The Effect of Dietary Replacement of Fish Meal with Whey Protein Concentrate on the Growth Performance, Fish Health, and Immune Status of Nile Tilapia Fingerlings, Oreochromis niloticus
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish and Experimental Design
2.2. Preparation and Characterization of Whey Protein Concentrate and Experimental Diet Formulation
2.3. Survival Percentage and Growth Performance Parameters
2.4. Bacterial Challenge Test
2.5. Sampling
2.6. Fish Whole Body Composition
2.7. Blood Biochemical Parameters
2.7.1. Phagocytic Capacity
2.7.2. The Blood Levels of IgM, Complement 3, and Lysozyme Activity
2.7.3. Liver and Kidney Function Tests
2.7.4. The Blood Levels of Growth Hormone and Nitric Oxide
2.8. Histology and Morphometric Methods
2.9. Immunohistochemistry (IHC)
2.10. Economic Efficiency
2.11. Statistical Analysis
3. Results
3.1. Characterization of Whey Protein Concentrate
3.2. Survival Percentage and Growth Performance
3.3. Fish Whole body Proximate Composition
3.4. Clinical Signs and Fish Behavior
3.5. Growth Hormone and Nitric Oxide (NO) Levels
3.6. Levels of IgM, Complement 3, and Lysozyme Activity
3.7. Liver Function Tests
3.8. Kidney Function Tests
3.9. Phagocytic Percentage and Phagocytic Index
3.10. Histological Findings
3.11. Morphometric Measures of the Intestine
3.12. Immunohistochemistry Results
3.13. Economic Efficiency of the Experimental Diets
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tacon, A.G.; Metian, M. Global overview on the use of fish meal and fish oil in industrially compounded aquafeeds: Trends and future prospects. Aquaculture 2008, 285, 146–158. [Google Scholar] [CrossRef]
- Bergheim, A.; Sveier, H. Replacement of fish meal in salmonid diets by soya meal reduces phosphorus excretion. Aquac. Int. 1995, 3, 265–268. [Google Scholar] [CrossRef]
- Jahan, P.; Watanabe, T.; Kiron, V.; Satoh, S. Improved carp diets based on plant protein sources reduce environmental phosphorus loading. Fish. Sci. 2003, 69, 219–225. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Wang, Y.-Y.; Lu, Y.; Li, Q.-Z. Effect of replacing fish meal with soybean meal on growth, feed utilization and nitrogen and phosphorus excretion on rainbow trout (Oncorhynchus mykiss). Aquac. Int. 2011, 19, 405–419. [Google Scholar] [CrossRef]
- dos Santos Barbosa, A.; Florentino, E.R.; Florêncio, I.M.; dos Santos Araújo, A. Utilização do soro como substrato para produção de aguardente: Estudo cinético da produção de etanol. Revista Verde de Agroecologia e Desenvolvimento Sustentável 2010, 5, 7–25. [Google Scholar]
- Brans, G.; Schroën, C.; Van der Sman, R.; Boom, R. Membrane fractionation of milk: State of the art and challenges. J. Membr. Sci. 2004, 243, 263–272. [Google Scholar] [CrossRef]
- Bounous, G.; Stevenson, M.M.; Kongshavn, P.A. Influence of dietary lactalbumin hydrolysate on the immune system of mice and resistance to Salmonellosis. J. Infect. Dis. 1981, 144, 281. [Google Scholar] [CrossRef]
- Stoeck, M.; Ruegg, C.; Miescher, S.; Carrel, S.; Cox, D.; Von Fliedner, V.; Alkan, S. Comparison of the immunosuppressive properties of milk growth factor and transforming growth factors beta 1 and beta 2. J. Immunol. 1989, 143, 3258–3265. [Google Scholar]
- Wong, C.; Seow, H.; Husband, A.; Regester, G.; Watson, D. Effects of purified bovine whey factors on cellular immune functions in ruminants. Vet. Immunol. Immunopathol. 1997, 56, 85–96. [Google Scholar] [CrossRef]
- Wong, K.; Middleton, N.; Montgomery, M.; Dey, M.; Carr, R. Immunostimulation of murine spleen cells by materials associated with bovine milk protein fractions. J. Dairy Sci. 1998, 81, 1825–1832. [Google Scholar] [CrossRef]
- Debbabi, H.; Dubarry, M.; Rautureau, M.; Tomé, D. Bovine lactoferrin induces both mucosal and systemic immune response in mice. J. Dairy Res. 1998, 65, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Cross, M.L.; Gill, H.S. Modulation of immune function by a modified bovine whey protein concentrate. Immunol. Cell Biol. 1999, 77, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Parodi, P.W. A role for milk proteins in cancer prevention. Aust. J. Dairy Technol. 1998, 53, 37. [Google Scholar]
- McIntosh, G.H.; Royle, P.J.; Le Leu, R.K.; Regester, G.O.; Johnson, M.A.; Grinsted, R.L.; Kenward, R.S.; Smithers, G.W. Whey proteins as functional food ingredients? Int. Dairy J. 1998, 8, 425–434. [Google Scholar] [CrossRef]
- Tavares, T.G.; Malcata, F.X. Whey proteins as source of bioactive peptides against hypertension. Bioact. Food Pept. Health Dis. 2013, 2013, 75–114. [Google Scholar]
- Yoshizawa, F. Regulation of protein synthesis by branched-chain amino acids in vivo. Biochem. Biophys. Res. Commun. 2004, 313, 417–422. [Google Scholar] [CrossRef]
- Tsakali, E.; Petrotos, K.; D’Allessandro, A.; Goulas, P. A review on whey composition and the methods used for its utilization for food and pharmaceutical products. In Proceedings of the 6th International Conference on Simulation and Modelling in the Food and Bio-industry: FOODSIM, Bragança, Portugal, 24–26 June 2010; pp. 195–201. [Google Scholar]
- Abdel-Tawwab, M.; Abbass, F.E. Dry whey meal as a protein source in practical diets for Nile tilapia, Oreochromis niloticus fingerlings. J. Appl. Aquac. 2016, 28, 276–284. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The genus Aeromonas: Taxonomy, pathogenicity, and infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef]
- Romero, J.; Feijoó, C.G.; Navarrete, P. Antibiotics in aquaculture–use, abuse and alternatives. In Health and Environment in Aquaculture; IntechOpen: London, UK, 2012; p. 159. [Google Scholar]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater; APHA: Washington, DC, USA; AWWA: Denver, CO, USA; WEF: Cologny, Switzerland, 1998. [Google Scholar]
- Ha, M.; Bekhit, A.E.-D.; McConnell, M.; Mason, S.; Carne, A. Fractionation of whey proteins from red deer (Cervus elaphus) milk and comparison with whey proteins from cow, sheep and goat milks. Small Rumin. Res. 2014, 120, 125–134. [Google Scholar] [CrossRef]
- Kishawy, A.T.; Amer, S.A.; Osman, A.; Elsayed, S.A.; El-Hack, M.E.A.; Swelum, A.A.; Ba-Awadh, H.; Saadeldin, I.M. Impacts of supplementing growing rabbit diets with whey powder and citric acid on growth performance, nutrient digestibility, meat and bone analysis, and gut health. AMB Express 2018, 8, 86. [Google Scholar] [CrossRef]
- Otte, J.; Abdel-Hamid, M.; Osman, A. Comparative assessment of peptide concentration in milk protein hydrolysates and fractions. Int. J. Dairy Sci. 2015, 10, 228–235. [Google Scholar]
- Nishanthi, M.; Chandrapala, J.; Vasiljevic, T. Compositional and structural properties of whey proteins of sweet, acid and salty whey concentrates and their respective spray dried powders. Int. Dairy J. 2017, 74, 49–56. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, M.; Goda, H.A.; De Gobba, C.; Jenssen, H.; Osman, A. Antibacterial activity of papain hydrolysed camel whey and its fractions. Int. Dairy J. 2016, 61, 91–98. [Google Scholar] [CrossRef]
- Souillac, P.O.; Middaugh, C.R.; Rytting, J.H. Investigation of protein/carbohydrate interactions in the dried state. 2. Diffuse reflectance FTIR studies. Int. J. Pharm. 2002, 235, 207–218. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2000. [Google Scholar]
- NRC. Nutrient Requirements of Fish and Shrimp; Wash; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Santiago, C.B.; Bañes-Aldaba, M.; Laron, M.A. Dietary crude protein requirement of Tilapia nilotica fry. Kalikasan: J. Philipp. Biol. 1982, 11, 255–265. [Google Scholar]
- Castell, J.; Tiews, K. Report of the EIFAC, IUNS and ICES Working Group on Standardization of Methodology in Fish Nutrition Research; Documents Techniques de la CECPI (FAO): Hamburg, Germany, 1980. [Google Scholar]
- Stuart, J.S.; Hung, S.S. Growth of juvenile white sturgeon (Acipenser transmontanus) fed different proteins. Aquaculture 1989, 76, 303–316. [Google Scholar] [CrossRef]
- Zhou, F.; Song, W.; Shao, Q.; Peng, X.; Xiao, J.; Hua, Y.; Owari, B.N.; Zhang, T.; Ng, W.K. Partial replacement of fish meal by fermented soybean meal in diets for black sea bream, Acanthopagrus schlegelii, juveniles. J. World Aquac. Soc. 2011, 42, 184–197. [Google Scholar] [CrossRef]
- Scheidegger, E.; Fracalanzza, S.; Teixeira, L.; Cardarelli-Leite, P. RFLP analysis of a PCR-amplified fragment of the 16S rRNA gene as a tool to identify Enterococcus strains. Memórias do Instituto Oswaldo Cruz 2009, 104, 1003–1008. [Google Scholar] [CrossRef]
- Lucky, Z. Methods for the Diagnosis of Fish Diseases; Amerind. Publishing Co., P V T. Ltd.: New Delhi, Bombay, India, 1977. [Google Scholar]
- Lima dos Santos, C.; James, D.; Teutscher, F. Guidelines for Chilled Fish Storage Experiments; FAO Fisheries Technical Papers: Québec, QC, Canada, 1981. [Google Scholar]
- Wilkinson, P. Neutrophil leucocyte function tests. Tech. Clin. Immunol. 1977, 2, 273–293. [Google Scholar]
- Rashid, M.M.; Nakai, T.; Muroga, K.; Miyazaki, T. Pathogenesis of experimental edwardsiellosis in Japanese flounder Paralichthys olivaceus. Fish. Sci. 1997, 63, 384–387. [Google Scholar] [CrossRef]
- Murray, R. Aspartate aminotransferase. In Clinical Chemistry; Kaplan, L.A., Pesce, A.J., Eds.; The CV Mosby Co.: Toronto, ON, Canada, 1984; pp. 418, 437, 1257–1260. [Google Scholar]
- Burtis, C.A.; Ashwood, E.R. Tietz Textbook of Clinical Chemistry; Amer Assn for Clinical Chemistry: Washington, DC, USA, 1994. [Google Scholar]
- Kaplan, A. Clinical Chemistry; Kaplan, L.A., Pesce, A.J., Eds.; The CV Mosby Co.: Toronto, ON, Canada, 1984; pp. 418, 437, 1257–1260. [Google Scholar]
- Fossati, P.; Prencipe, L.; Berti, G. Enzymic creatinine assay: A new colorimetric method based on hydrogen peroxide measurement. Clin. Chem. 1983, 29, 1494–1496. [Google Scholar] [PubMed]
- Sun, J.; Zhang, X.; Broderick, M.; Fein, H. Measurement of nitric oxide production in biological systems by using Griess reaction assay. Sensors 2003, 3, 276–284. [Google Scholar] [CrossRef]
- Suvarna, S.; Layton, C.; Bancroft, J. The hematoxylins and eosin. In Bancroft’s Theory and Practice of Histological Techniques, 7th ed.; Churchill Livingstone: London, UK, 2013; pp. 172–186. [Google Scholar]
- Hsu, S.-M.; Raine, L.; Fanger, H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. J. Histochem. Cytochem. 1981, 29, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Rizzardi, A.E.; Johnson, A.T.; Vogel, R.I.; Pambuccian, S.E.; Henriksen, J.; Skubitz, A.P.; Metzger, G.J.; Schmechel, S.C. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn. Pathol. 2012, 7, 42. [Google Scholar] [CrossRef]
- El-Telbany, M.; Atallah, S. Some Culture Factors Affecting the Productive and Economic Efficiency of Mugil Capito Nursing in Earthen Pond System 9 th Scientific Cingrees. Assiut Vet. J. 2000, 46, 19–20. [Google Scholar]
- Dunning, R.; Daniels, H. Hybrid Striped Bass Production in Ponds: Enterprise Budget; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2001. [Google Scholar]
- North, M.; Bell, D. Breeder management. In Commercial Chicken Production Manual; The Avi. Publishing Company. Inc.: Westport, CT, USA, 1984; pp. 240–321. [Google Scholar]
- Agyare, K.K.; Damodaran, S. pH-stability and thermal properties of microbial transglutaminase-treated whey protein isolate. J. Agric. Food Chem. 2010, 58, 1946–1953. [Google Scholar] [CrossRef]
- Jiang, S.; Altaf Hussain, M.; Cheng, J.; Jiang, Z.; Geng, H.; Sun, Y.; Sun, C.; Hou, J. Effect of heat treatment on physicochemical and emulsifying properties of polymerized whey protein concentrate and polymerized whey protein isolate. LWT 2018, 98, 134–140. [Google Scholar] [CrossRef]
- Andrade, J.; Pereira, C.G.; de Almeida Junior, J.C.; Viana, C.C.R.; de Oliveira Neves, L.N.; da Silva, P.H.F.; Bell, M.J.V.; dos Anjos, V.D.C. FTIR-ATR determination of protein content to evaluate whey protein concentrate adulteration. LWT 2019, 99, 166–172. [Google Scholar] [CrossRef]
- Dixon, T.; Perkins, H. Citric acid and bone metabolism. Biochem. J. 1952, 52, 260. [Google Scholar] [CrossRef]
- Huss, H.H. Quality and Quality Changes in Fresh Fish; FAO: Rome, Italy, 1995; Volume 348. [Google Scholar]
- Rutherfurd-Markwick, K.J.; Johnson, D.; Cross, M.L.; Gill, H.S. Modified milk powder supplemented with immunostimulating whey protein concentrate (IMUCARE) enhances immune function in mice. Nutr. Res. 2005, 25, 197–208. [Google Scholar] [CrossRef]
- Galeotti, M.; Romano, N.; Volpatti, D.; Bulfon, C.; Brunetti, A.; Tiscar, P.G.; Mosca, F.; Bertoni, F.; Marchetti, M.G.; Abelli, L. Innovative vaccination protocol against vibriosis in Dicentrarchus labrax (L.) juveniles: Improvement of immune parameters and protection to challenge. Vaccine 2013, 31, 1224–1230. [Google Scholar] [CrossRef] [PubMed]
- Clare, D.; Catignani, G.; Swaisgood, H. Biodefense properties of milk: The role of antimicrobial proteins and peptides. Curr. Pharm. Des. 2003, 9, 1239–1255. [Google Scholar] [CrossRef] [PubMed]
- Expósito, I.L.; Recio, I. Antibacterial activity of peptides and folding variants from milk proteins. Int. Dairy J. 2006, 16, 1294–1305. [Google Scholar] [CrossRef]
- Brück, W.; Graverholt, G.; Gibson, G. A two-stage continuous culture system to study the effect of supplemental α-lactalbumin and glycomacropeptide on mixed cultures of human gut bacteria challenged with enteropathogenic Escherichia coli and Salmonella serotype Typhimurium. J. Appl. Microbiol. 2003, 95, 44–53. [Google Scholar] [CrossRef]
- Pellegrini, A.; Dettling, C.; Thomas, U.; Hunziker, P. Isolation and characterization of four bactericidal domains in the bovine β-lactoglobulin. Biochim. Biophys. Acta 2001, 1526, 131–140. [Google Scholar] [CrossRef]
- Groleau, P.E.; Morin, P.; Gauthier, S.F.; Pouliot, Y. Effect of physicochemical conditions on peptide-peptide interactions in a tryptic hydrolysate of β-lactoglobulin and identification of aggregating peptides. J. Agric. Food Chem. 2003, 51, 4370–4375. [Google Scholar] [CrossRef]
- Pellegrini, A.; Thomas, U.; Bramaz, N.; Hunziker, P.; von Fellenberg, R. Isolation and identification of three bactericidal domains in the bovine α-lactalbumin molecule. Biochim. Biophys. Acta 1999, 1426, 439–448. [Google Scholar] [CrossRef]
- Low, P.; Rutherfurd, K.; Cross, M.; Gill, H. Enhancement of mucosal antibody responses by dietary whey protein concentrate. Food Agric. Immunol. 2001, 13, 255–264. [Google Scholar] [CrossRef]
- Wong, C.W.; Watson, D.L. Immunomodulatory effects of dietary whey proteins in mice. J. Dairy Res. 1995, 62, 359–368. [Google Scholar] [CrossRef]
- De Wit, J. Nutritional and functional characteristics of whey proteins in food products. J. Dairy Sci. 1998, 81, 597–608. [Google Scholar] [CrossRef]
- Gauthier, S.F.; Pouliot, Y.; Saint-Sauveur, D. Immunomodulatory peptides obtained by the enzymatic hydrolysis of whey proteins. Int. Dairy J. 2006, 16, 1315–1323. [Google Scholar] [CrossRef]
- Gill, H. Dairy Products and Immune Health; Leatherhead Publishing, Surrey, UK, 2000; pp. 268–284.
- Heine, W.; Radke, M.; Wutzke, K.; Peters, E.; Kundt, G. α-Lactalbumin-enriched low-protein infant formulas: A comparison to breast milk feeding. Acta Paediatr. 1996, 85, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Knowles, G.; Gill, H. Immunomodulation by dairy ingredients: Potential for improving health. In Handbook of Functional Dairy Products; CRC Press: Boca Raton, FL, USA, 2004; pp. 125–153. [Google Scholar]
- Low, P.; Rutherfurd, K.; Gill, H.; Cross, M. Effect of dietary whey protein concentrate on primary and secondary antibody responses in immunized BALB/c mice. Int. Immunopharmacol. 2003, 3, 393–401. [Google Scholar] [CrossRef]
- Prates, J.M.; Mateus, C. Functional foods from animal sources and their physiologically active components. Rev. De Médecine Vétérinaire 2002, 153, 155–160. [Google Scholar]
- Chi, C.C.; Shiu, Y.L.; Lin, H.L.; Liu, C.H. Immune Response and Disease Resistance of Barramundi, Lates calcarifer (Bloch), Offered Diets Supplemented with Replete Levels of Tryptophan or Whey. J. World Aquac. Soc. 2018, 49, 127–140. [Google Scholar] [CrossRef]
- Uthaisangsook, S.; Day, N.K.; Bahna, S.L.; Good, R.A.; Haraguchi, S. Innate immunity and its role against infections. Ann. Allergy Asthma Immunol. 2002, 88, 253–265. [Google Scholar] [CrossRef]
- Chun, S.-H.; Lee, H.A.; Lee, K.B.; Kim, S.H.; Park, K.-Y.; Lee, K.-W. Effects of glycated whey protein concentrate on pro-inflammatory cytokine expression and phagocytic activity in RAW264. 7 macrophages. Biol. Pharm. Bull. 2016, 39, 199–206. [Google Scholar] [CrossRef]
- Lowenstein, C.J. Nitric oxide, a novel biologic messenger. Cell 1992, 70, 705–707. [Google Scholar] [CrossRef]
- Pihlanto, A. Antioxidative peptides derived from milk proteins. Int. Dairy J. 2006, 16, 1306–1314. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Food protein-derived bioactive peptides: Production, processing, and potential health benefits. J. Food Sci. 2012, 77, R11–R24. [Google Scholar] [CrossRef]
- Kew, M.C. Serum aminotransferase concentration as evidence of hepatocellular damage. Lancet 2000, 355, 591–592. [Google Scholar] [CrossRef]
- Cocchetto, D.M.; Tschanz, C.; Bjornsson, T.D. Decreased rate of creatinine production in patients with hepatic disease: Implications for estimation of creatinine clearance. Ther. Drug Monit. 1983, 5, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Terjung, R.; Baldwin, K.; Winder, W.; Holloszy, J. Glycogen repletion in different types of muscle and in liver after exhausting exercise. Am. J. Physiol. -Leg. Content 1974, 226, 1387–1391. [Google Scholar] [CrossRef] [PubMed]
- Morifuji, M.; Sakai, K.; Sugiura, K. Dietary whey protein modulates liver glycogen level and glycoregulatory enzyme activities in exercise-trained rats. Exp. Biol. Med. 2005, 230, 23–30. [Google Scholar] [CrossRef]
- BouNoUsº, G.; Batist, G.; Gold, P. MICE: ROLE OF GLUTATHIONE. Clin. Investig. Med. 1989, 12, 154–161. [Google Scholar]
- AnvariFar, H.; Amirkolaie, A.; Jalali, A.M.; Miandare, H.; Sayed, A.H.; Üçüncü, S.İ.; Ouraji, H.; Ceci, M.; Romano, N. Environmental pollution and toxic substances: Cellular apoptosis as a key parameter in a sensible model like fish. Aquat. Toxicol. 2018, 204, 144–159. [Google Scholar] [CrossRef]
- Kerr, J.F.; Winterford, C.M.; Harmon, B.V. Apoptosis. Its significance in cancer and cancer therapy. Cancer 1994, 73, 2013–2026. [Google Scholar] [CrossRef]
- Bratton, D.L.; Fadok, V.A.; Richter, D.A.; Kailey, J.M.; Guthrie, L.A.; Henson, P.M. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J. Biol. Chem. 1997, 272, 26159–26165. [Google Scholar] [CrossRef]
- Ekert, P.G.; Vaux, D.L. Apoptosis and the immune system. Br. Med Bull. 1997, 53, 591–603. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Hengartner, M.O. Programmed cell death: Alive and well in the new millennium. Trends Cell Biol. 2001, 11, 526–534. [Google Scholar] [CrossRef]
- Gao, Z.; Shao, Y.; Jiang, X. Essential roles of the Bcl-2 family of proteins in caspase-2-induced apoptosis. J. Biol. Chem. 2005, 280, 38271–38275. [Google Scholar] [CrossRef] [PubMed]
- Gürgen, S.; Yücel, A.; Karakuş, A.; Ceçen, D.; Özen, G.; Koçtürk, S. Usage of whey protein may cause liver damage via inflammatory and apoptotic responses. Hum. Exp. Toxicol. 2015, 34, 769–779. [Google Scholar] [CrossRef] [PubMed]
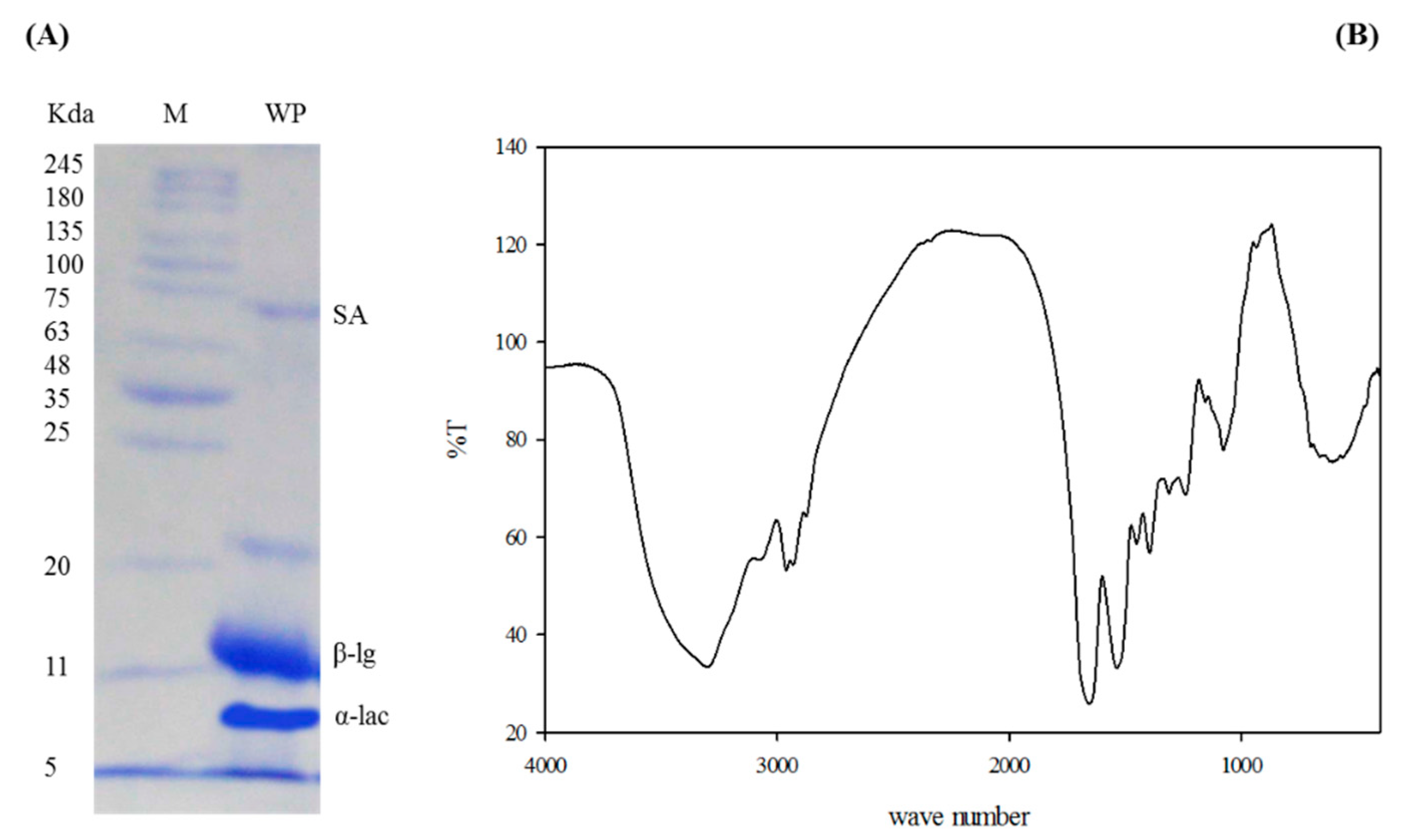


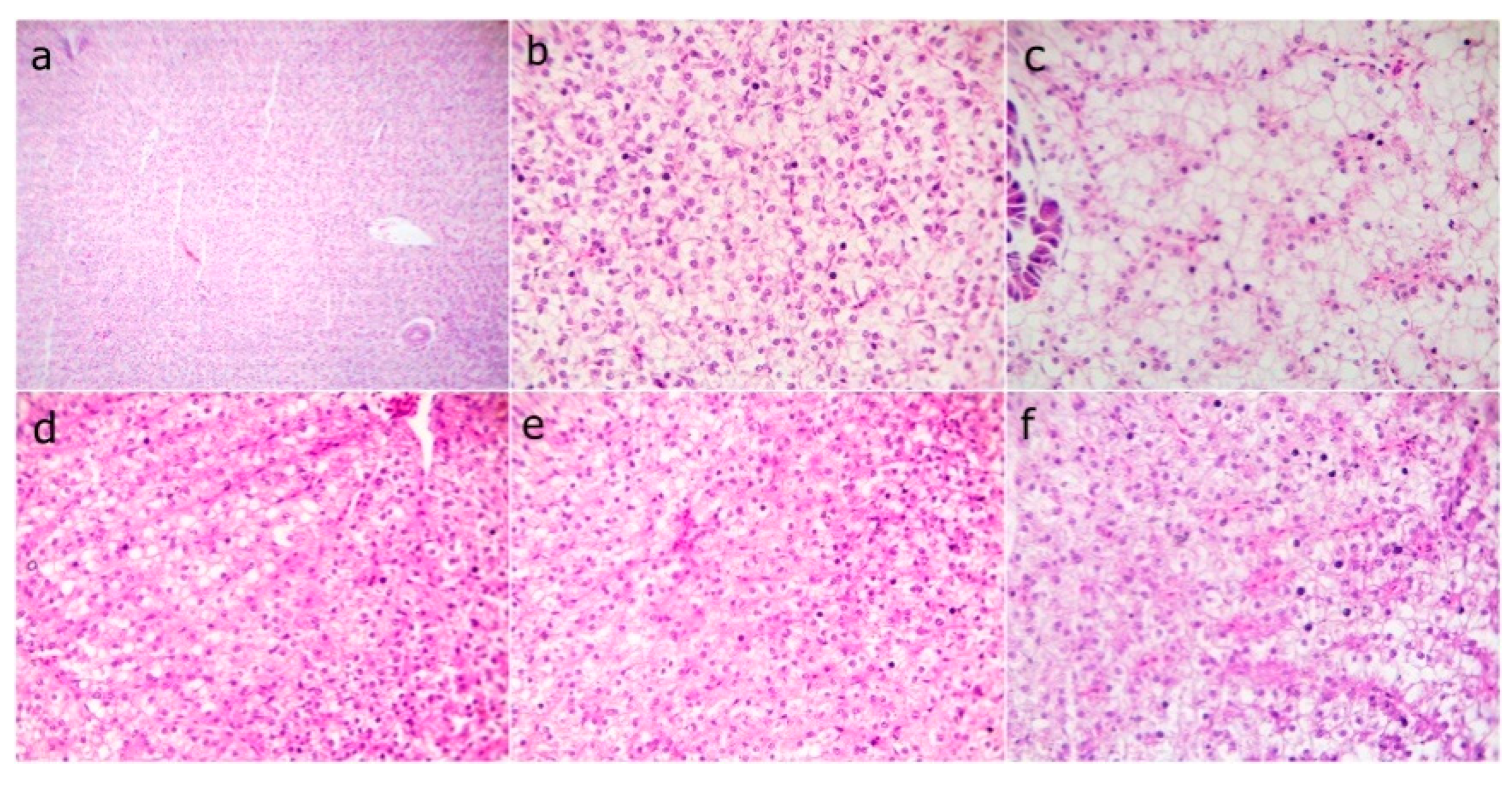
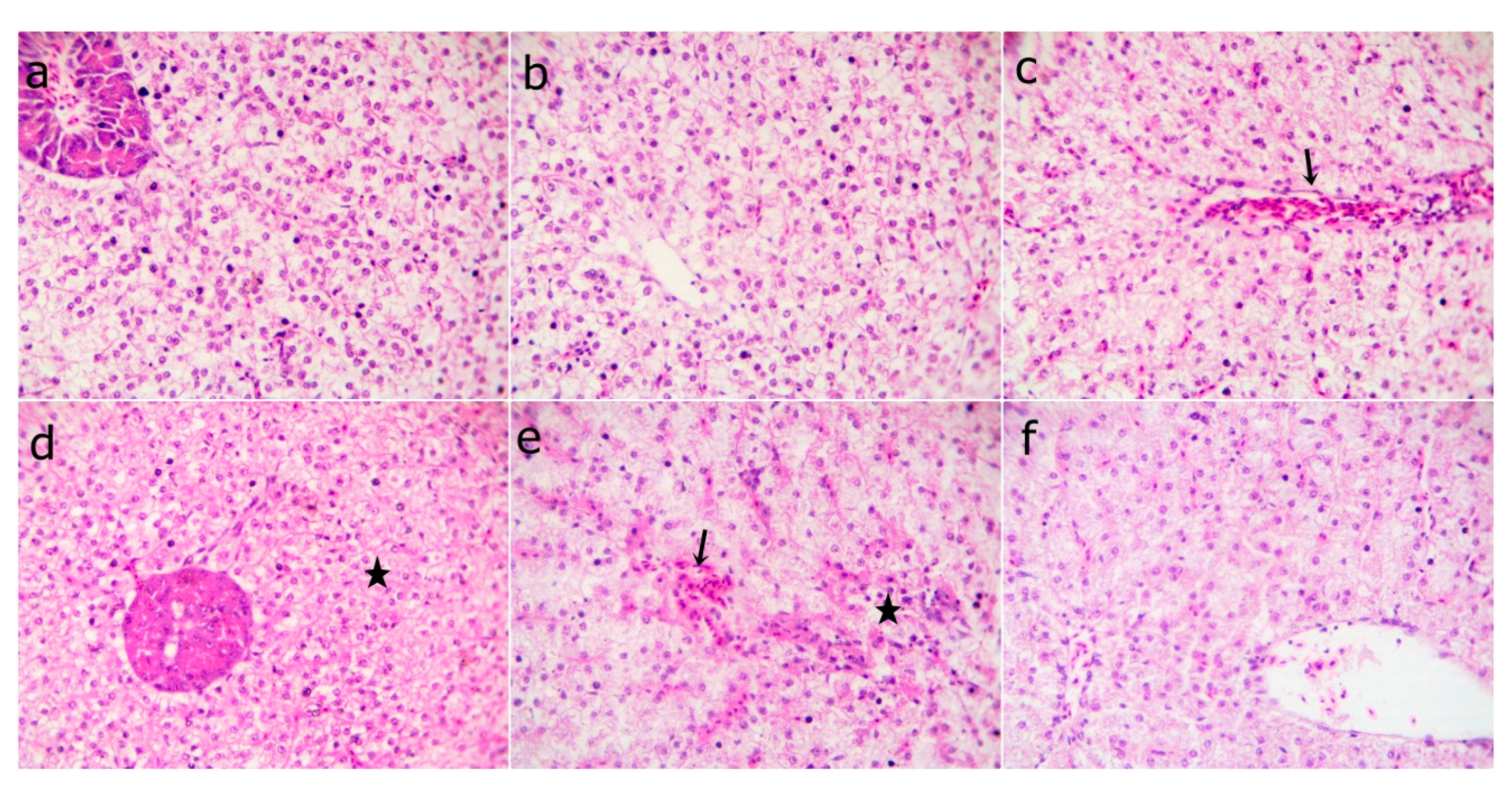

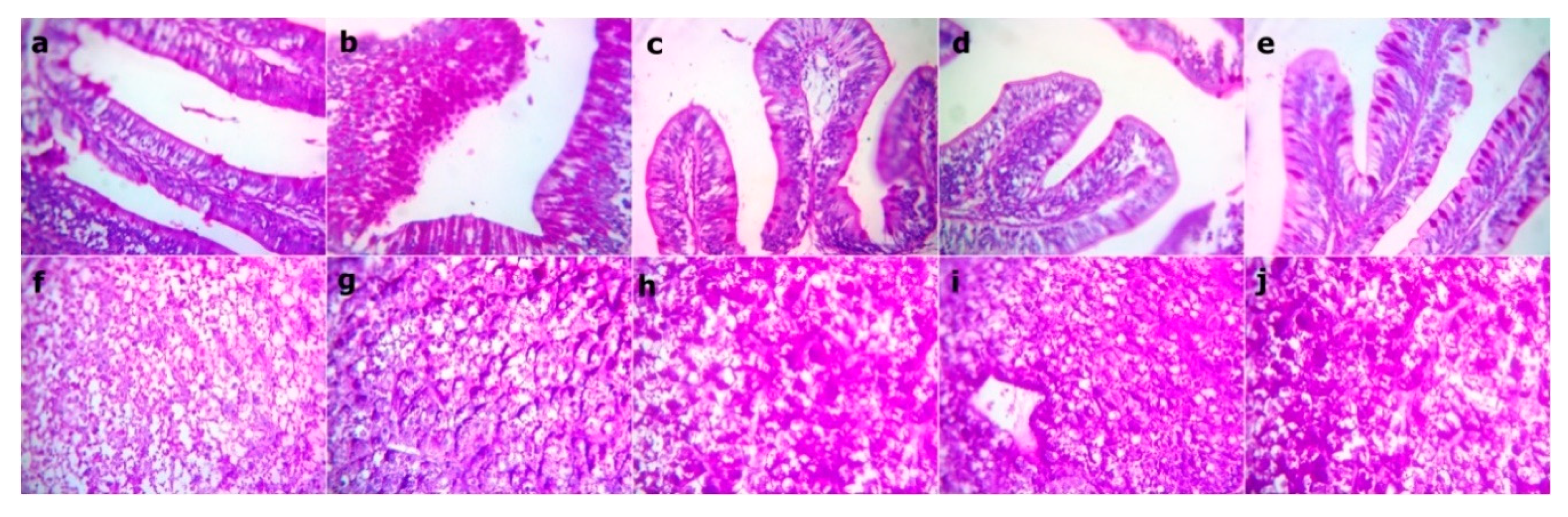

| Ingredients | WPC0 | WPC13.8 | WPC27.7 | WPC41.6 | WPC55.5 |
|---|---|---|---|---|---|
| Fish meal | 180 | 150 | 120 | 90 | 60 |
| DWPC | - | 25 | 50 | 75 | 100 |
| SBM 44% | 255 | 258 | 290 | 333 | 355 |
| Ground corn | 243 | 236 | 212 | 164.5 | 130 |
| Corn gluten 60% cp | 110 | 110 | 110 | 110 | 110 |
| Wheat bran | 90 | 100 | 90 | 90 | 100 |
| Wheat | 50 | 50 | 50 | 50 | 50 |
| Fish oil | 60 | 58 | 61 | 65 | 68 |
| Dical. ph. | 0 | 1 | 4.5 | 10 | 14 |
| Methionine | 0 | - | 0.5 | 0.5 | 1 |
| Vitamin and mineral premix # | 12 | 12 | 12 | 12 | 12 |
| Chemical composition ## | |||||
| Crude Protein | 336.2 | 335.8 | 335.6 | 336.1 | 336.5 |
| Fat | 94.6 | 90.8 | 91.4 | 92.8 | 93.5 |
| Crude Fiber | 37.4 | 38.5 | 38.9 | 40.7 | 42.5 |
| NFE * | 385.6 | 397.5 | 397.8 | 390.1 | 389.0 |
| DE kcal/kg | 2907.3 | 2905.1 | 2910.6 | 2904.1 | 2908.2 |
| Lysine | 18.3 | 17.8 | 17.9 | 18.4 | 18.3 |
| Methionine | 7.1 | 6.7 | 7.0 | 6.8 | 7.1 |
| Calcium | 10.4 | 9.2 | 8.9 | 8.7 | 8.9 |
| AP ** | 9.1 | 8.7 | 8.8 | 8.9 | 8.8 |
| Parameters | WPC0 | WPC13.8 | WPC27.7 | WPC41.6 | WPC55.5 | Regression Analysis ** | |
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Growth performance parameters | |||||||
| Initial wt. (g/fish) | 18.62 ± 0.19 | 18.43 ± 0.04 | 18.71 ± 0.04 | 18.77 ± 0.07 | 18.73 ± 0.07 | 0.11 | 0.84 |
| Final BW (g/fish) | 30.08 ± 0.89 | 30.01 ± 0.79 | 28.62 ± 0.21 | 29.69 ± 1.16 | 28.61 ± 0.48 | 0.21 | 0.88 |
| Daily BWG (g/fish) | 0.16 ± 0.01 | 0.16 ± 0.01 | 0.14 ± 0.003 | 0.15 ± 0.01 | 0.14 ± 0.007 | 0.13 | 0.89 |
| Total BWG (g/fish) | 11.46 ± 0.74 | 11.57 ± 0.76 | 9.91 ± 0.25 | 10.92 ± 1.11 | 9.87 ± 0.55 | 0.13 | 0.89 |
| Total FI (g/fish) | 30.23 ± 1.64 | 27.40 ± 1.65 | 26.23 ± 1.01 | 30.62 ± 1.02 | 29.33 ± 0.60 | 0.72 | 0.09 |
| FCR | 2.67 ± 0.28 | 2.37 ± 0.06 | 2.65 ± 0.16 | 2.86 ± 0.32 | 2.98 ± 0.16 | 0.14 | 0.37 |
| PER | 1.14 ± 0.13 | 1.25 ± 0.03 | 1.13 ± 0.07 | 1.06 ± 0.11 | 1.001 ± 0.05 | 0.01 | 0.98 |
| SGR (%/day) | 0.68 ± 0.03 | 0.69 ± 0.03 | 0.60 ± 0.01 | 0.65 ± 0.05 | 0.60 ± 0.02 | 0.09 | 0.90 |
| Survival% after bacterial challenge | 66.66 ± 7.95 | 88.88 ± 2.63 | 91.11 ± 2.22 | 95.55 ± 2.47 | 97.77 ± 2.22 | 0.00 | 0.03 |
| Total wt. of the surviving fish after bacterial challenge (g) | 299.15 ± 27.89 | 409.81 ± 9.84 | 381.72 ± 10.17 | 425.74 ± 20.19 | 419.83 ± 15.02 | 0.001 | 0.03 |
| Whole body composition (g/kg) | |||||||
| Dry Matter # | 222.6 ± 0.8 | 222.6 ± 8.7 | 229.0 ± 3.1 | 226.3 ± 3.5 | 230.1 ± 3.8 | 0.29 | 0.70 |
| Crude protein * | 652.8 ± 5.83 | 683.3 ± 21.73 | 661.0 ± 21.10 | 660.1 ± 11.3 | 683.3 ± 15.9 | 0.481 | 0.912 |
| Fat * | 156.6 ± 8.8 | 156.6 ± 3.3 | 170.0 ± 5.7 | 133.3 ± 8.8 | 136.6 ± 8.8 | 0.02 | 0.15 |
| Ash * | 113.0 ± 3.8 | 113.3 ± 8.4 | 100.06 ± 1.3 | 139.1 ± 0. 9 | 121.8 ± 2.5 | 0.01 | 0.31 |
| Parameters | WPC0 | WPC13.8 | WPC27.7 | WPC41.6 | WPC55.5 | Regression Analysis * | |
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Immunological tests | |||||||
| IgM (µg/mL) | 237.33 ±2.02 | 251.00 ±6.80 | 256.66 ±10.03 | 255.66 ±12.12 | 260.33 ±12.12 | 0.120 | 0.501 |
| Lysozymes (µg/mL) | 14.89 ±0.29 | 18.48 ± 0.32 | 21.06 ± 3.32 | 22.22 ± 1.68 | 26.03 ±2.85 | 0.003 | 0.905 |
| Complement 3 (µg/mL) | 106.66 ±2.40 | 111.66 ±1.76 | 114.33 ±2.33 | 121.00 ±3.21 | 127.33 ±3.28 | 0.00 | 0.519 |
| Phagocytic % | 35.33 ±2.40 | 38.33 ± 2.02 | 52.66 ± 0.66 | 45.66 ± 1.20 | 42.66 ±1.76 | 0.002 | 0.00 |
| Phagocytic index | 1.25 ± 0.08 | 1.53 ± 0.14 | 2.98 ± 0.10 | 2.29 ± 0.10 | 1.84 ± 0.07 | 0.00 | 0.00 |
| Growth hormone (ng/mL) | 1.59 ± 0.05 | 2.04 ± 0.14 | 2.55 ± 0.28 | 3.03 ± 0.03 | 4.44 ± 0.29 | 0.00 | 0.02 |
| NO (µmol/L) | 55.34 ±2.24 | 61.34 ± 1.62 | 60.19 ± 2.86 | 65.94 ± 3.59 | 69.44 ±6.99 | 0.02 | 0.90 |
| Liver function tests | |||||||
| ALT (U/L) | 12.03 ±0.11 | 11.03 ± 0.08 | 11.91 ± 0.02 | 12.80 ± 0.02 | 13.12 ±0.04 | 0.14 | 0.00 |
| AST (U/L) | 9.12 ± 0.04 | 7.90 ± 0.03 | 9.30 ± 0.02 | 9.73 ± 0.04 | 13.85 ±0.02 | 0.00 | 0.00 |
| Kidney function tests | |||||||
| Urea (mg/dL) | 7.14 ± 0.06 | 6.92 ± 0.018 | 5.92 ± 0.03 | 13.86 ± 1.55 | 3.38 ± 0.06 | 0.00 | 0.001 |
| Creatinine (mg/dL) | 0.53 ±0.002 | 0.52 ± 0.002 | 0.53 ± 0.002 | 0.62 ± 0.004 | 0.56 ±0.001 | 0.002 | 0.07 |
| Parameters | WPC0 | WPC13.8 | WPC27.7 | WPC41.6 | WPC55.5 | Regression Analysis * | |
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Immunological tests | |||||||
| IgM (µg/mL) | 234.00 ±6.24 | 240.00 ±4.72 | 289.66 ±1.76 | 231.00 ±5.03 | 235.00 ±4.72 | 0.65 | 0.00 |
| Lysozymes (µg/mL) | 13.11 ±1.04 | 16.53 ± 0.28 | 26.18 ± 1.55 | 12.46 ± 0.96 | 16.22 ±0.13 | 0.49 | 0.00 |
| Complement 3 (µg/mL) | 103.66 ±3.17 | 113.33 ±2.33 | 141.33 ±4.97 | 103.00 ±2.51 | 106.00 ±4.58 | 0.63 | 0.00 |
| Phagocytic % | 50.16 ±2.48 | 59.33 ± 2.96 | 70.33 ± 2.02 | 64.66 ± 2.02 | 64.00 ±3.60 | 0.003 | 0.005 |
| Phagocytic index | 3.25 ± 0.05 | 3.34 ± 0.06 | 4.20 ± 0.04 | 3.73 ± 0.08 | 3.66 ± 0.07 | 0.00 | 0.00 |
| Growth hormone (ng/mL) | 1.57 ± 0.07 | 2.08 ± 0.40 | 9.97 ± 1.37 | 2.52 ± 0.35 | 2.02 ± 0.26 | 0.53 | 0.00 |
| NO (µmol/L) | 55.14 ±2.41 | 60.09 ± 0.64 | 89.11 ± 5.83 | 64.20 ± 3.61 | 62.97 ±2.93 | 0.53 | 0.00 |
| Liver function tests | |||||||
| ALT (U/L) | 12.12 ±0.05 | 11.84 ± 0.06 | 10.10 ± 0.03 | 10.77 ± 0.03 | 10.35 ±0.03 | 0.00 | 0.00 |
| AST (U/L) | 7.52 ± 0.02 | 7.50 ± 0.01 | 7.31 ± 0.02 | 7.45 ± 0.03 | 8.01 ± 0.03 | 0.00 | 0.00 |
| Kidney function tests | |||||||
| Urea (mg/dL) | 4.20 ± 0.03 | 5.50 ± 0.01 | 9.93 ± 0.24 | 4.82 ± 0.02 | 2.49 ± 0.02 | 0.00 | 0.00 |
| Creatinine (mg/dL) | 0.75 ±0.002 | 0.64 ± 0.004 | 0.66 ± 0.002 | 0.60 ± 0.003 | 0.64 ±0.003 | 0.00 | 0.00 |
| Parameters | WPC0 | WPC13.8 | WPC27.7 | WPC41.6 | WPC55.5 | Regression Analysis * | |
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| At the experimental end | |||||||
| Villus height | 158.92 ± 12.28 | 174.47 ± 19.47 | 179.86 ± 16.11 | 237.86 ± 12.76 | 267.98 ± 37.05 | 0.001 | 0.323 |
| Villus width | 83.13 ± 5.57 | 92.01 ± 17.69 | 92.57 ± 8.68 | 69.13 ± 9.94 | 57.62 ± 9.59 | 0.047 | 0.133 |
| Crypt depth | 93.18 ± 6.41 | 68.01 ± 12.92 | 61.23 ± 6.04 | 58.15 ± 7.73 | 48.43 ± 6.12 | 0.001 | 0.277 |
| Goblet cells | 14.40 ± 1.43 | 15.20 ± 2.26 | 9.60 ± 0.67 | 16.00 ± 2.70 | 14.20 ± 2.05 | 0.94 | 0.36 |
| Lymphocytic count ** | 193.20 ± 16.746 | 205.00 ± 7.328 | 216.00 ± 12.075 | 131.60 ± 19.816 | 55.20 ± 1.960 | 0.00 | 0.00 |
| After bacterial challenge | |||||||
| Villus height | 188.97 ± 12.04 | 183.99 ± 21.56 | 165.01 ± 7.511 | 240.56 ± 10.65 | 270.50 ± 46.42 | 0.01 | 0.08 |
| Villus width | 72.21 ± 10.36 | 57.10 ± 2.96 | 129.88 ± 33.54 | 81.69 ± 16.99 | 94.60 ± 15.34 | 0.25 | 0.36 |
| Crypt depth | 56.63 ± 7.13 | 60.10 ± 10.21 | 49.40 ± 7.20 | 50.08 ± 10.48 | 37.38 ± 3.61 | 0.07 | 0.49 |
| Goblet cells | 54.00 ± 6.26 | 97.20 ± 8.35 | 7.80 ± 0.73 | 24.60 ± 1.46 | 12.40 ± 2.11 | 0.00 | 0.80 |
| Lymphocytic count ** | 269.20 ± 20.901 | 242.20 ± 19.871 | 276.20 ± 23.155 | 289.20 ± 66.560 | 102.60 ± 11.303 | 0.01 | 0.01 |
| Parameters | WPC0 | WPC13.8 | WPC27.7 | WPC41.6 | WPC55.5 | Regression Analysis * | |
|---|---|---|---|---|---|---|---|
| Linear | Quadratic | ||||||
| Net profit (LE) | 9.02 ±1.19 | 11.19 ±0.25 | 10.04 ±0.81 | 9.64 ±1.04 | 9.21 ±0.57 | 0.67 | 0.18 |
| Total return (LE) | 15.68 ± 1.13 | 18.01 ± 0.47 | 16.42 ± 0.86 | 16.66 ± 1.17 | 16.01 ± 0.66 | 0.81 | 0.25 |
| Feed cost (LE) | 4.66 ± 0.28 | 4.81 ± 0.11 | 4.37 ± 0.15 | 5.01 ± 0.09 | 4.80 ± 0.12 | 0.36 | 0.58 |
| Total cost (LE) | 6.66 ± 0.06 | 6.81 ± 0.28 | 6.37 ± 0.11 | 7.01 ± 0.15 | 6.80 ± 0.09 | 0.36 | 0.58 |
| Feed cost (LE)/kg gain | 45.73 ± 9.77 | 27.74 ± 0.73 | 36.09 ± 6.91 | 41.52 ± 10.03 | 41.97 ± 6.13 | 0.79 | 0.25 |
| EE # | 1.94 ± 0.28 | 2.34 ± 0.11 | 2.29 ± 0.17 | 1.91 ± 0.16 | 1.91 ± 0.08 | 0.41 | 0.12 |
| PI % ## | 11.77 ± 3.72 | 19.02 ± 0.87 | 14.41 ± 2.96 | 13.37 ± 3.33 | 11.73 ± 1.89 | 0.52 | 0.19 |
| Total return after bacterial infection (LE) ** | 10.34 ± 0.91 | 16.01 ± 0.69 | 15.00 ± 1.13 | 15.95 ± 1.36 | 15.65 ± 0.53 | 0.005 | 0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amer, S.A.; Osman, A.; Al-Gabri, N.A.; Elsayed, S.A.M.; Abd El-Rahman, G.I.; Elabbasy, M.T.; Ahmed, S.A.A.; Ibrahim, R.E. The Effect of Dietary Replacement of Fish Meal with Whey Protein Concentrate on the Growth Performance, Fish Health, and Immune Status of Nile Tilapia Fingerlings, Oreochromis niloticus. Animals 2019, 9, 1003. https://doi.org/10.3390/ani9121003
Amer SA, Osman A, Al-Gabri NA, Elsayed SAM, Abd El-Rahman GI, Elabbasy MT, Ahmed SAA, Ibrahim RE. The Effect of Dietary Replacement of Fish Meal with Whey Protein Concentrate on the Growth Performance, Fish Health, and Immune Status of Nile Tilapia Fingerlings, Oreochromis niloticus. Animals. 2019; 9(12):1003. https://doi.org/10.3390/ani9121003
Chicago/Turabian StyleAmer, Shimaa A., Ali Osman, Naif A. Al-Gabri, Shafika A. M. Elsayed, Ghada I. Abd El-Rahman, Mohamed Tharwat Elabbasy, Shaimaa A. A. Ahmed, and Rowida E. Ibrahim. 2019. "The Effect of Dietary Replacement of Fish Meal with Whey Protein Concentrate on the Growth Performance, Fish Health, and Immune Status of Nile Tilapia Fingerlings, Oreochromis niloticus" Animals 9, no. 12: 1003. https://doi.org/10.3390/ani9121003
APA StyleAmer, S. A., Osman, A., Al-Gabri, N. A., Elsayed, S. A. M., Abd El-Rahman, G. I., Elabbasy, M. T., Ahmed, S. A. A., & Ibrahim, R. E. (2019). The Effect of Dietary Replacement of Fish Meal with Whey Protein Concentrate on the Growth Performance, Fish Health, and Immune Status of Nile Tilapia Fingerlings, Oreochromis niloticus. Animals, 9(12), 1003. https://doi.org/10.3390/ani9121003







