Myoinositol Supplementation of Freezing Medium Improves the Quality-Related Parameters of Dog Sperm
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals Used and Semen Collection
2.2. Determination of Optimal Myo-Ins Concentration
2.3. Semen Cryopreservation and Thawing
2.4. Assessment of Sperm Plasma Membrane Integrity
2.5. Assessment of Sperm Acrosome Membrane Integrity
2.6. Mucus Penetration Test
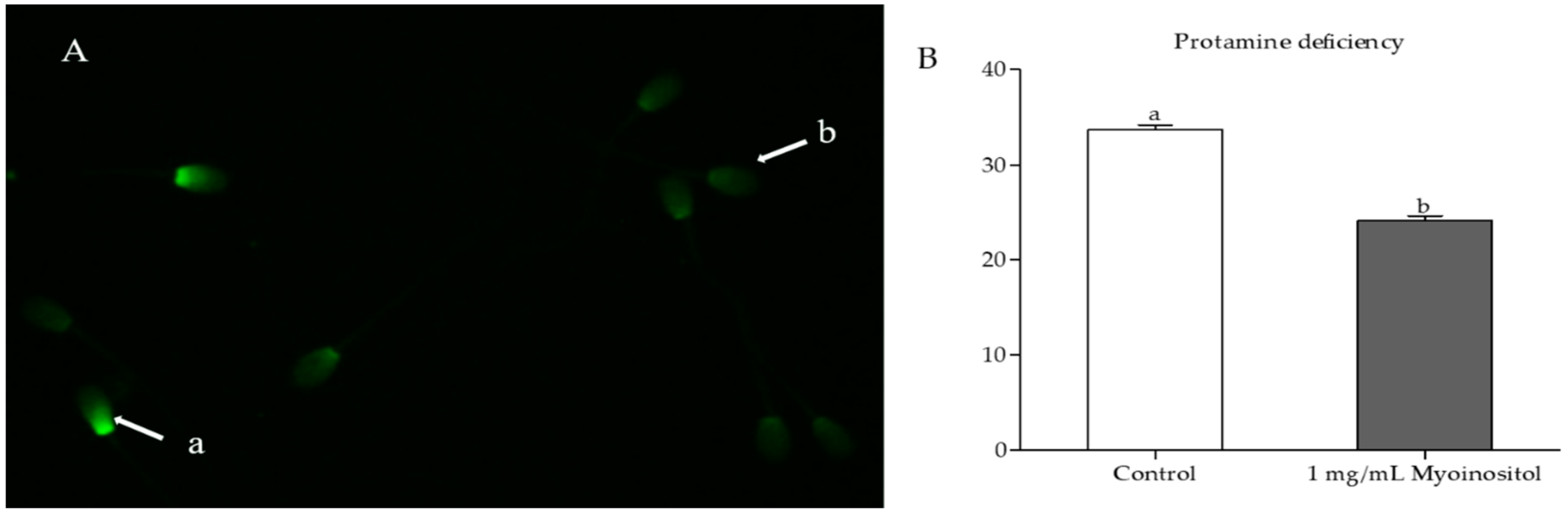
2.7. Protamine Deficiency Test
2.8. Assessment of Gene Expression
2.9. Statistical Analysis
3. Results
3.1. Determination of Optimal Myo-Ins Concentration
3.2. Effect of Myo-Ins on Post-Thaw Sperm Motility and Kinematic Parameters
3.3. Effect of Myo-Ins on Sperm Viability and Plasma Membrane Integrity
3.4. Effect of Myo-Ins on Sperm Acrosome Integrity
3.5. Effect of Myo-Ins on Mucus Penetration
3.6. Effect of Myo-Ins on Chromatin Integrity
3.7. Effect of Myo-Ins on Gene Expression
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jang, G.; Kim, M.K.; Lee, B.C. Current status and applications of somatic cell nuclear transfer in dogs. Theriogenology 2010, 74, 1311–1320. [Google Scholar] [CrossRef] [PubMed]
- Thomassen, R.; Farstad, W. Artificial insemination in canids: A useful tool in breeding and conservation. Theriogenology 2009, 71, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Tahir, M.; Khalid, A.; Sattar, A.; Ahmad, N. Effect of cholesterol-loaded cyclodextrins on cryosurvival of dog spermatozoa. Reprod. Domest. Anim. 2017, 52, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Axner, E.; Lagerson, E. Cryopreservation of dog semen in a tris extender with 1% or 2% soya bean lecithin as a replacement of egg yolk. Reprod. Domest. Anim. 2016, 51, 262–268. [Google Scholar] [CrossRef]
- Hong, H.M.; Sim, G.Y.; Park, S.M.; Lee, E.J.; Kim, D.Y. Ameliorative effect of chitosan complex on miniature pig sperm cryopreservation. J. Anim. Reprod. Biotechnol. 2018, 33, 337–342. [Google Scholar] [CrossRef]
- Silva, A.R.; de Cassia Soares Cardoso, R.; Uchoa, D.C.; MacHado da Silva, L.D. Effect of tris-buffer, egg yolk and glycerol on canine semen freezing. Vet. J. 2002, 164, 244–246. [Google Scholar] [CrossRef]
- Michael, A.; Alexopoulos, C.; Pontiki, E.; Hadjipavlou-Litina, D.; Saratsis, P.; Boscos, C. Effect of antioxidant supplementation on semen quality and reactive oxygen species of frozen-thawed canine spermatozoa. Theriogenology 2007, 68, 204–212. [Google Scholar] [CrossRef]
- Johnston, S.D.; Satake, N.; Zee, Y.; Lopez-Fernandez, C.; Holt, W.V.; Gosalvez, J. Osmotic stress and cryoinjury of koala sperm: An integrative study of the plasma membrane, chromatin stability and mitochondrial function. Reproduction 2012, 143, 787–797. [Google Scholar] [CrossRef]
- Dejarkom, S.; Kunathikom, S. Evaluation of cryo-injury of sperm chromatin according to computer controlled rate freezing method part 2. J. Med. Assoc. Thai. 2007, 90, 852–856. [Google Scholar]
- O’Flaherty, C.; de Lamirande, E.; Gagnon, C. Positive role of reactive oxygen species in mammalian sperm capacitation: Triggering and modulation of phosphorylation events. Free Radic. Biol. Med. 2006, 41, 528–540. [Google Scholar] [CrossRef]
- De Leeuw, F.E.; Chen, H.C.; Colenbrander, B.; Verkleij, A.J. Cold-induced ultrastructural changes in bull and boar sperm plasma membranes. Cryobiology 1990, 27, 171–183. [Google Scholar] [CrossRef]
- John Morris, G.; Acton, E.; Murray, B.J.; Fonseca, F. Freezing injury: The special case of the sperm cell. Cryobiology 2012, 64, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.L.; Bilodeau, J.F.; Cormier, N. Semen cryopreservation in domestic animals: A damaging and capacitating phenomenon. J. Androl. 2000, 21, 1–7. [Google Scholar] [PubMed]
- Aitken, R.J.; Baker, M.A.; Nixon, B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J. Androl. 2015, 17, 633. [Google Scholar] [CrossRef]
- Mohammadi, F.; Varanloo, N.; Heydari Nasrabadi, M.; Vatannejad, A.; Amjadi, F.S.; Javedani Masroor, M.; Bajelan, L.; Mehdizadeh, M.; Aflatoonian, R.; Zandieh, Z. Supplementation of sperm freezing medium with myoinositol improve human sperm parameters and protects it against DNA fragmentation and apoptosis. Cell Tissue Bank. 2019, 20, 77–86. [Google Scholar] [CrossRef]
- Santamaria, A.; Giordano, D.; Corrado, F.; Pintaudi, B.; Interdonato, M.L.; Vieste, G.D.; Benedetto, A.D.; D’Anna, R. One-year effects of myo-inositol supplementation in postmenopausal women with metabolic syndrome. Climacteric 2012, 15, 490–495. [Google Scholar] [CrossRef]
- Foskett, J.K. Inositol trisphosphate receptor Ca2+ release channels in neurological diseases. Pflügers Arch. 2010, 460, 481–494. [Google Scholar] [CrossRef]
- Eisenberg, F.; Parthasarathy, R. Measurement of biosynthesis of myo-inositol from glucose 6-phosphate. In Methods in Enzymology; Academic Press: Amsterdam, The Netherlands, 1987; Volume 141, pp. 127–143. [Google Scholar] [CrossRef]
- Condorelli, R.A.; La Vignera, S.; Di Bari, F.; Unfer, V.; Calogero, A.E. Effects of myoinositol on sperm mitochondrial function in-vitro. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 129–134. [Google Scholar]
- Voglmayr, J.; Amann, R. The distribution of free myo-inositol in fluids, spermatozoa, and tissues of the bull genital tract and observations on its uptake by the rabbit epididymis. Biol. Reprod. 1973, 8, 504–513. [Google Scholar] [CrossRef]
- Bevilacqua, A.; Carlomagno, G.; Gerli, S.; Montanino Oliva, M.; Devroey, P.; Lanzone, A.; Soulange, C.; Facchinetti, F.; Carlo Di Renzo, G.; Bizzarri, M.; et al. Results from the International Consensus Conference on myo-inositol and D-chiro-inositol in Obstetrics and Gynecology-assisted reproduction technology. Gynecol. Endocrinol. 2015, 31, 441–446. [Google Scholar] [CrossRef]
- Chauvin, T.R.; Griswold, M.D. Characterization of the expression and regulation of genes necessary for myo-inositol biosynthesis and transport in the seminiferous epithelium. Biol. Reprod. 2004, 70, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Korosi, T.; Barta, C.; Rokob, K.; Torok, T. Physiological Intra-Cytoplasmic Sperm Injection (PICSI) outcomes after oral pretreatment and semen incubation with myo-inositol in oligoasthenoteratozoospermic men: Results from a prospective, randomized controlled trial. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 66–72. [Google Scholar] [PubMed]
- Rubino, P.; Palini, S.; Chigioni, S.; Carlomagno, G.; Quagliariello, A.; De Stefani, S.; Baglioni, A.; Bulletti, C. Improving fertilization rate in ICSI cycles by adding myoinositol to the semen preparation procedures: A prospective, bicentric, randomized trial on sibling oocytes. J. Assist. Reprod. Genet. 2015, 32, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.A.; La Vignera, S.; Bellanca, S.; Vicari, E.; Calogero, A.E. Myoinositol: Does it improve sperm mitochondrial function and sperm motility? Urology 2012, 79, 1290–1295. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.F.; Hand, S.C.; Crowe, L.M.; Crowe, J.H. Cryoprotection of phosphofructokinase with organic solutes: Characterization of enhanced protection in the presence of divalent cations. Arch. Biochem. Biophys. 1986, 250, 505–512. [Google Scholar] [CrossRef]
- Molinia, F.; Evans, G.; Maxwell, W. Effect of polyols on the post-thawing motility of pellet-frozen ram spermatozoa. Theriogenology 1994, 42, 15–23. [Google Scholar] [CrossRef]
- Qamar, A.Y.; Fang, X.; Kim, M.J.; Cho, J. Improved post-thaw quality of canine semen after treatment with exosomes from conditioned medium of adipose-derived mesenchymal stem cells. Animals 2019, 9, 865. [Google Scholar] [CrossRef]
- Saleh, R.; Assaf, H.; El Maged, A.; Wafaa, M.; Elsuity, M.; Fawzy, M. Increased cryo-survival rate in ejaculated human sperm from infertile men following pre-freeze in vitro myo-inositol supplementation. Clin. Exp. Reprod. Med. 2018, 45, 177–182. [Google Scholar] [CrossRef]
- Nagashima, J.B.; Sylvester, S.R.; Nelson, J.L.; Cheong, S.H.; Mukai, C.; Lambo, C.; Flanders, J.A.; Meyers-Wallen, V.N.; Songsasen, N.; Travis, A.J. Live births from domestic dog (Canis familiaris) embryos produced by In Vitro Fertilization. PLoS ONE 2015, 10, e0143930. [Google Scholar] [CrossRef]
- Abdillah, D.A.; Setyawan, E.M.N.; Oh, H.J.; Ra, K.; Lee, S.H.; Kim, M.J.; Lee, B.C. Iodixanol supplementation during sperm cryopreservation improves protamine level and reduces reactive oxygen species of canine sperm. J. Vet. Sci. 2019, 20, 79–86. [Google Scholar] [CrossRef]
- Setyawan, E.M.; Kim, M.J.; Oh, H.J.; Kim, G.A.; Jo, Y.K.; Lee, S.H.; Choi, Y.B.; Lee, B.C. Maintaining canine sperm function and osmolyte content with multistep freezing protocol and different cryoprotective agents. Cryobiology 2015, 71, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Root Kustritz, M.V. The value of canine semen evaluation for practitioners. Theriogenology 2007, 68, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Petrunkina, A.M.; Gropper, B.; Gunzel-Apel, A.R.; Topfer-Petersen, E. Functional significance of the cell volume for detecting sperm membrane changes and predicting freezability in dog semen. Reproduction 2004, 128, 829–842. [Google Scholar] [CrossRef] [PubMed]
- Iranpour, F.G.; Nasr-Esfahani, M.H.; Valojerdi, M.R.; al-Taraihi, T.M. Chromomycin A3 staining as a useful tool for evaluation of male fertility. J. Assist. Reprod. Genet. 2000, 17, 60–66. [Google Scholar] [CrossRef]
- Holt, W.V. Alternative strategies for the long-term preservation of spermatozoa. Reprod. Fertil. Dev. 1997, 9, 309–319. [Google Scholar] [CrossRef]
- Miguel-Jiménez, S.; Mogas, T.; Peña, A.; Tamargo, C.; Hidalgo, C.; Muiño, R.; Rodríguez-Gil, J.; Morató, R. Post-thaw changes in sperm membrane and ROS following cryopreservation of dairy bull semen using four different commercial extenders. In Proceedings of the Physiology of Reproduction in Male and Semen Technology (Abstracts A191E to A205E): 32nd Meeting of the European Embryo Transfer Association (AETE), Barcelona, Spain, 9–10 August 2016; pp. 9–10. [Google Scholar]
- Alvarez, J.G.; Storey, B.T. Taurine, hypotaurine, epinephrine and albumin inhibit lipid peroxidation in rabbit spermatozoa and protect against loss of motility. Biol. Reprod. 1983, 29, 548–555. [Google Scholar] [CrossRef]
- Sang-Hyoun Park, I.-J.Y. Effect of antioxidant supplementation in freezing extender on porcine sperm viability, motility and reactive oxygen species. J. Anim. Reprod. Biotechnol. 2017, 32, 9–15. [Google Scholar]
- Park, S.H.; Jeon, Y.; Yu, I.J. Effects of antioxidants supplement in porcine sperm freezing on in vitro fertilization and the glutathione and reactive oxygen species level of presumptive zygotes. J. Anim. Reprod. Biotechnol. 2017, 32, 337–342. [Google Scholar] [CrossRef]
- Aitken, R.J.; Krausz, C. Oxidative stress, DNA damage and the Y chromosome. Reprod. Camb. 2001, 122, 497–506. [Google Scholar] [CrossRef]
- Ghallab, A.M.; Shahat, A.M.; Fadl, A.M.; Ayoub, M.M.; Moawad, A.R. Impact of supplementation of semen extender with antioxidants on the quality of chilled or cryopreserved Arabian stallion spermatozoa. Cryobiology 2017, 79, 14–20. [Google Scholar] [CrossRef]
- Aitken, R.J.; Clarkson, J.S. Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. Reproduction 1987, 81, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Baumber, J.; Ball, B.A.; Linfor, J.J. Assessment of the cryopreservation of equine spermatozoa in the presence of enzyme scavengers and antioxidants. Am. J. Vet. Res. 2005, 66, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.; Papale, P.; Della Ragione, A.; Quaranta, G.; Russo, G.; Russo, S. In vitro antioxidant treatment of semen samples in assisted reproductive technology: Effects of myo-inositol on nemaspermic parameters. Int. J. Endocrinol. 2016, 2016, 2839041. [Google Scholar] [CrossRef] [PubMed]
- Boni, R.; Gallo, A.; Cecchini, S. Kinetic activity, membrane mitochondrial potential, lipid peroxidation, intracellular pH and calcium of frozen/thawed bovine spermatozoa treated with metabolic enhancers. Andrology 2017, 5, 133–145. [Google Scholar] [CrossRef]
- Bucak, M.N.; Tuncer, P.B.; Sariozkan, S.; Baspinar, N.; Taspinar, M.; Coyan, K.; Bilgili, A.; Akalin, P.P.; Buyukleblebici, S.; Aydos, S.; et al. Effects of antioxidants on post-thawed bovine sperm and oxidative stress parameters: Antioxidants protect DNA integrity against cryodamage. Cryobiology 2010, 61, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.A.; Chung, J.S.; Cho, S.-H.; Kim, H.J.; Do Yoo, Y. Romo1 expression contributes to oxidative stress-induced death of lung epithelial cells. Biochem. Biophys. Res. Commun. 2013, 439, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Brewer, L.; Corzett, M.; Balhorn, R. Condensation of DNA by spermatid basic nuclear proteins. J. Biol. Chem. 2002, 277, 38895–38900. [Google Scholar] [CrossRef]
- Boissonneault, G. Chromatin remodeling during spermiogenesis: A possible role for the transition proteins in DNA strand break repair. FEBS Lett. 2002, 514, 111–114. [Google Scholar] [CrossRef]
- Yanagimachi, R. The Sperm Cell: Production, Maturation, Fertilization, Regeneration; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Schulte, R.T.; Ohl, D.A.; Sigman, M.; Smith, G.D. Sperm DNA damage in male infertility: Etiologies, assays, and outcomes. J. Assist. Reprod. Genet. 2010, 27, 3–12. [Google Scholar] [CrossRef]
- Cox, J.F.; Zavala, A.; Saravia, F.; Rivas, C.; Gallardo, P.; Alfaro, V.C. Differences in sperm migration through cervical mucus in vitro relates to sperm colonization of the oviduct and fertilizing ability in goats. Theriogenology 2002, 58, 9–18. [Google Scholar] [CrossRef]
- Aitken, R.J.; Bowie, H.; Buckingham, D.; Harkiss, D.; Richardson, D.W.; West, K.M. Sperm penetration into a hyaluronic acid polymer as a means of monitoring functional competence. J. Androl. 1992, 13, 44–54. [Google Scholar] [PubMed]
- Johnston, R.C.; Mbizvo, M.T.; Summerbell, D.; Kovacs, G.T.; Baker, H.W. Relationship between stimulated hyperactivated motility of human spermatozoa and pregnancy rate in donor insemination: A preliminary report. Hum. Reprod. 1994, 9, 1684–1687. [Google Scholar] [CrossRef]

| Gene | Primer Sequence (5′–3′) | Product Size (bp) | NCBI Accession No. |
|---|---|---|---|
| BACT | F: GAGGCATCCTGACTCTGA | 87 | XM_544346.3 |
| R: TCTGGCACCACACTTTCT | |||
| BCL2 | F: GACAGAGAGGATCATGCTGT | 141 | NM_001002949.1 |
| R: TGGCATGAGATGCAGGAAAT | |||
| BAX | F: CCAAGAAGCTGAGCGAATG | 123 | NM_001003011.1 |
| R: CTGCCACTCGGAAGAAGAC | |||
| PRM2 | F: CTCCAGAAGGGTCAGGAG | 169 | NM_001287148.1 |
| R: GGCTCCTTGCAAACTCAG | |||
| PRM3 | F: TCTGGAGAGGCAGCCAGA | 101 | XM_022420065.1 |
| R: AGGCCATGAGCTTCTTCA | |||
| ROMO1 | F: CTACGTGCTCCCGGAAGT | 100 | XM_534406.6 |
| R: TCGCTCAGTTCTACGTCTCAC |
| Group | Motility (%) | Linearity (%) | Straightness (%) | ALH (µm) | Live Sperm (%) |
|---|---|---|---|---|---|
| 0 mg/mL (control) | 73.8 ± 0.3 a | 24.1 ± 0.3 b | 48.4 ± 0.5 | 5.0 ± 0.1 a | 69.2 ± 0.2 a |
| 1 mg/mL | 71.5 ± 0.4 b | 25.7 ± 0.4 a,b | 49.5 ± 0.5 | 4.6 ± 0.1 a | 68.7 ± 0.2 a |
| 2 mg/mL | 64.7 ± 0.7 c | 27.0 ± 0.5 a | 49.5 ± 0.9 | 4.1 ± 0.1 b | 61.2 ± 0.3 b |
| Group | Motility (%) | Linearity (%) | Straightness (%) | ALH (µm) | Live Sperm (%) | Membrane Integrity (%) |
|---|---|---|---|---|---|---|
| Control (0 mg/mL) | 47.8 ± 0.2 b | 23.9 ± 0.3 b | 50.8 ± 0.9 b | 2.5 ± 0.0 b | 44.6 ± 0.6 b | 46.2 ± 0.6 b |
| Treatment (1 mg/mL) | 57.9 ± 0.4 a | 29.6 ± 1.1 a | 54.4 ± 0.6 a | 3.3 ± 0.0 a | 57.5 ± 0.5 a | 56.6 ± 0.4 a |
| Group | Acrosome Integrity (%) | Sperm Count at Mucus Penetration Distance | |
|---|---|---|---|
| 1 cm | 3 cm | ||
| Control | 51.8 ± 0.5 b | 139.6 ± 0.5 b | 48.1 ± 0.7 b |
| Treatment (1 mg/mL) | 59.3 ± 0.5 a | 150.4 ± 0.6 a | 57.0 ± 0.4 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qamar, A.Y.; Fang, X.; Kim, M.J.; Cho, J. Myoinositol Supplementation of Freezing Medium Improves the Quality-Related Parameters of Dog Sperm. Animals 2019, 9, 1038. https://doi.org/10.3390/ani9121038
Qamar AY, Fang X, Kim MJ, Cho J. Myoinositol Supplementation of Freezing Medium Improves the Quality-Related Parameters of Dog Sperm. Animals. 2019; 9(12):1038. https://doi.org/10.3390/ani9121038
Chicago/Turabian StyleQamar, Ahmad Yar, Xung Fang, Min Jung Kim, and Jongki Cho. 2019. "Myoinositol Supplementation of Freezing Medium Improves the Quality-Related Parameters of Dog Sperm" Animals 9, no. 12: 1038. https://doi.org/10.3390/ani9121038
APA StyleQamar, A. Y., Fang, X., Kim, M. J., & Cho, J. (2019). Myoinositol Supplementation of Freezing Medium Improves the Quality-Related Parameters of Dog Sperm. Animals, 9(12), 1038. https://doi.org/10.3390/ani9121038






