Quality of Adherence to the ARRIVE Guidelines in the Material and Methods Section in Studies Where Swine Were Used as Surgical Biomodels: A Systematic Review (2013–2018)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
- Original articles published in English
- Use of swine models in vivo
- Studies that included surgery as an experimental procedure
- Studies published between 2013 and 2018. This period was selected to sample the recent biomedical literature (for the last 5 years), considering a suitable period for the ARRIVE guidelines to be implemented
- Studies that described painful experimental surgical procedures like skin incision, craniotomies, thoracotomies, laparotomies, laparoscopies, dental surgeries, and orthopedic surgeries
2.3. Evaluation of Publication Quality
2.4. Statistics
3. Results
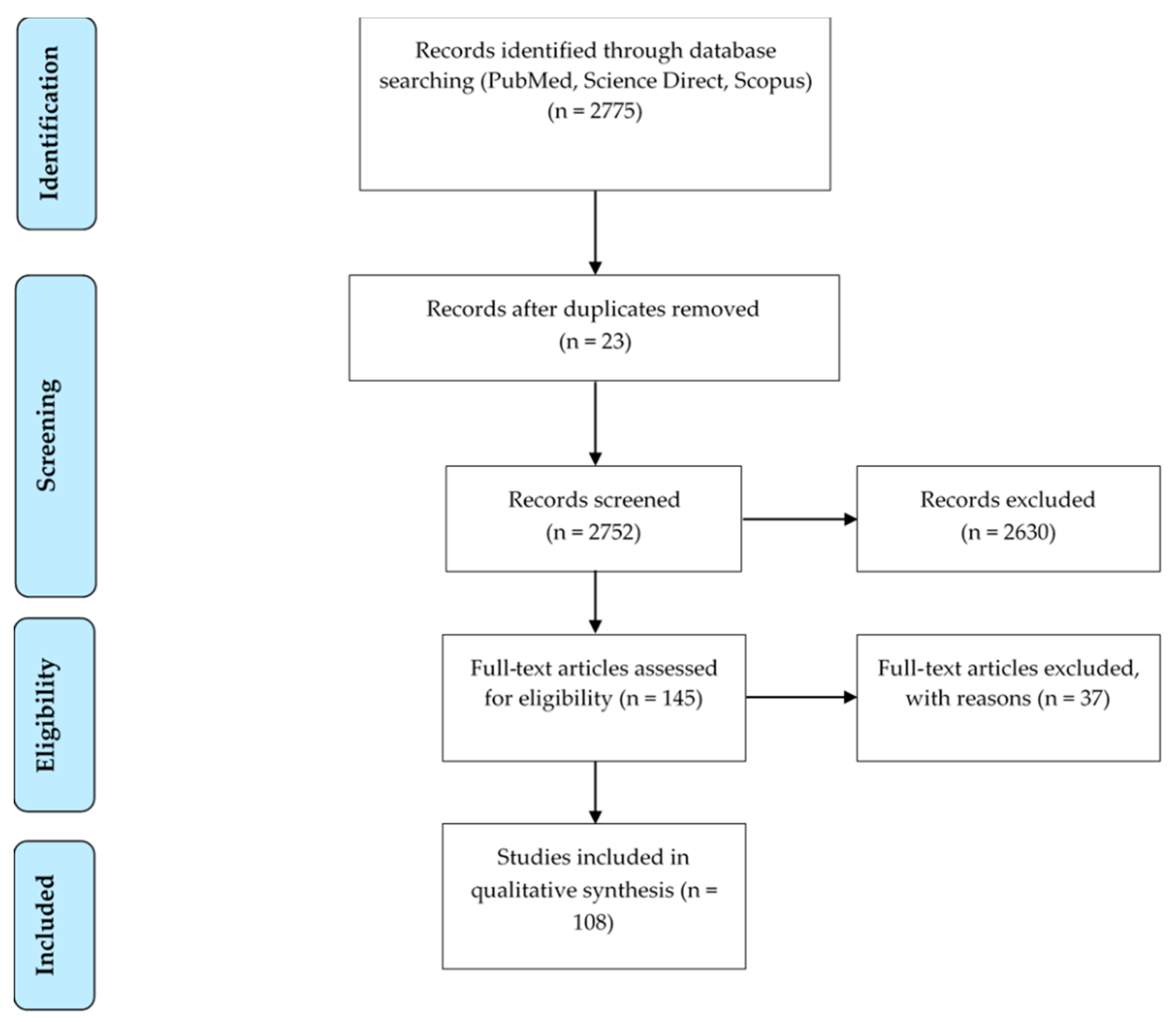
3.1. Study Selection
3.2. Information Analyzed in Each Article
3.3. Surgery and Anesthesia
3.4. Anesthesia
3.5. Analgesia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Swindle, M.M.; Makin, A.; Herron, A.J.; Clubb, F.J.; Frazier, K.S. Swine as Models in Biomedical Research and Toxicology Testing. Vet. Pathol. 2012, 49, 344–356. [Google Scholar] [CrossRef] [PubMed]
- Bradbury, A.G.; Eddleston, M.; Clutton, R.E. Pain management in pigs undergoing experimental surgery; a literature review (2012–4). Br. J. Anaesth. 2017, 116, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Ting, K.H.J.; Hill, C.L.; Whittle, S.L. Quality of reporting of interventional animal studies in rheumatology: A systematic review using the ARRIVE guidelines. Int. J. Rheum. Dis. 2015, 18, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012, 20, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Blomme, E.A.G. The ARRIVE guidelines: A resource for authors and reviewers to ensure that submissions to The Veterinary Journal meet minimal expectations of completeness, accuracy and transparency. Vet. J. 2011, 189, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Gulin, J.E.N.; Rocco, D.M.; García-Bournissen, F. Quality of Reporting and Adherence to ARRIVE Guidelines in Animal Studies for Chagas Disease Preclinical Drug Research: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9, e0004194. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, F.; Iglhaut, G.; Becker, J. Quality assessment of reporting of animal studies on pathogenesis and treatment of peri-implant mucositis and peri-implantitis. A systematic review using the ARRIVE guidelines. J. Clin. Periodontol. 2012, 39, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 6, e1000097. [Google Scholar]
- Baker, D.; Lidster, K.; Sottomayor, A.; Amor, S. Two Years Later: Journals Are Not Yet Enforcing the ARRIVE Guidelines on Reporting Standards for Pre-Clinical Animal Studies. PLoS Biol. 2014, 12, e1001756. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, B.G.; Koustova, E.; Wang, Y. Getting personal with the “reproducibility crisis”: Interviews in the animal research community. Lab. Anim. 2018, 47, 175–177. [Google Scholar] [CrossRef] [PubMed]
- Council, N.R. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Andrews, N.A.; Latrémolière, A.; Basbaum, A.I.; Mogil, J.S.; Porreca, F.; Rice, A.S.C.; Woolf, C.J.; Currie, G.L.; Dworkin, R.H.; Eisenach, J.C.; et al. Ensuring transparency and minimization of methodologic bias in preclinical pain research. Pain 2016, 157, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Gumz, M.L. Taking into account circadian rhythm when conducting experiments on animals. Am. J. Physiol. Physiol. 2016, 310, F454–F455. [Google Scholar] [CrossRef] [PubMed]
- Bardal, S.; Waechter, J.; Martin, D. Applied Pharmacology; Elsevier Saunders: St. Louis, MO, USA, 2011; ISBN 9781437703108. [Google Scholar]
- Ramamoorthi, M.; Bakkar, M.; Jordan, J.; Tran, S.D. Osteogenic Potential of Dental Mesenchymal Stem Cells in Preclinical Studies: A Systematic Review Using Modified ARRIVE and CONSORT Guidelines. Stem Cells Int. 2015, 2015, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Kohn, D.F.; Martin, T.E.; Foley, P.L.; Morris, T.H.; Swindle, M.M.; Vogler, G.A.; Wixson, S.K. Guidelines for the assessment and management of pain in rodents and rabbits. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 97–108. [Google Scholar] [PubMed]
- Richardson, C.A.; Flecknell, P.A. Anaesthesia and Post-operative Analgesia following Experimental Surgery in Laboratory Rodents: Are we Making Progress? Altern. to Lab. Anim. 2005, 33, 119–127. [Google Scholar] [CrossRef]
- Molina, A.; Moyano, M.; Serrano-Rodriguez, J.; Ayala, N.; Lora, A.; Serrano-Caballero, J. Analyses of anaesthesia with ketamine combined with different sedatives in rats. Vet. Med. 2016, 60, 368–375. [Google Scholar] [CrossRef]
- Asres, A.; Amha, N. Effect of Stress on Animal Health: A Review. J. Biol. Agric. Healthc. 2014, 4, 116–122. [Google Scholar]
- Coulter, C.A.; Flecknell, P.A.; Leach, M.C.; Richardson, C.A. Reported analgesic administration to rabbits undergoing experimental surgical procedures. Vet. Res. 2017, 7, 1–6. [Google Scholar] [CrossRef]
- Hermansen, K.; Pedersen, L.E.; Olesen, H.O. The Analgesic Effect of Buprenorphine, Etorphine and Pethidine in the Pig: A Randomized Double Blind Cross-over Study. Acta Pharmacol. Toxicol. 1986, 59, 27–35. [Google Scholar] [CrossRef]
| Category | 0 | 1 | 2 |
|---|---|---|---|
| 1. Ethical Statement | 6% | 80% | 14% |
| 2. Study Design | 21% | 53% | 26% |
| 3. Experimental procedures | 0% | 100% | 0% |
| 4. Experimental animals | 3% | 90% | 7% |
| 5. Housing and Husbandry | 43% | 57% | 0% |
| 6. Sample size | 7% | 90% | 3% |
| 7. Allocating animals to experimental groups | 19% | 74% | 7% |
| 8. Experimental outcomes | 95% | N/A | 5% |
| 9. Statistical methods | 37% | 49% | 14% |
| Category | Subcategory | Items | Mean (%) | Max. (%, Year) | Min. (%, Year) |
|---|---|---|---|---|---|
| 1. Ethical Statement | 1.1. Ethical review permissions | 1.1.1. Refers to guidelines | 52 | 69 (2014) | 35 (2017) |
| 1.1.2. Approved by ethical committee | 92 | 100 (2016) | 81 (2014) | ||
| 1.1.3. Protocol number | 20 | 57(2018) | 17 (2013) | ||
| 2. Study Design | 2.1. Number of experimental and control groups | 71 | 88 (2014) | 57 (2018) | |
| 2.2. Steps taken to minimize the effects of subjective bias | 27 | 40 (2016) | 11 (2013) | ||
| 2.3. The experimental unit | 79 | 94 (2014) | 57 (2018) | ||
| 3. Experimental procedures | 3.1. How | 3.1.1. Anesthesia drugs | 81 | 94 (2015) | 71 (2017) |
| 3.1.2. Dose of anesthesia | 71 | 83 (2015) | 57 (2018) | ||
| 3.1.3. Route | 68 | 78 (2015) | 60 (2016) | ||
| 3.1.4. Monitoring during anesthesia | 30 | 48 (2016) | 21 (2018) | ||
| 3.1.5. Analgesia drugs | 41 | 56 (2014) | 28 (2016) | ||
| 3.1.6. Dose of analgesia | 32 | 39 (2015) | 21 (2018) | ||
| 3.1.7. Route | 24 | 33 (2015) | 7 (2018) | ||
| 3.1.8. Surgical procedure | 100 | 100 (All) | 0 (None) | ||
| 3.1.9. Method of euthanasia | 32 | 43 (2018) | 22 (2013) | ||
| 3.2. When | 3.2.1. Time of the day | 0 | 0 (None) | 0 (All) | |
| 3.3. Where | 3.3.1. Home cage | 13 | 22 (2015) | 6 (2014, 2017) | |
| 3.4. Why | 3.4.1. Choice of a specific anesthetic, route and dose | 1 | 6 (2014) | 0 (All except 2014) | |
| 4. Experimental animals | 4.1. Details of animals | 4.1.1. Breed | 69 | 83 (2015) | 59 (2017) |
| 4.1.2. Sex | 58 | 88 (2014) | 40 (2016) | ||
| 4.1.3. Age/Weight | 86 | 100 (2014) | 78 (2013) | ||
| 4.2. Further information | 4.2. Source of animals | 48 | 64 (2018) | 22 (2013) | |
| 5. Housing and Husbandry | 5.1. Housing | 5.1.1. Type of facility | 6 | 14 (2018) | 0 (2015, 2016) |
| 5.1.2. Type of cage | 19 | 33 (2015) | 6 (2017) | ||
| 5.1.3. Bedding material | 3 | 11 (2015) | 0 (2013, 2014, 2016, 2017) | ||
| 5.1.4. Number of cage companions | 13 | 31 (2014) | 0 (2018) | ||
| 5.2. Husbandry conditions | 5.2.1. Light/Dark cycle | 9 | 19 (2014) | 4 (2016) | |
| 5.2.2. Temperature | 10 | 19 (2014) | 4 (2016) | ||
| 5.2.3. Humidity | 6 | 13 (2014) | 0 (2017) | ||
| 5.2.4. Type of food | 36 | 63 (2014) | 22 (2013) | ||
| 5.2.5. Access to water or food | 31 | 56 (2014) | 22 (2013) | ||
| 5.2.6. Environmental enrichment | 3 | 11 (2015) | 0 (2013, 2017, 2018) | ||
| 5.2.7. Adaptation | 12 | 18 (2017) | 4 (2016) | ||
| 5.3. Welfare related assessment | 5.3.1. Welfare intervention | 24 | 44 (2017) | 12 (2015) | |
| 6. Sample size | 6.1. Total number of animals used | 94 | 100 (all except 2017) | 76 (2017) | |
| 6.2. Explanation how the number of animals was arrived at | 3 | 6 (2015, 2016) | 0 (2013, 2014, 2018) | ||
| 6.3. Indicate the number of independent replications of each experiment | 11 | 18 (2016) | 0 (2013, 2014) | ||
| 7. Allocating animals to experimental groups | 7.1. Details of how animals where allocated | 24 | 38 (2014) | 6 (2013) | |
| 7.2. Order of experimental treatment | 48 | 68 (2016) | 18 (2017) | ||
| 8. Experimental outcomes | 8.1. Primary and secondary experimental outcomes assessed | 8 | 13 (2014) | 0 (2018) | |
| 9. Statistical methods | 9.1. Details of statistical methods | 76 | 88 (2014) | 65 (2017) | |
| 9.2. Unit of analysis for each dataset | 59 | 72 (2015) | 41 (2016) | ||
| 9.3. Methods used to assess whether the data met assumptions of the statistical approach | 49 | 56 (2015) | 41 (2016) |
| Type of Surgery | n of Journal | % of Journals |
|---|---|---|
| Craniotomies | 3 | 2.8% |
| Dental surgeries | 15 | 13.9% |
| Laparoscopies | 28 | 25.9% |
| Laparoscopy + Thoracotomy | 1 | 0.9% |
| Laparotomies | 12 | 11.1% |
| Orthopedic surgeries | 22 | 20.4% |
| Skin incisions | 11 | 10.2% |
| Thoracotomies | 16 | 14.8% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alemán-Laporte, J.; Alvarado, G.; SA Garcia-Gomes, M.; Fonseca Brasil Antiorio, A.T.; Zúñiga-Montero, M.; Cabrera Mori, C.M. Quality of Adherence to the ARRIVE Guidelines in the Material and Methods Section in Studies Where Swine Were Used as Surgical Biomodels: A Systematic Review (2013–2018). Animals 2019, 9, 947. https://doi.org/10.3390/ani9110947
Alemán-Laporte J, Alvarado G, SA Garcia-Gomes M, Fonseca Brasil Antiorio AT, Zúñiga-Montero M, Cabrera Mori CM. Quality of Adherence to the ARRIVE Guidelines in the Material and Methods Section in Studies Where Swine Were Used as Surgical Biomodels: A Systematic Review (2013–2018). Animals. 2019; 9(11):947. https://doi.org/10.3390/ani9110947
Chicago/Turabian StyleAlemán-Laporte, Jilma, Gilbert Alvarado, Mariana SA Garcia-Gomes, Ana Tada Fonseca Brasil Antiorio, Marco Zúñiga-Montero, and Claudia Madalena Cabrera Mori. 2019. "Quality of Adherence to the ARRIVE Guidelines in the Material and Methods Section in Studies Where Swine Were Used as Surgical Biomodels: A Systematic Review (2013–2018)" Animals 9, no. 11: 947. https://doi.org/10.3390/ani9110947
APA StyleAlemán-Laporte, J., Alvarado, G., SA Garcia-Gomes, M., Fonseca Brasil Antiorio, A. T., Zúñiga-Montero, M., & Cabrera Mori, C. M. (2019). Quality of Adherence to the ARRIVE Guidelines in the Material and Methods Section in Studies Where Swine Were Used as Surgical Biomodels: A Systematic Review (2013–2018). Animals, 9(11), 947. https://doi.org/10.3390/ani9110947





