 37.03 | 43.03 | 55.55 | 8.55 | 0.26 | 0.14 | 0.77 |
| ALT, U/L | 23.93 | 20.67 | 28.20 | 26.12 | 30.03 | 32.40 | 2.64 | 0.06 | 0.21 | 0.06 |
| ALP, U/L | 257.00 | 300.43 | 339.25 | 325.08 | 412.87 | 406.92 | 46.03 | 0.16 | 0.32 | 0.15 |
| CREA, μmol/L | 84.02 | 83.13 | 88.43 | 80.63 | 89.40 | 90.25 | 4.54 | 0.60 | 0.11 | 0.65 |
| UN, mmol/L | 2.45 | 2.93 | 2.48 | 2.50 | 2.91 | 3.72 | 0.34 | 0.11 | 0.74 | 0.33 |
| 28 d | ||||||||||
| GLU, mmol/L | 3.68 | 3.29 | 3.95 | 4.83 | 3.68 | 4.45 | 0.36 | 0.06 | 0.45 | 0.15 |
| TP, g/L | 39.57 | 40.90 | 48.25 | 45.43 | 47.30 | 45.90 | 2.30 | 0.07 | 0.07 | 0.42 |
| ALB, g/L | 27.27 | 25.92 | 31.78 | 30.58 | 29.97 | 27.10 | 1.57 | 0.09 | 0.11 | 0.71 |
| AST, U/L | 39.97 | 43.58 | 44.33 | 37.78 | 33.15 | 35.38 | 4.94 | 0.55 | 0.96 | 0.08 |
| ALT, U/L | 24.98 | 27.88 | 36.58 | 33.43 | 33.43 | 36.48 | 3.29 | 0.11 | 0.06 | 0.54 |
| ALP, U/L | 271.43 | 284.27 | 332.00 | 340.22 | 324.23 | 311.12 | 23.73 | 0.28 | 0.36 | 0.43 |
| CREA, μmol/L | 90.27 | 88.83 | 105.18 | 103.85 | 102.98 | 103.71 | 5.65 | 0.16 | 0.11 | 0.28 |
| UN, mmol/L | 3.02 | 2.98 | 3.38 | 2.85 | 3.33 | 3.43 | 0.21 | 0.29 | 0.07 | 0.77 |
| Items 2 | CHC, mg kg−1 | SEM | p Value | ||
|---|---|---|---|---|---|
| 0 | 1000 | 10,000 | |||
| Body weight | |||||
| 0 d, kg | 8.57 | 8.58 | 8.59 | 0.04 | 0.97 |
| 14 d, kg | 12.81 b | 13.45 a | 12.31 b | 0.17 | <0.01 |
| 28 d, kg | 20.57 b | 21.54 a | 20.20 b | 0.28 | 0.02 |
| 0–14 d | |||||
| ADG, kg | 0.30 b | 0.35 a | 0.27 b | 0.01 | <0.01 |
| ADFI, kg | 0.53 a | 0.55 a | 0.46 b | 0.02 | 0.02 |
| F: G | 1.74 a | 1.57 b | 1.75 a | 0.03 | <0.01 |
| 15–28 d | |||||
| ADG, kg | 0.55 | 0.58 | 0.57 | 0.02 | 0.63 |
| ADFI, kg | 0.86 | 0.86 | 0.85 | 0.03 | 0.96 |
| F: G | 1.57 | 1.48 | 1.50 | 0.06 | 0.54 |
| 0–28 d | |||||
| ADG, kg | 0.43 ab | 0.46 a | 0.41 b | 0.01 | <0.05 |
| ADFI, kg | 0.70 | 0.70 | 0.66 | 0.02 | 0.36 |
| F: G | 1.63 a | 1.51 b | 1.59 a | 0.03 | 0.04 |
| Visceral index, g kg−1 | |||||
| Heart | 5.62 | 5.57 | 5.60 | 0.21 | 0.99 |
| Liver | 29.12 | 27.67 | 28.70 | 1.31 | 0.73 |
| Spleen | 2.45 | 2.28 | 2.60 | 0.17 | 0.47 |
| Lung | 11.14 | 11.04 | 11.48 | 0.54 | 0.84 |
| Kidney | 5.27 | 5.29 | 5.70 | 0.26 | 0.43 |
| Items 2 | CHC, mg kg−1 | SEM | p Value | ||
|---|---|---|---|---|---|
| 0 | 1000 | 10,000 | |||
| 14 d | |||||
| GLU, mmol/L | 4.40 | 4.07 | 4.04 | 0.19 | 0.46 |
| TP, g/L | 34.48 | 38.08 | 44.83 | 3.54 | 0.18 |
| ALB, g/L | 28.35 | 29.53 | 33.23 | 2.45 | 0.38 |
| AST, U/L | 36.92 | 38.28 | 43.75 | 8.34 | 0.83 |
| ALT, U/L | 22.12 | 25.53 | 31.00 | 2.63 | 0.10 |
| ALP, U/L | 261.67 | 346.83 | 378.48 | 36.14 | 0.11 |
| CREA, μmol/L | 84.87 | 80.62 | 89.53 | 5.18 | 0.50 |
| UN, mmol/L | 2.96 | 2.67 | 3.45 | 0.33 | 0.30 |
| TC, mmol/L | 1.07 | 1.39 | 1.26 | 0.09 | 0.07 |
| TG, mmol/L | 0.31 | 0.29 | 0.40 | 0.06 | 0.42 |
| TBILI, μmol/L | 0.85 | 0.80 | 0.80 | 0.13 | 0.95 |
| 28 d | |||||
| GLU, mmol/L | 3.93 | 4.28 | 4.83 | 0.30 | 0.14 |
| TP, g/L | 60.28 | 65.50 | 61.35 | 1.80 | 0.15 |
| ALB, g/L | 23.88 | 28.73 | 27.02 | 1.24 | 0.06 |
| AST, U/L | 33.35 | 35.73 | 34.68 | 3.97 | 0.91 |
| ALT, U/L | 33.02 | 35.27 | 32.27 | 2.41 | 0.67 |
| ALP, U/L | 221.27 b | 312.33 a | 325.52 a | 25.29 | 0.03 |
| CREA, μmol/L | 89.72 | 105.22 | 98.75 | 5.17 | 0.15 |
| UN, mmol/L | 3.47 | 3.35 | 3.23 | 0.23 | 0.76 |
| TC, mmol/L | 1.29 | 1.37 | 1.40 | 0.10 | 0.76 |
| TG, mmol/L | 0.30 | 0.28 | 0.38 | 0.05 | 0.32 |
| TBILI, μmol/L | 1.20 | 1.07 | 0.95 | 0.13 | 0.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Zhu, L.; Gong, L.; Zhang, X.; Wang, Y.; Liao, J.; Ke, L.; Dong, B. Effects of Activated Charcoal-Herb Extractum Complex on Antioxidant Status, Lipid Metabolites and Safety of Excess Supplementation in Weaned Piglets. Animals 2019, 9, 1151. https://doi.org/10.3390/ani9121151
Wang L, Zhu L, Gong L, Zhang X, Wang Y, Liao J, Ke L, Dong B. Effects of Activated Charcoal-Herb Extractum Complex on Antioxidant Status, Lipid Metabolites and Safety of Excess Supplementation in Weaned Piglets. Animals. 2019; 9(12):1151. https://doi.org/10.3390/ani9121151
Chicago/Turabian StyleWang, Liqi, Lin Zhu, Limin Gong, Xin Zhang, Yubo Wang, Jianling Liao, Linfu Ke, and Bing Dong. 2019. "Effects of Activated Charcoal-Herb Extractum Complex on Antioxidant Status, Lipid Metabolites and Safety of Excess Supplementation in Weaned Piglets" Animals 9, no. 12: 1151. https://doi.org/10.3390/ani9121151
APA StyleWang, L., Zhu, L., Gong, L., Zhang, X., Wang, Y., Liao, J., Ke, L., & Dong, B. (2019). Effects of Activated Charcoal-Herb Extractum Complex on Antioxidant Status, Lipid Metabolites and Safety of Excess Supplementation in Weaned Piglets. Animals, 9(12), 1151. https://doi.org/10.3390/ani9121151






