Copy Number Variation of the CADM2 Gene and Its Association with Growth Traits in Yak
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection and Growth Traits
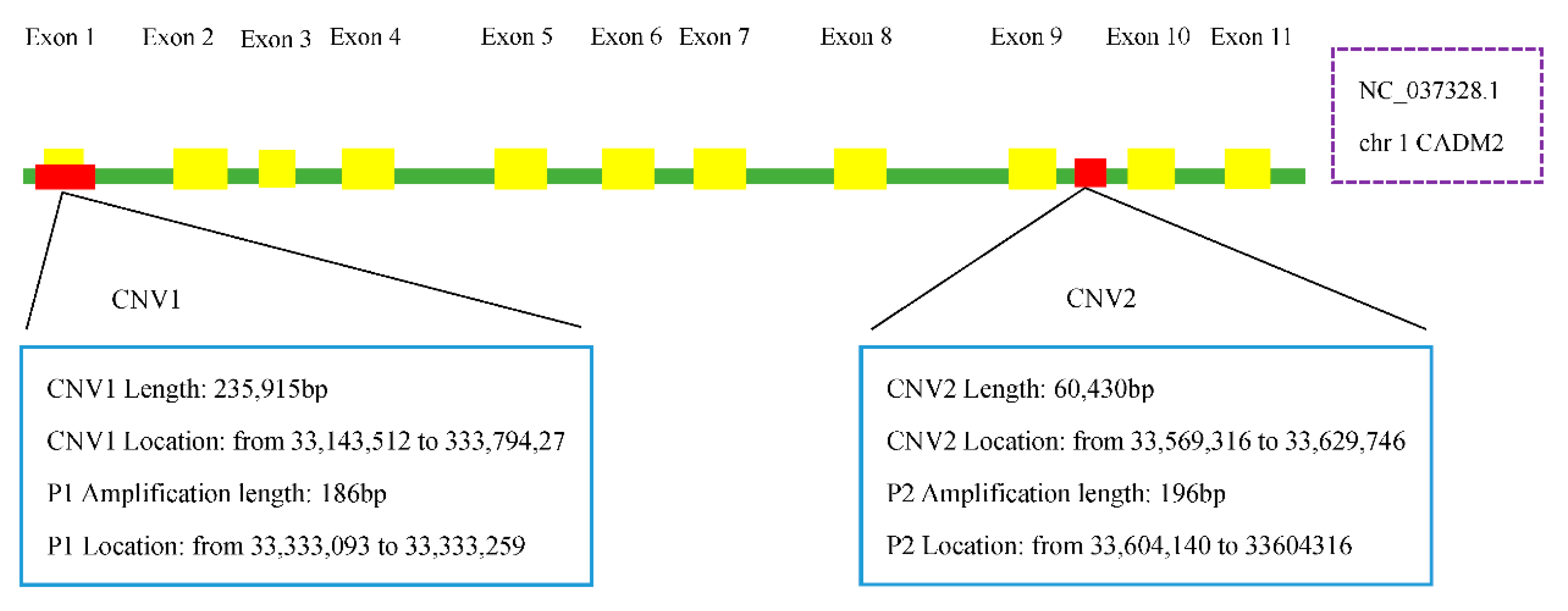
2.3. Genomic DNA Extraction and Copy Number Identification
2.4. Primer Design
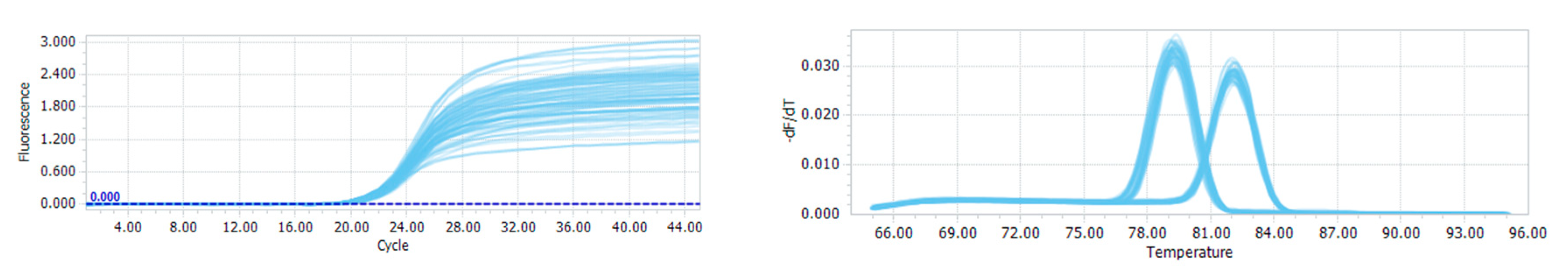
2.5. Quantitative PCR Analysis
2.6. Statistical Analysis
3. Results
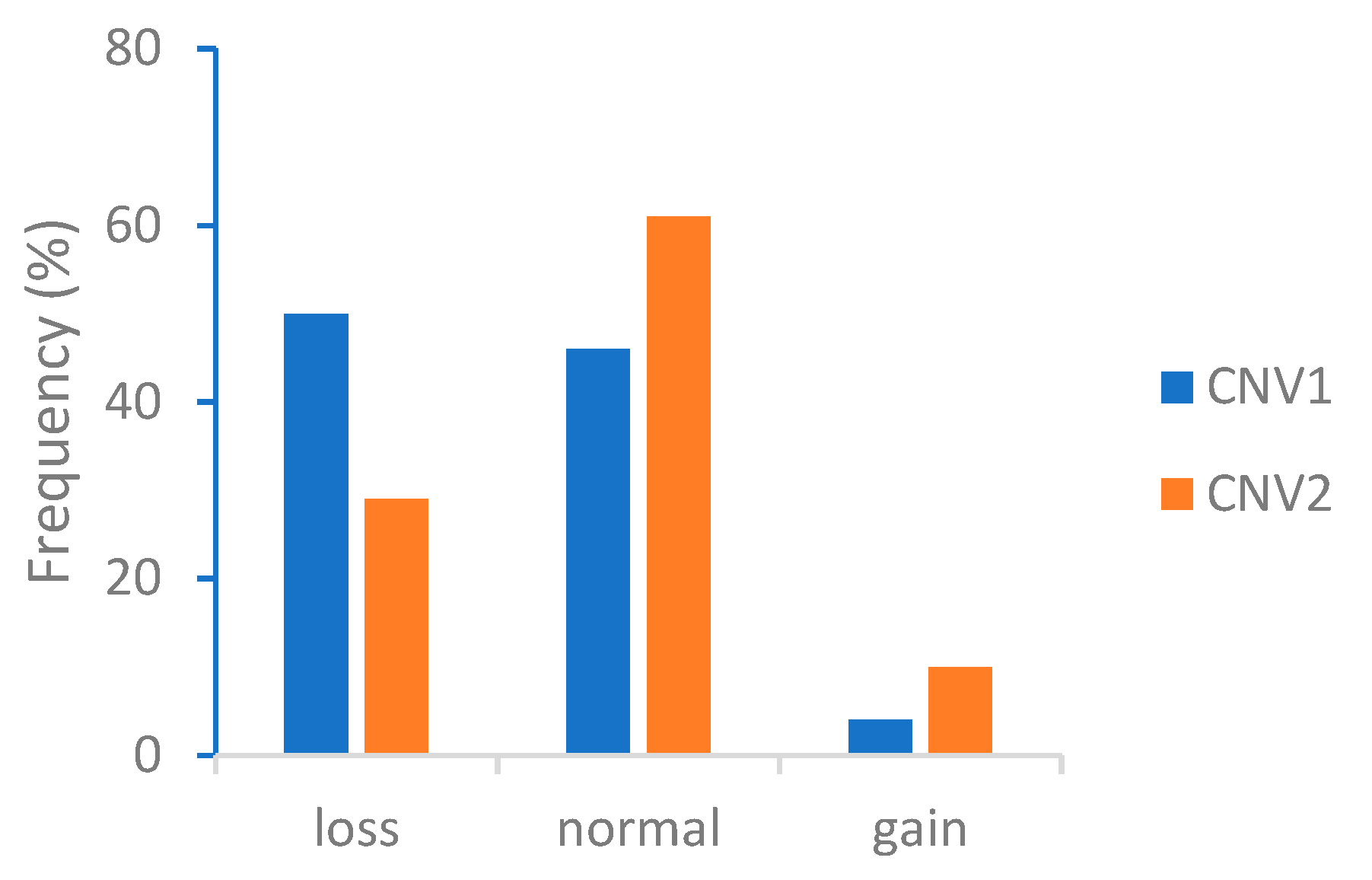
3.1. Quantitative PCR and the Distribution of CADM2-CNVs
3.2. Association Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sebat, J.; Lakshmi, B.; Troge, J.; Alexander, J.; Young, J.; Lundin, P.; Månér, S.; Massa, H.; Walker, M.; Chi, M.; et al. Large-scale copy number polymorphism in the human genome. Science 2004, 305, 525–528. [Google Scholar] [CrossRef]
- Chung, E.R.; Kim, W.T. Association of SNP marker in IGF-I and MYF5 candidate genes with growth traits in Korean cattle. Asian Australas. J. Anim. Sci. 2005, 18, 1061–1065. [Google Scholar] [CrossRef]
- Wang, A.; Zhang, Y.; Li, M.; Lan, X.; Wang, J.; Chen, H. SNP identification in FBXO32 gene and their associations with growth traits in cattle. Gene 2013, 515, 181–186. [Google Scholar] [CrossRef]
- Wu, J.; Qiao, L.; Liu, J.; Yuan, Y.; Liu, W. SNP variation in ADRB3 gene reflects the breed difference of sheep populations. Mol. Biol. Rep. 2012, 39, 8395–8403. [Google Scholar] [CrossRef]
- Wang, X.; Yang, Q.; Zhang, S.; Zhang, X.; Pan, C.; Chen, H.; Zhu, H.; Lan, X. Genetic effects of single nucleotide polymorphisms in the goat GDF9 gene on prolificacy: True or false positive? Animals 2019, 9, 886. [Google Scholar] [CrossRef]
- Britten, R.J.; Rowen, L.; Williams, J.; Cameron, R.A. Majority of divergence between closely related DNA samples is due to indels. Proc. Natl. Acad. Sci. USA 2003, 100, 4661–4665. [Google Scholar] [CrossRef]
- Alkan, C.; Coe, B.P.; Eichler, E.E. Genome structural variation discovery and genotyping. Nat. Rev. Genet. 2011, 12, 363–376. [Google Scholar] [CrossRef]
- Tuzun, E.; Sharp, A.J.; Bailey, J.A.; Kaul, R.; Morrison, V.A.; Pertz, L.M.; Haugen, E.; Hayden, H.; Albertson, D.; Pinkel, D. Fine-scale structural variation of the human genome. Nat. Genatics 2005, 37, 727–732. [Google Scholar] [CrossRef]
- Han, S.H.; Shin, K.Y.; Lee, S.S.; Ko, M.S.; Cho, I.C. SINE indel polymorphism of AGL gene association with growth and carcass traits in Landrace X Jeju black pig F2 population. Mol. Biol. Rep. 2010, 37, 467–471. [Google Scholar] [CrossRef]
- Zhang, W.; Fang, M.; Li, Y.; Nie, Q.; Zhang, X. Identification of TDRP1 gene and its association with pig reproduction traits. DNA Cell Biol. 2012, 31, 371–377. [Google Scholar] [CrossRef]
- Dong-Mei, X.I.; Liu, Q.; Hong-Man, Y.U.; Yang, Y.A.; Mao, H.M.; Deng, W.D. Research progress in the relationship of polymorphism of prion protein gene (PRNP) with disease resistance for sheep and cattle. J. Yunnan Agric. Univ. 2011, 26, 418–425. [Google Scholar]
- Iafrate, A.J.; Feuk, L.; Rivera, M.N.; Listewnik, M.L.; Donahoe, P.K.; Qi, Y.; Scherer, S.W.; Lee, C. Detection of large-scale variation in the human genome. Nat Genet 2004, 36, 949–951. [Google Scholar] [CrossRef]
- Lars, F.; Carson, A.R.; Scherer, S.W. Structural variation in the human genome. Nat. Rev. Genet. 2006, 7, 85–97. [Google Scholar]
- Kidd, J.M.; Cooper, G.M.; Donahue, W.F.; Hayden, H.S.; Nick, S.; Tina, G.; Nancy, H.; Brian, T.; Can, A.; Francesca, A. Mapping and sequencing of structural variation from eight human genomes. Nature 2008, 453, 56–64. [Google Scholar] [CrossRef]
- Gu, W.; Zhang, F.; Lupski, J.R. Mechanisms for human genomic rearrangements. Pathogenetics 2008, 1, 4. [Google Scholar] [CrossRef]
- Gonzalez, E.; Kulkarni, H.; Bolivar, H.; Mangano, A.; Sanchez, R.; Catano, G.; Nibbs, R.J.; Freedman, B.I.; Quinones, M.P.; Bamshad, M.J. The influence of CCL3L1 gene-containing segmental duplications on HIV-1/AIDS susceptibility. Science 2005, 307, 1434–1440. [Google Scholar] [CrossRef]
- Dalila, P.; Pagnamenta, A.T.; Lambertus, K.; Richard, A.; Daniele, M.; Regina, R.; Judith, C.; Magalhaes, T.R.; Catarina, C.; Abrahams, B.S. Functional impact of global rare copy number variation in autism spectrum disorders. Nature 2010, 466, 368–372. [Google Scholar]
- Bassett, A.S.; Scherer, S.W. Copy number variation in tourette syndrome. Neuron 2017, 94, 1041. [Google Scholar] [CrossRef]
- Hou, Y.; Liu, G.E.; Bickhart, D.M.; Matukumalli, L.K.; Li, C.; Song, J.; Gasbarre, L.C.; Tassell, C.P.V.; Sonstegard, T.S. Genomic regions showing copy number variations associate with resistance or susceptibility to gastrointestinal nematodes in Angus cattle. Funct. Integr. Genom. 2012, 12, 81–92. [Google Scholar] [CrossRef]
- Zhang, G.M.; Zheng, L.; He, H.; Song, C.C.; Zhang, Z.J.; Cao, X.K.; Lei, C.Z.; Lan, X.Y.; Qi, X.L.; Chen, H. Associations of GBP2 gene copy number variations with growth traits and transcriptional expression in Chinese cattle. Gene 2018, 647, 101–106. [Google Scholar] [CrossRef]
- Yao, X.; Tao, S.; Hanfang, C.; Yang, Z.; Xianyong, L.; Chunlei, Z.; Chuzhao, L.; Xinglei, Q.; Hong, C. Associations of MYH3 gene copy number variations with transcriptional expression and growth traits in Chinese cattle. Gene 2014, 535, 106–111. [Google Scholar]
- Chen, C.; Qiao, R.; Wei, R.; Guo, Y.; Ai, H.; Ma, J.; Ren, J.; Huang, L. A comprehensive survey of copy number variation in 18 diverse pig populations and identification of candidate copy number variable genes associated with complex traits. BMC Genom. 2012, 13, 733. [Google Scholar] [CrossRef]
- Santana, M.H.D.A.; Junior, G.A.O.; Cesar, A.S.M.; Freua, M.C.; Gomes, R.D.C.; Silva, S.D.L.E.; Leme, P.R.; Fukumasu, H.; Carvalho, M.E.; Ventura, R.V. Copy number variations and genome-wide associations reveal putative genes and metabolic pathways involved with the feed conversion ratio in beef cattle. J. Appl. Genet. 2016, 57, 1–10. [Google Scholar]
- Wiener, G.; Han, J.L.; Long, R.J.; Wiener, G.; Han, J.L.; Long, R.J. The yak. RAP Publ. 2011, 44, 57–58. [Google Scholar]
- Zhang, X.; Wang, K.; Wang, L.; Yang, Y.; Ni, Z.; Xie, X.; Shao, X.; Han, J.; Wan, D.; Qiu, Q. Genome-wide patterns of copy number variation in the Chinese yak genome. BMC Genom. 2016, 17, 379. [Google Scholar] [CrossRef]
- Goshu, H.; Wu, X.; Chu, M.; Bao, P.; Ding, X.; Yan, P. Copy number variations of KLF6 modulate gene transcription and growth traits in Chinese Datong Yak (Bos Grunniens). Animals 2018, 8, 145. [Google Scholar] [CrossRef]
- Goshu, H.A.; Chu, M.; Xiaoyun, W.; Pengjia, B.; Zhi, D.X.; Yan, P. Genomic copy number variation of the CHKB gene alters gene expression and affects growth traits of Chinese domestic yak (Bos grunniens) breeds. Mol. Genet. Genom. 2019, 294, 549–561. [Google Scholar] [CrossRef]
- Jia, C.; Wang, H.; Li, C.; Wu, X.; Zan, L.; Ding, X.; Guo, X.; Bao, P.; Pei, J.; Chu, M. Genome-wide detection of copy number variations in polled yak using the Illumina BovineHD BeadChip. BMC Genom. 2019, 20, 376. [Google Scholar] [CrossRef]
- Speliotes, E.K.; Willer, C.J.; Berndt, S.I.; Monda, K.L.; Thorleifsson, G.; Jackson, A.U.; Allen, H.L.; Lindgren, C.M.; Luan, J.; Magi, R.; et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010, 42, 937–953. [Google Scholar] [CrossRef]
- Speakman, J.R.; Loos, R.J.F.; O’Rahilly, S.; Hirschhorn, J.N.; Allison, D.B. GWAS for BMI: A treasure trove of fundamental insights into the genetic basis of obesity. Int. J. Obes. 2018, 42, 1524–1531. [Google Scholar] [CrossRef]
- Locke, A.E.; Bratati, K.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Corey, P.; Sailaja, V.; Buchkovich, M.L.; Jian, Y. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Wang, Z.; Schmidt, V.; Gauert, A.; Willnow, T.E.; Heinig, M.; Poy, M.N. Cadm2 regulates body weight and energy homeostasis in mice. Mol. Metab. 2017, 8, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Snelling, W.M.; Allan, M.F.; Keele, J.W.; Kuehn, L.A.; Mcdaneld, T.; Smith, T.P.L.; Sonstegard, T.S.; Thallman, R.M.; Bennett, G.L. Genome-wide association study of growth in crossbred beef cattle. J. Anim. Sci. 2010, 88, 837–848. [Google Scholar] [CrossRef]
- Gilbert, R.P.; Bailey, D.R.; Shannon, N.H. Linear body measurements of cattle before and after 20 years of selection for postweaning gain when fed two different diets. J. Anim. Sci. 1993, 71, 1712. [Google Scholar] [CrossRef]
- Bickhart, D.M.; Hou, Y.L.; Schroeder, S.G.; Alkan, C.; Cardone, M.F.; Matukumalli, L.K.; Song, J.Z.; Schnabe, R.D.; Ventura, M.; Taylor, J.F.; et al. Copy number variation of individual cattle genomes using next-generation sequencing. Genome Res. 2012, 22, 778–790. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Cheong, H.S.; Kim, L.H.; Namgung, S.; Park, T.J.; Chun, J.Y.; Kim, J.Y.; Pasaje, C.F.A.; Jin, S.L.; Shin, H.D. Identification of copy number variations and common deletion polymorphisms in cattle. BMC Genom. 2010, 11, 232. [Google Scholar] [CrossRef]
- Yoav, B. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 1995, 57, 289–300. [Google Scholar]
- Ekegbu, U.J.; Burrows, L.; Amirpour-Najafabadi, H.; Zhou, H.; Hickford, J.G. Gene polymorphisms in PROP1 associated with growth traits in sheep. Gene 2019, 683, 41–46. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Guo, Y.; Wang, Y.; Sun, G.; Jiang, R.; Kang, X.; Han, R. Identification of a novel 43-bp insertion in the heparan sulfate 6-O-sulfotransferase 3 (HS6ST3) gene and its associations with growth and carcass traits in chickens. Anim. Biotechnol. 2019, 30, 252–259. [Google Scholar] [CrossRef]
- Zhou, T.; Wei, H.; Li, D.; Yang, W.; Cui, Y.; Gao, J.; Yu, T.; Lv, X.; Pan, C. A novel missense mutation within the domain of lysine demethylase 4D (KDM4D) gene is strongly associated with testis morphology traits in pigs. Anim. Biotechnol. 2019, 1–7. [Google Scholar] [CrossRef]
- Henrichsen, C.N.; Vinckenbosch, N.; Zöllner, S.; Chaignat, E.; Pradervand, S.; Schütz, F.; Ruedi, M.; Kaessmann, H.; Reymond, A. Segmental copy number variation shapes tissue transcriptomes. Nat. Genet. 2009, 41, 424. [Google Scholar] [CrossRef] [PubMed]
- Conrad, D.F.; Christine, B.; Ben, B.; Sarah, L.; Lira, M.; Charles, L.; Turner, D.J.; Hurles, M.E. Mutation spectrum revealed by breakpoint sequencing of human germline CNVs. Nat. Genet. 2010, 42, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, L.; Xu, L.; Ren, H.; Lu, J.; Zhang, X.; Zhang, S.; Zhou, X.; Wei, C.; Zhao, F. Analysis of copy number variations in the sheep genome using 50K SNP BeadChip array. BMC Genom. 2013, 14, 229. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Wang, J.; Wang, H.; Zhang, Y.; Kang, H.; Feng, X.; Wang, J.; Yin, Z.; Bao, W.; Zhang, Q. Global copy number analyses by next generation sequencing provide insight into pig genome variation. BMC Genom. 2014, 15, 593. [Google Scholar] [CrossRef][Green Version]
- Yang, M.; Lv, J.; Zhang, L.; Li, M.; Zhou, Y.; Lan, X.; Lei, C.; Chen, H. Association study and expression analysis of CYP4A11 gene copy number variation in Chinese cattle. Sci. Rep. 2017, 7, 46599. [Google Scholar] [CrossRef]
- Revilla, M.; Puig-Oliveras, A.; Castelló, A.; Crespo-Piazuelo, D.; Paludo, E.; Fernández, A.I.; Ballester, M.; Folch, J.M. A global analysis of CNVs in swine using whole genome sequence data and association analysis with fatty acid composition and growth traits. PLoS ONE 2017, 12, e0177014. [Google Scholar] [CrossRef]
- Zhang, R.-Q.; Wang, J.-J.; Zhang, T.; Zhai, H.-L.; Shen, W. Copy-number variation in goat genome sequence: A comparative analysis of the different litter size trait groups. Gene 2019, 696, 40–46. [Google Scholar] [CrossRef]
- Sundström, E.; Imsland, F.; Mikko, S.; Wade, C.; Sigurdsson, S.; Pielberg, G.R.; Golovko, A.; Curik, I.; Seltenhammer, M.H.; Sölkner, J. Copy number expansion of the STX17 duplication in melanoma tissue from Grey horses. BMC Genom. 2012, 13, 365. [Google Scholar] [CrossRef]
- Cantsilieris, S.; White, S.J. Correlating multiallelic copy number polymorphisms with disease susceptibility. Hum. Mutat. 2013, 34, 1–13. [Google Scholar] [CrossRef]
- Thomas, L.A.; Akins, M.R.; Thomas, B. Expression and adhesion profiles of SynCAM molecules indicate distinct neuronal functions. J. Comp. Neurol. 2010, 510, 47–67. [Google Scholar] [CrossRef]
- Rathjen, T.; Yan, X.; Kononenko, N.L.; Ku, M.C.; Song, K.; Ferrarese, L.; Tarallo, V.; Puchkov, D.; Kochlamazashvili, G.; Brachs, S. Regulation of body weight and energy homeostasis by neuronal cell adhesion molecule 1. Nat. Neurosci. 2017, 20, 1096. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim-Verbaas, C.A.; Bressler, J.; Debette, S.; Schuur, M.; Smith, A.V.; Bis, J.C.; Davies, G.; Trompet, S.; Smith, J.A.; Wolf, C. GWAS for executive function and processing speed suggests involvement of the CADM2 gene. Mol. Psychiatry 2016, 21, 189. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.; Bailey, M.E.; Baldassarre, D.; Cullen, B.; de Faire, U.; Ferguson, A.; Gigante, B.; Giral, P.; Goel, A.; Graham, N. Genetic variation in CADM2 as a link between psychological traits and obesity. Sci. Rep. 2019, 9, 7339. [Google Scholar] [CrossRef] [PubMed]
- Lamiquiz-Moneo, I.; Mateo-Gallego, R.; Bea, A.M.; Dehesa-García, B.; Pérez-Calahorra, S.; Marco-Benedí, V.; Baila-Rueda, L.; Laclaustra, M.; Civeira, F.; Cenarro, A. Genetic predictors of weight loss in overweight and obese subjects. Sci. Rep. 2019, 9, 10770. [Google Scholar] [CrossRef]
- Rout, P.K.; Matika, O.; Kaushik, R.; Dass, G.; Singh, M.K.; Dige, M.S.; Bhusan, S. Genetic analysis of growth parameters and survival potential of Jamunapari goats in semiarid tropics. Small Rumin. Res. 2018, 165, 124–130. [Google Scholar] [CrossRef]
- Shi, T.; Xu, Y.; Yang, M.; Huang, Y.; Lan, X.; Lei, C.; Qi, X.; Yang, X.; Chen, H. Copy number variations at LEPR gene locus associated with gene expression and phenotypic traits in Chinese cattle. Anim. Sci. J. 2016, 87, 336–343. [Google Scholar] [CrossRef]
- Jiang, R.; Cheng, J.; Cao, X.-K.; Ma, Y.-L.; Chaogetu, B.; Huang, Y.-Z.; Lan, X.-Y.; Lei, C.-Z.; Hu, L.-Y.; Chen, H. Copy number variation of the SHE gene in sheep and its association with economic traits. Animals 2019, 9, 531. [Google Scholar] [CrossRef]
- Wright, D.; Boije, H.; Meadows, J.R.; Bed’Hom, B.; Gourichon, D.; Vieaud, A.; Tixier-Boichard, M.; Rubin, C.-J.; Imsland, F.; Hallböök, F. Copy number variation in intron 1 of SOX5 causes the Pea-comb phenotype in chickens. PLoS Genet. 2009, 5, e1000512. [Google Scholar] [CrossRef]
| Gene | Primer Pairs Sequence (5′-3′) | Amplicon Length (bp) | Tm (°C) |
|---|---|---|---|
| CADM2-P1 | F: GACTTCCCAGGATTGCCTGT R: CCCTGGGAGCACAGTTGTTT | 186 | 62 °C |
| CADM2-P2 | F: GGCTGTCACGTTCTTCTCTCA R: AGGGTTCATCCTGGAGGCTT | 196 | 62 °C |
| BTF3 | F: AACCAGGAGAAACTCGCCAA R: TTCGGTGAAATGCCCTCTCG | 166 | 62 °C |
| Type | Loss | Normal | Gain |
|---|---|---|---|
| CNV1 | 176 (0.50) | 161 (0.46) | 13 (0.04) |
| CNV2 | 101 (0.29) | 215 (0.61) | 34 (0.1) |
| Type | CNV1 | CNV2 |
|---|---|---|
| CNV1 | 37.445 (p = 0.000 **) | |
| CNV2 |
| Age | Growth Trait | CNV Type (Mean ± SE) | p-Value | ||
|---|---|---|---|---|---|
| Loss (0.50) | Normal (0.46) | Gain (0.04) | |||
| 6 months | Body weight (kg) | 83.14 ± 0.773 a | 85.60 ± 0.833 b | 81.15 ± 2.207 a,b | 0.052 |
| Withers height (cm) | 94.50 ± 0.376 | 94.27 ± 0.435 | 95.31 ± 1.491 | 0.765 | |
| Body length (cm) | 91.46 ± 0.557 | 92.53 ± 0.594 | 90.54 ± 1.228 | 0.325 | |
| Chest girth(cm) | 124.21 ± 0.567 | 123.66 ± 0.662 | 124.38 ± 1.607 | 0.802 | |
| 12 months | Body weight (kg) | 82.17 ± 0.784 | 83.14 ± 0.881 | 80.64 ± 3.058 | 0.584 |
| Withers height (cm) | 90.62 ± 0.324 | 90.28 ± 0.335 | 90.31 ± 0.894 | 0.755 | |
| Body length (cm) | 95.98 ± 0.380 | 95.74 ± 0.387 | 96.92 ± 1.719 | 0.682 | |
| Chest girth(cm) | 117.35 ± 0.379 | 116.93 ± 0.428 | 116.92 ± 0.843 | 0.747 | |
| 18 months | Body weight (kg) | 121.82 ± 1.119 | 122.59 ± 1.258 | 126.25 ± 3.371 | 0.516 |
| Withers height (cm) | 101.71 ± 0.452 | 101.73 ± 0.476 | 105.54 ± 3.098 | 0.180 | |
| Body length (cm) | 101.22 ± 0.464 | 102.07 ± 0.460 | 101.69 ± 1.784 | 0.439 | |
| Chest girth(cm) | 137.97 ± 0.840 | 138.57 ± 0.795 | 141.54 ± 3.580 | 0.464 | |
| Age | Growth Trait | CNV Type (Mean ± SE) | p-Value | ||
|---|---|---|---|---|---|
| Loss (0.29) | Normal (0.61) | Gain (0.1) | |||
| 6 months | Body weight (kg) | 82.22 ± 1.004 a | 84.75 ± 0.723 b | 86.61 ± 1.559 c | 0.048 * |
| Withers height (cm) | 94.27 ± 0.544 | 94.28 ± 0.357 | 95.82 ± 0.785 | 0.263 | |
| Body length (cm) | 90.93 ± 0.743 | 92.30 ± 0.499 | 92.74 ± 1.270 | 0.278 | |
| Chest girth(cm) | 123.56 ± 0.877 a | 123.66 ± 0.518 a | 127.08 ± 1.013 b | 0.051 | |
| 12 months | Body weight (kg) | 82.53 ± 1.107 | 82.43 ± 0.726 | 84.53 ± 2.013 | 0.579 |
| Withers height (cm) | 90.09 ± 0.456 | 90.54 ± 0.278 | 90.94 ± 0.729 | 0.534 | |
| Body length (cm) | 95.64 ± 0.491 | 96.09 ± 0.333 | 95.47 ± 1.038 | 0.656 | |
| Chest girth(cm) | 116.74 ± 0.525 | 117.24 ± 0.355 | 117.65 ± 0.778 | 0.606 | |
| 18 months | Body weight (kg) | 122.71 ± 1.510 | 121.71 ± 1.076 | 125.19 ± 1.996 | 0.428 |
| Withers height (cm) | 101.49 ± 0.640 | 102.31 ± 0.414 | 100.34 ± 1.299 | 0.180 | |
| Body length (cm) | 101.11 ± 0.667 | 101.79 ± 0.396 | 102.13 ± 0.913 | 0.567 | |
| Chest girth(cm) | 137.99 ± 1.165 | 138.45 ± 0.698 | 139.19 ± 1.923 | 0.844 | |
| Kendall Tau correlation coefficient | 0.261 |
| Significance | 0.007 ** |
| Age | Growth Trait | CNVs (P Value) | ||
|---|---|---|---|---|
| CNV1 | CNV2 | CNV1 × CNV2 | ||
| 6 months | Body weight (kg) | 0.129 | 0.485 | 0.884 |
| Withers height (cm) | 0.439 | 0.295 | 0.418 | |
| Body length (cm) | 0.470 | 0.960 | 0.370 | |
| Chest girth(cm) | 0.905 | 0.652 | 0.387 | |
| 12 months | Body weight (kg) | 0.447 | 0.958 | 0.859 |
| Withers height (cm) | 0.870 | 0.944 | 0.735 | |
| Body length (cm) | 0.631 | 0.891 | 0.996 | |
| Chest girth(cm) | 0.997 | 0.995 | 0.845 | |
| 18 months | Body weight (kg) | 0.622 | 0.941 | 0.354 |
| Withers height (cm) | 0.003 ** | 0.039 * | 0.190 | |
| Body length (cm) | 0.508 | 0.821 | 0.750 | |
| Chest girth(cm) | 0.122 | 0.253 | 0.168 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, F.; Jia, C.; Chu, M.; Liang, C.; Yan, P. Copy Number Variation of the CADM2 Gene and Its Association with Growth Traits in Yak. Animals 2019, 9, 1008. https://doi.org/10.3390/ani9121008
Ge F, Jia C, Chu M, Liang C, Yan P. Copy Number Variation of the CADM2 Gene and Its Association with Growth Traits in Yak. Animals. 2019; 9(12):1008. https://doi.org/10.3390/ani9121008
Chicago/Turabian StyleGe, Fei, Congjun Jia, Min Chu, Chunnian Liang, and Ping Yan. 2019. "Copy Number Variation of the CADM2 Gene and Its Association with Growth Traits in Yak" Animals 9, no. 12: 1008. https://doi.org/10.3390/ani9121008
APA StyleGe, F., Jia, C., Chu, M., Liang, C., & Yan, P. (2019). Copy Number Variation of the CADM2 Gene and Its Association with Growth Traits in Yak. Animals, 9(12), 1008. https://doi.org/10.3390/ani9121008




