Effect of Fermented Cottonseed Meal on the Lipid-Related Indices and Serum Metabolic Profiles in Broiler Chickens
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Substrate Preparation and Fermentation
2.2. Animals and Experimental Design
2.3. Sampling, Data Collection, and Chemical Analyses
2.3.1. Chemical Composition, Fatty Acid and Amino Acid Analyze of FCSM and Soybean Meal
2.3.2. Growth Performance
2.3.3. Blood Samples
2.3.4. Carcass Trait
2.3.5. Histopathological Analysis
2.3.6. Tissue Samples
2.3.7. qRT-PCR Analysis
2.3.8. LC-MS/MS
2.3.9. Correlations among Fat Deposition, Lipid-Related Indices, and Serum Metabolites
2.4. Statistical Analysis
3. Results
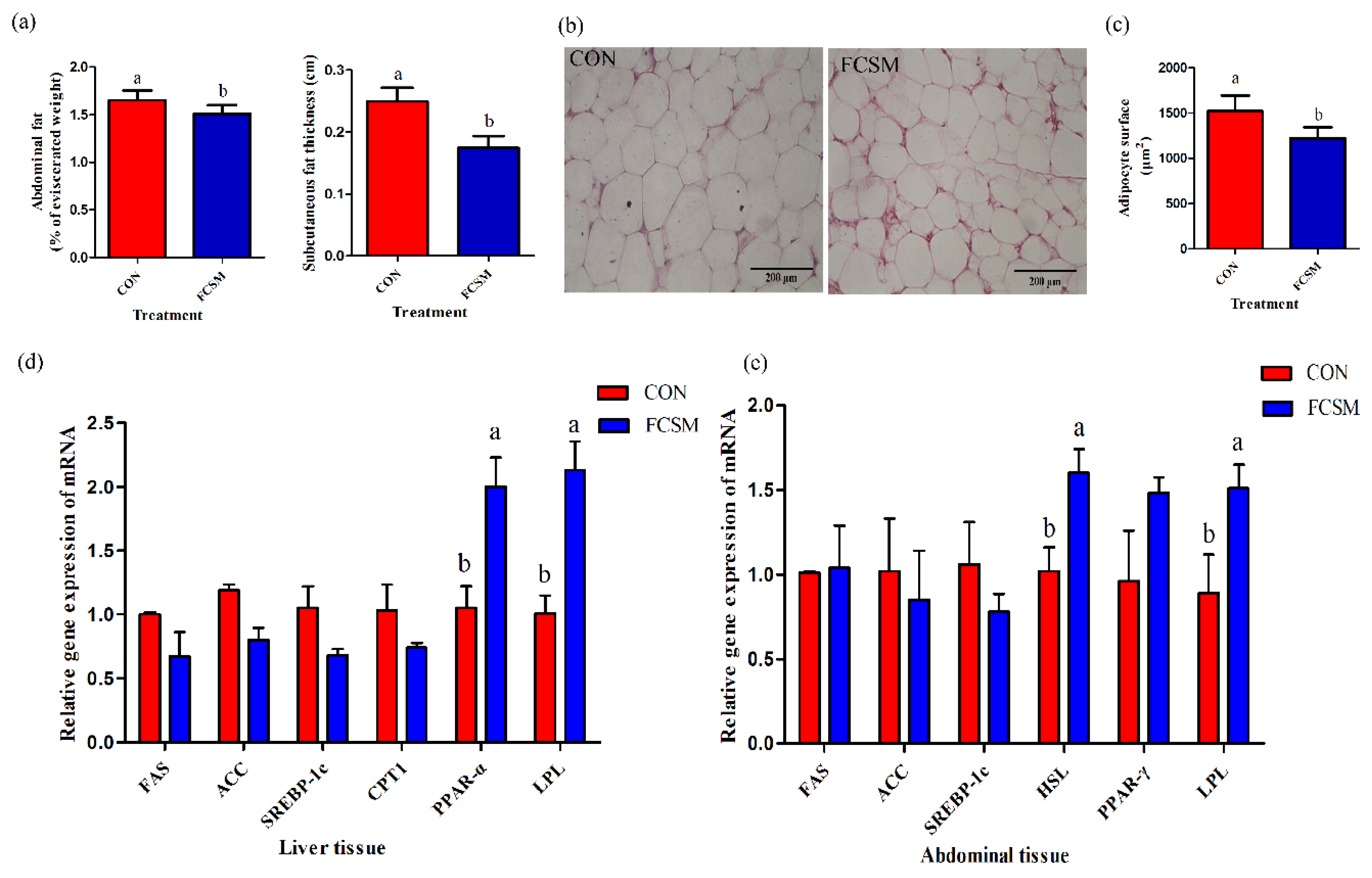
3.1. Growth Performance and Fat Deposition
3.2. Lipid-Related Metabolites’ Indices in Serum, Liver, and Abdominal Fat
3.3. Lipid-Related Metabolites’ Enzyme Activity and Hormone Level in Serum, Liver, and Abdominal Fat
3.4. Expression of mRNA in the Liver and Abdominal Fat Tissues
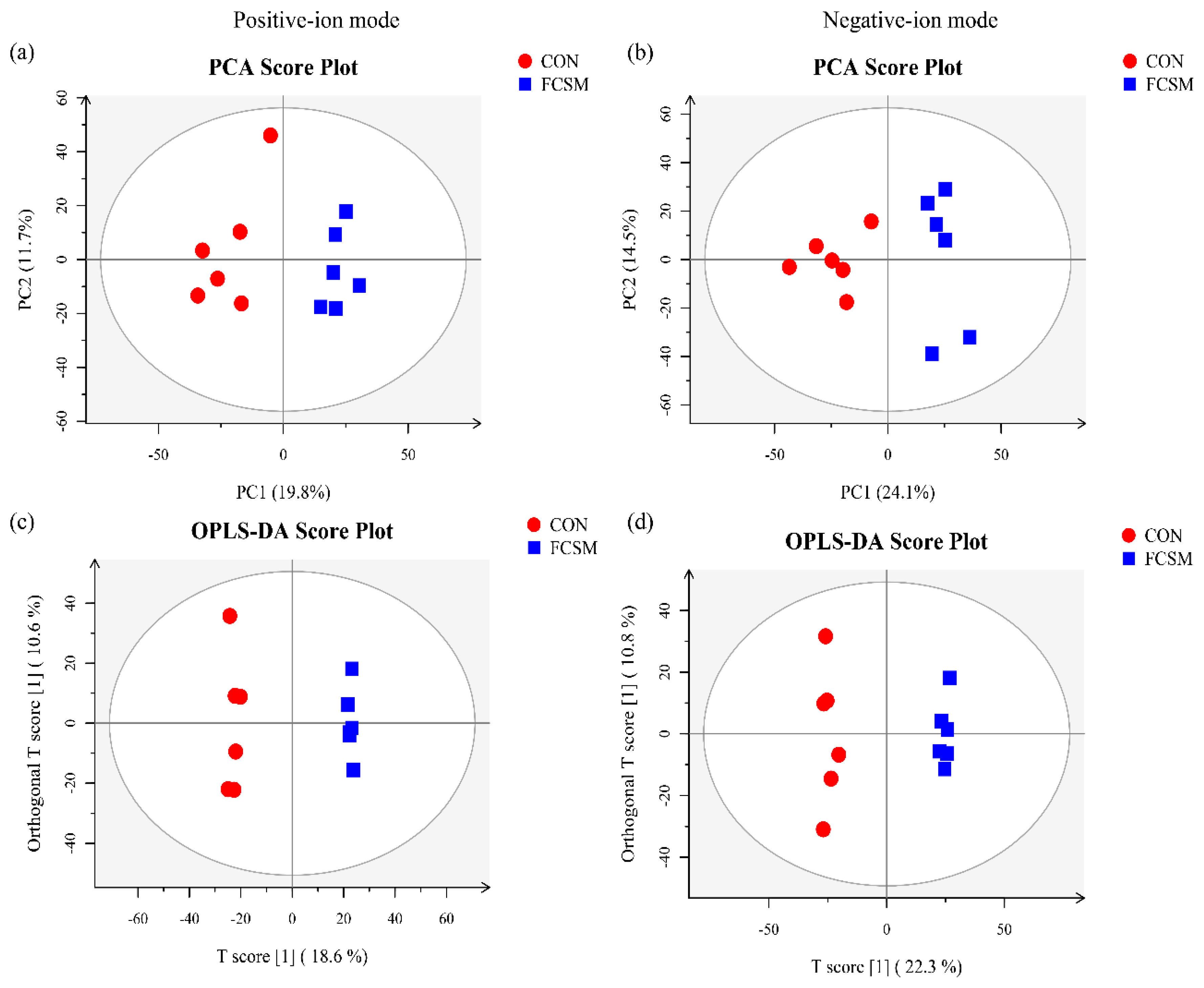
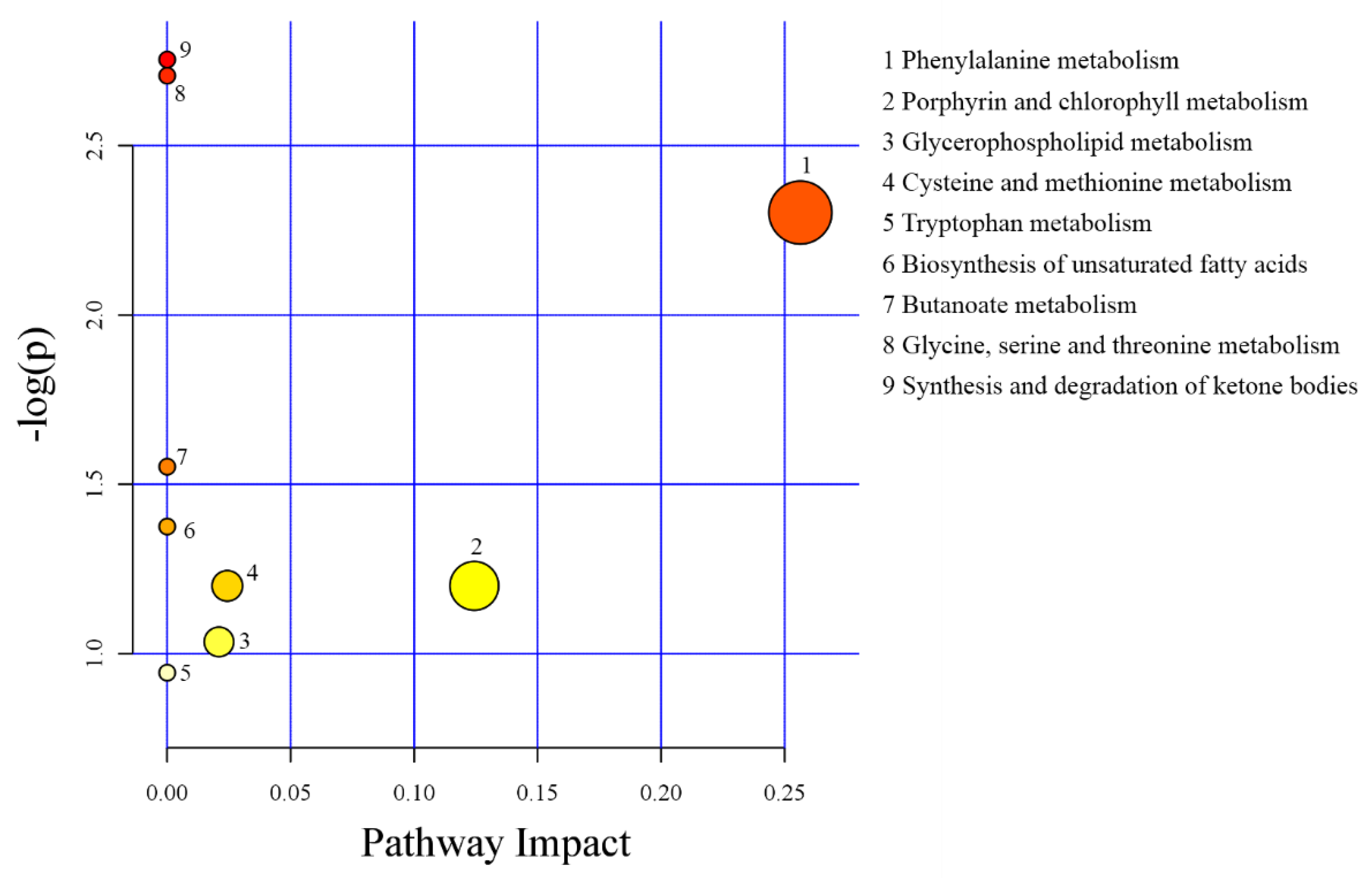
3.5. Metabolomics Profiling in Serum
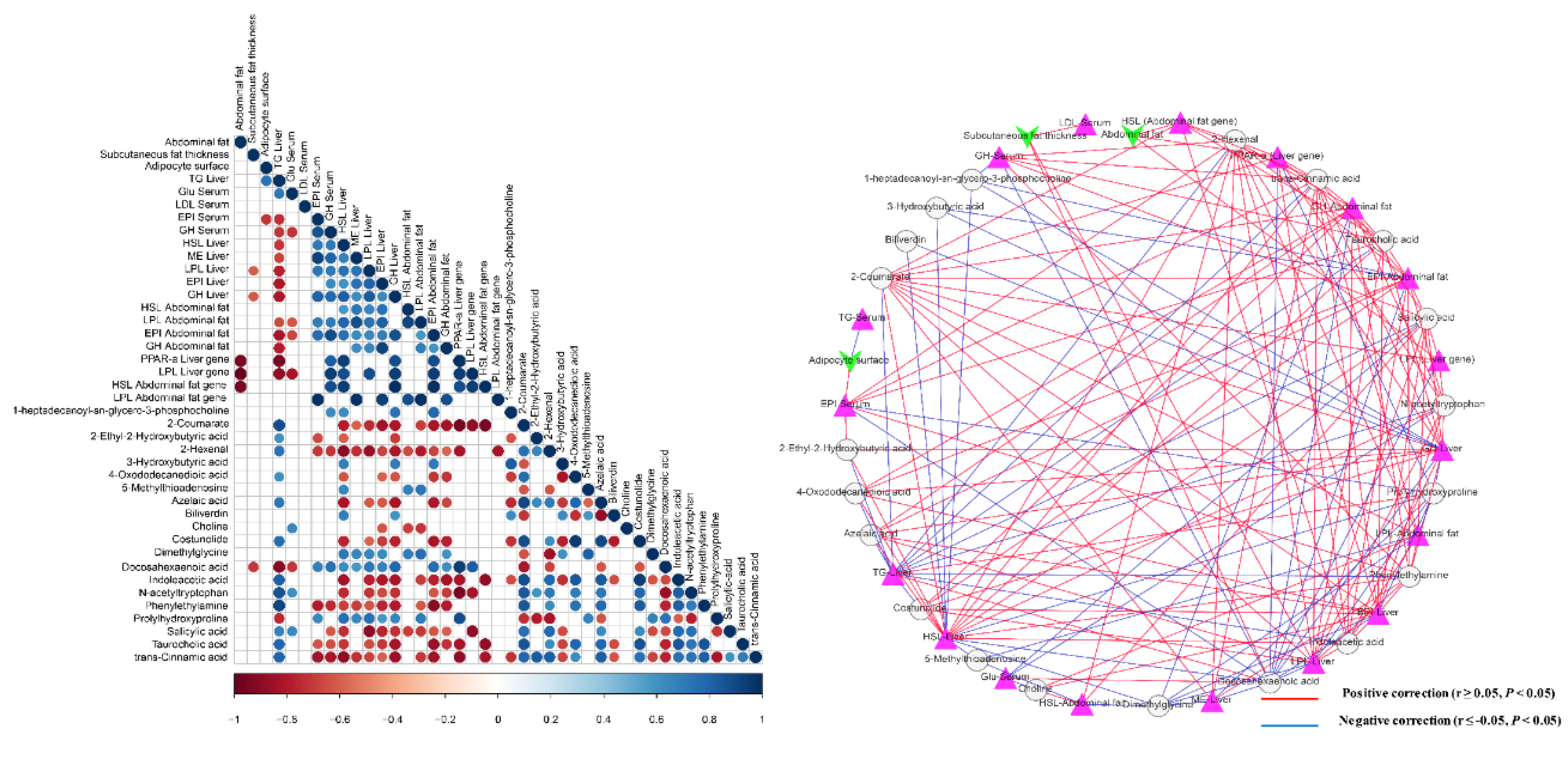
3.6. Interactions of Serum Metabolites and Lipid-Related Indices
4. Discussion
4.1. Growth Performance and Fat Deposition
4.2. Lipid-Related Metabolites’ Indices
4.3. Lipid-Related mRNA Expression
4.4. Metabolomics Profiling in Serum
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gaíva, M.H.G.; Couto, R.C.; Oyama, L.M.; Couto, G.E.C.; Silveria, V.L.F.; Roberio, E.B.; Nascimento, C.M. Polyunsaturated fatty acid-rich diets: effect on adipose tissue metabolism in rats. Br. J. Nutr. 2001, 86, 371–377. [Google Scholar] [CrossRef]
- Stanislawski, M.A.; Dabelea, D.; Lange, L.A.; Wagner, B.D.; Lozupone, C.A. Gut microbiota phenotypes of obesity. NPJ Biofilms Microb. 2019, 5, 18. [Google Scholar] [CrossRef]
- Pandit, R.J.; Hinsu, A.T.; Patel, N.V.; Koringa, P.G.; Jakhesara, S.J.; Thakkar, J.R.; Shah, T.M.; Limon, G.; Psifidi, A.; Guitian, J.; et al. Microbial diversity and community composition of caecal microbiota in commercial and indigenous Indian chickens determined using 16s rDNA amplicon sequencing. Microbiome 2018, 6, 115. [Google Scholar] [CrossRef]
- Latshaw, J.D.; Bishop, B.L. Estimating body weight and body composition of chickens by using noninvasive measurements. Poult. Sci. 2001, 80, 868–873. [Google Scholar] [CrossRef]
- Havenstein, G.B.; Ferketm, P.R.; Qureshi, A. Growth, livability, and feed conversion of 1957 versus 2001 broilers when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003, 82, 1500–1508. [Google Scholar] [CrossRef]
- Homma, H.; Shinohara, T. Effects of probiotic Bacillus cereus toyoi on abdominal fat accumulation in the Japanese quail (Coturnix japonica). Anim. Sci. J. 2015, 75, 37–41. [Google Scholar] [CrossRef]
- Nie, C.X.; Zhang, W.J.; Ge, W.X.; Liu, Y.F.; Wang, Y.Q.; Liu, J.C. Effect of cottonseed meal fermented with yeast on the lipid-related gene expression in broiler chickens. Braz. J. Poult. Sci. 2015, 17, 57–64. [Google Scholar] [CrossRef]
- Nikolova, N.; Pavlovski, Z.; Milosevic, N.; Peric, L. The quantity of abdominal fat in broiler chicken of different genotypes from fifth to seventh week of age. Biotechnol. Anim. Husb. 2007, 23, 331–338. [Google Scholar] [CrossRef]
- Jazi, V.; Boldaji, F.; Dastar, B.; Hashemi, S.R.; Ashayerizadeh, A. Effects of fermented cottonseed meal on the growth performance, gastrointestinal microflora population and small intestinal morphology in broiler chickens. Br. Poult. Sci. 2017, 58, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.X.; Zhang, W.J.; Wang, Y.Q.; Liu, Y.F.; Ge, W.X.; Liu, J.C. Tissue lipid metabolism and hepatic metabolomic profiling in response to supplementation of fermented cottonseed meal in the diets of broiler chickens. J. Zhejiang Univ. Sci. B 2015, 16, 447–455. [Google Scholar] [CrossRef]
- Sun, H.; Tand, J.W.; Fang, C.L.; Yao, X.H.; Wu, Y.F.; Wang, X.; Feng, J. Molecular analysis of intestinal bacterial microbiota of broiler chickens fed diets containing fermented cottonseed meal. Poult. Sci. 2013, 92, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Xu, Z.R.; Sun, J.Y.; Yang, X. Effect of selected fungi on the reduction of gossypol levels and nutritional value during solid substrate fermentation of cottonseed meal. J. Zhejiang Univ. Sci. B 2006, 7, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Xu, Z.R.; Zhao, S.H.; Sun, J.Y.; Yang, X. Development of a microbial fermentation process for detoxification of gossypol in cottonseed meal. Anim. Feed Sci. Tech. 2007, 135, 176–186. [Google Scholar] [CrossRef]
- Sun, H.; Yao, X.H.; Wang, X.; Wu, Y.F.; Liu, Y.; Tang, J.W.; Feng, J. Chemical composition and in vitro antioxidant property of peptides produced from cottonseed meal by solid-state fermentation. CyTA J. Food 2014, 13, 264–272. [Google Scholar] [CrossRef]
- Kalavathy, R.; Abdullah, N.; Jalaludin, S.; Ho, Y.W. Effects of Lactobacillus cultures on growth performance, abdominal fat deposition, serum lipids and weight of organs of broiler chickens. Br. Poult. Sci. 2003, 44, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Aluwong, T.; Hassan, F.; Dzenda, T.; Kawu, M.; Ayo, J. Effect of different levels of supplemental yeast on body weight, thyroid hormone metabolism and lipid profile of broiler chickens. J. Vet. Med. Sci. 2013, 75, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.X.; Zhang, W.J.; Ge, W.X.; Wang, Y.Q.; Liu, Y.F.; Liu, J.C. Effects of fermented cottonseed meal on the growth performance, apparent digestibility, carcass traits, and meat composition in yellow-feathered broilers. Turk. J. Vet. Anim. Sci. 2015, 39, 350–356. [Google Scholar] [CrossRef]
- Nie, C.X.; Zhang, W.J.; Yan, L.D.; Jiang, L.X.; Ma, G.J. A study of metabolites of protein feed fermented with cottonseed meal mixed substrates. Chin. J. Anim. Nutr. 2012, 24, 1602–1609. [Google Scholar]
- Zhang, J.; Shi, H.T.; Wang, Y.J.; Li, S.L.; Cao, Z.J.; Ji, S.; He, Y.; Zhang, H.T. Effect of Dietary Forage to Concentrate Ratios on Dynamic Profile Changes and Interactions of Ruminal Microbiota and Metabolites in Holstein Heifer. Front. Microbiol. 2017, 8, 2206. [Google Scholar] [CrossRef]
- Hea, Q.; Ren, P.; Kong, X.; Wuc, Y.; Wua, G. Comparison of serum metabolite compositions between obese and lean growing pigs using an NMR-based metabonomic approach. J. Nutr. Biochem. 2012, 23, 133–139. [Google Scholar] [CrossRef]
- AOAC. Association of Official Analytical Chemists, 17th ed.; AOAC International: Arlington, MA, USA, 2000. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- AOCS. Official Methods and Recommended Practices of the AOCS, 6th ed.; American Oil Chemists Society: Chigago, IL, USA, 2009. [Google Scholar]
- Liu, J.C.; Sun, H.; Nie, C.X.; Ge, W.X.; Wang, Y.Q.; Zhang, W.J. Oligopeptide derived from solid-state fermented cottonseed meal significantly affect the immunomodulatory in BALB/c mice treated with cyclophosphamide. Food Sci. Biotechnol. 2018, 27, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Baziz, H.A.; Geraert, P.A.; Padilha, J.C.F.; Guillaumin, S. Chronic heat exposure enhances fat deposition and modifies muscle and fat partition in broiler carcasses. Poult. Sci. 1996, 75, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Siegel, P.B.; Liu, Y.P.; Wang, Y.; Gilbert, E.R.; Zhou, Q.; Zhang, L. Housing system affects broiler characteristics of local Chinese breed reciprocal crosses. Poult. Sci. 2012, 91, 2405–2410. [Google Scholar] [CrossRef] [PubMed]
- Chambers, M.C.; Maclean, B.; Burke, R.; Amodei, D.; Ruderman, D.L.; Neumann, S.; Gatto, L.; Fischer, B.; Pratt, B.; Egertson, J.; et al. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918. [Google Scholar] [CrossRef]
- Jia, H.; Shen, X.; Guan, Y.; Xu, M.; Tu, J.; Mo, M.; Xie, L.; Yuan, J.; Zhang, Z.; Cai, S.; et al. Predicting the pathological response to neoadjuvant chemoradiation using untargeted metabolomics in locally advanced rectal cancer. Radiother. Oncol. 2018, 128, 548–556. [Google Scholar] [CrossRef]
- Xia, J.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—Making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Nagalakshmi, D.; Rao, S.V.R.; Panda, A.K.; Sastry, V.R.B. Cottonseed meal in poultry diets: A review. J. Poult. Sci. 2007, 44, 119–134. [Google Scholar] [CrossRef]
- Dong, X.F.; Gao, W.W.; Tong, J.M.; Jia, H.Q.; Sa, R.N.; Zhang, Q. Effect of polysavone (alfalfa extract) on abdominal fat deposition and immunity in broiler chickens. Poult. Sci. 2007, 86, 1955–1959. [Google Scholar] [CrossRef]
- Cha, Y.S.; Kim, S.R.; Yang, J.A.; Back, H.I.; Kim, M.G.; Jung, S.J.; Song, W.O.; Chae, S.W. Kochujang, fermented soybean-based red pepper paste, decreases visceral fat and improves blood lipid profiles in overweight adults. Nutr. Metab. 2013, 10, 244. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.W.; Sun, H.; Yao, X.H.; Wu, Y.F.; Wang, X.; Feng, J. Effects of replacement of soybean meal by fermented cottonseed meal on growth performance, serum biochemical parameters and immune function of yellow-feathered broilers. Asian Austral. J. Anim. Sci. 2012, 25, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Leenstra, F.R. Effect of age, sex, genotype and environment on fat deposition in broiler chickens-A review. World Poult. Sci J. 1986, 42, 12–25. [Google Scholar] [CrossRef]
- Wang, Y.W.; Deng, Q.Q.; Song, D.; Wang, W.W.; Zhou, H.; Wang, L.; Li, A.K. Effects of fermented cottonseed meal on growth performance, serum biochemical parameters, immune functions, antioxidative abilities, and cecal microflora in broilers. Food Agr. Immunol. 2017, 28, 725–738. [Google Scholar] [CrossRef]
- Obun, C.O.; Yahaya, M.S.; Olafadehan, O.A.; Kehinde, A.S.; Allison, D.S.; Yusuf, A.M.; Farouk, I.U. Dietary value of honey and it effects on abdominal fat deposit, blood and serum profile of finisher broiler chicks. J. Agr. Forest Soc. Sci. 2010, 6, 173–181. [Google Scholar] [CrossRef]
- Sang, I.L.; Clarissa, V.; Patrick, J.; Le, H.D.; Jonathan, M.; Danielle, A.A.; Gura, K.M. Impact of fish oil-based lipid emulsion on serum triglyceride, bilirubin, and albumin levels in children with parenteral nutrition-associated liver disease. Pediatr. Res. 2009, 66, 698–703. [Google Scholar]
- Donsmark, M.; Langfort, J.; Ploug, T.; Holm, C.; Enevoldsen, L.H.; Stallknecht, B.; Kjaer, M.; Ihlemann, J.; Galbo, H. Hormone-sensitive lipase (HSL) expression and regulation by epinephrine and exercise in skeletal muscle. Eur. J. Sport Sci. 2002, 2, 1–10. [Google Scholar] [CrossRef]
- Su, C.C.; Chang, C.S.; Chou, C.H.; Wu, Y.H.S.; Yang, K.T.; Tseng, J.K.; Chang, Y.Y.; Chen, Y.C. L-carnitine ameliorates dyslipidemic and hepatic disorders induced by a high-fat diet via regulating lipid metabolism, self-antioxidant capacity, and inflammatory response. J. Funct. Foods 2015, 15, 497–508. [Google Scholar] [CrossRef]
- Lin, P.J.; Chang, C.H. Endothelium dysfunction in cardiovascular disease. J. Food Sci. 1994, 17, 198–210. [Google Scholar]
- Schaefer, E.J.; Gleasonm, J.A.; Dansinger, L. Dietary fructose and glucose differentially affect lipid and glucose homeostasis. J. Nutr. 2009, 139, 1257S–1262S. [Google Scholar] [CrossRef]
- Heffernan, M.; Thorburn, A.; Fam, B. Increase of fat oxidation and weight loss in obese mice caused by chronic treatment with human growth hormone or a modified C-terminal fragment. Int. J. Obes. 2001, 25, 1442–1449. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kjell, M.; Nanni, D.; Thue, J.; Pedersen, S.B. Growth hormone affects both adiposity and voluntary food intake in old and obese female rats. Eur. J. Endocrinol. 2002, 146, 121–128. [Google Scholar]
- Naaz, A.; Yellayi, S.; Zakroczymski, M.A.; Bunick, D.; Doerge, D.R.; Lubahn, D.B.; Helferich, W.G.; Cooke, P.S. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology 2003, 144, 3315–3320. [Google Scholar] [CrossRef] [PubMed]
- Oscai, L.B.; Essig, D.A.; Palmer, W.K. Lipase regulation of muscle triglyceride hydrolysis. J. Appl. Physiol. 1990, 69, 1571. [Google Scholar] [CrossRef] [PubMed]
- Mersmann, H.J. Lipoprotein and hormone-sensitive lipases in porcine adipose tissue. J. Anim. Sci. 1998, 76, 1396–1404. [Google Scholar] [CrossRef]
- Lu, L.; Ji, C.; Luo, X.G.; Liu, B.; Yu, S.X. The effect of supplemental manganese in broiler diets on abdominal fat deposition and meat quality. Anim. Feed Sci. Tech. 2006, 129, 49–59. [Google Scholar] [CrossRef]
- Yeaman, S.J. Hormone-sensitive lipase—A multipurpose enzyme in lipid metabolism. BBA Mol. Cell Res. 1990, 1052, 128–132. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, X.; Guo, Y.; Long, F. Effects of dietary lipids and Clostridium butyricum on serum lipids and lipid-related gene expression in broiler chickens. Animal 2011, 5, 1909–1915. [Google Scholar] [CrossRef]
- Lee, S.S.; Pineau, T.; Drago, J.; Lee, E.J.; Owens, J.W.; Kroetz, D.L.; Fernandez-Salguero, P.M.; Westphal, H.; Gonzalez, F.J. Targeted disruption of the alpha isoform of the peroxisome proliferator-activated receptor gene in mice results in abolishment of the pleiotropic effects of peroxisome proliferators. Mol. Cell Boil. 1995, 15, 3012–3322. [Google Scholar] [CrossRef]
- Huang, J.B.; Zhang, Y.; Zhou, Y.B.; Zhang, Z.Z.; Xie, Z.W.; Zhang, J.S.; Wan, X.C. Green tea polyphenols alleviate obesity in broiler chickens through the regulation of lipid-metabolism-related genes and transcription factor expression. J. Agri. Food Chemis. 2013, 61, 8565–8572. [Google Scholar] [CrossRef]
- Zhang, Y.; MA, K.; Song, S.; Elam, M.B.; Cook, G.A.; Park, E.A. Peroxisomal proliferator-activated receptor-gamma coactivator-1 alpha (PGC-1 alpha) enhances the thyroid hormone induction of carnitine palmitoyltransferase I (CPT-I alpha). J. Biol. Chem. 2004, 279, 53963–53971. [Google Scholar] [CrossRef] [PubMed]
- Finck, B.N.; Kelly, D.P. Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation 2007, 115, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Preiss, L.K.; Zimmermann, R.; Hammerle, G.; Zechner, R. Lipoprotein lipase: the regulation of tissue specific expression and its role in lipid and energy metabolism. Curr. Opin. Lipidol. 2002, 13, 471–481. [Google Scholar] [CrossRef]
- Lafontan, M.; Langin, D. Lipolysis and lipid mobilization in human adipose tissue. Prog. Lipid Res. 2009, 48, 275–297. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.Y.; Xiong, J.; Wang, F.; Yuan, B.F.; Feng, U.Q. Chiral derivatization coupled with liquid chromatography/mass spectrometry for determining ketone metabolites of hydroxybutyrate enantiomers. Chin. Chem. Lett. 2018, 29, 115–118. [Google Scholar] [CrossRef]
- Newman, J.; Verdin, C.E. Ketone bodies as signaling metabolites. Trends Endocrinol. Metab. 2014, 25, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Cotter, D.G.; Schugar, R.C.; Wentz, A.E.; André, D.D.; Crawford, P.A. Successful adaptation to ketosis by mice with tissue-specific deficiency of ketone body oxidation. Am. J. Physiol. Endocrinol. Metab. 2013, 304, E363–E374. [Google Scholar] [CrossRef] [PubMed]
- Puchalska, P.; Crawford, P.A. Multi-dimensional roles of ketone bodies in fuel metabolism, signaling, and therapeutics. Cell Metab. 2017, 25, 262–284. [Google Scholar] [CrossRef] [PubMed]
- Inouye, M.; Mio, T.; Sumino, K. Dicarboxylic acids as markers of fatty acid peroxidation in diabetes. Atherosclerosis. 2000, 148, 197–202. [Google Scholar] [CrossRef]
- Amer, B.; Clausen, M.R.; Bertram, H.C.; Bohl, M.; Nebel, C.; Zheng, H.; Skov, T.; Larsen, M.K.; Gregersen, S.; Hermansen, K.; et al. Consumption of whey in combination with dairy medium-chain fatty acids (mcfas) may reduce lipid storage due to urinary loss of tricarboxylic acid cycle intermediates and increased rates of mcfas oxidation. Mol. Nutr. Food Res. 2017, 61, 12. [Google Scholar] [CrossRef]
- Mastrofrancesco, A.; Ottaviani, M.; Aspite, N.; Cardinali, G.; Izzo, E.; Graupe, K.; Zouboulis, C.C.; Camera, E.; Picardo, M. Azelaic acid modulates the inflammatory response in normal human keratinocytes through PPAR-gamma activation. Exp. Dermatol. 2010, 19, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Stec, D.E.; John, K.; Trabbic, C.J.; Luniwal, A.; Hankins, M.W.; Baum, J.; Hinds, T.D. Bilirubin binding to PPAR-alpha inhibits lipid accumulation. PLoS ONE 2016, 11, e0153427. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, K.; Hayashi, T.; Nagotani, S.; Sehara, Y.; Zhang, H.; Tsuchiya, A.; Ohta, Y.; Tomiyama, K.; Morimoto, N.; Miyazaki, M.; et al. Reduction of cerebral infarction in rats by biliverdin associated with amelioration of oxidative stress. Brain Res. 2008, 1188, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Gaíva, M.H.; Couto, R.C.; Oyama, L.M.; Couto, G.E.C.; Silveira, V.L.F.; Ribeiro, E.B.; Nascimento, C.M.O. Diets rich in polyunsaturated fatty acids: Effect on hepatic metabolism in rats. Nutrition 2003, 19, 144–149. [Google Scholar] [CrossRef]
- Jump, D.B.; Clarke, S.D.; Thelen, A.; Liimatta, M.; Ren, B.; Badin, M. Dietary polyunsaturated fatty acid regulation of gene transcription. Lipids 1996, 31, 7–11. [Google Scholar]
- Tsuboyama-Kasaoka, N.; Takahashi, M.; Kim, H.; Ezaki, O. Up-regulation of liver uncoupling protein-2 mRNA by either fish oil feeding or fibrate administration in mice. Biochem. Biophys. Res. Commun. 1999, 257, 879–885. [Google Scholar] [CrossRef]
| Item | CON | FCSM |
|---|---|---|
| Ingredients, %DM | ||
| Yellow corn | 54.50 | 54.30 |
| Soybean meal | 33.50 | 27.40 |
| Fermented cottonseed meal | 0.00 | 6.00 |
| Sunflower oil | 3.00 | 3.30 |
| Cottonseed protein | 4.00 | 4.00 |
| Premix 2 | 5.00 | 5.00 |
| Chemical compositions | ||
| Metabolizable energy, Mcal/kg | 2.95 | 2.95 |
| Crude protein, % of DM | 21.23 | 21.23 |
| Ether extract, % of DM | 5.8 | 5.5 |
| Crude fiber, % of DM | 2.4 | 2.5 |
| Calcium, % of DM | 1.04 | 1.03 |
| Total phosphorus, % of DM | 0.69 | 0.71 |
| Available phosphate, % of DM | 0.45 | 0.45 |
| Methionine, % of DM | 0.50 | 0.50 |
| Methionine + cysteine, % of DM | 0.86 | 0.86 |
| Threonine, % of DM | 0.78 | 0.76 |
| Lysine, % of DM | 1.12 | 1.08 |
| Item | FCSM | Soybean Meal |
|---|---|---|
| Chemical Composition | ||
| Dry matter, % | 91.26 | 90.52 |
| Crude protein, % of DM | 44.42 | 44.26 |
| Ether extract, % of DM | 0.86 | 1.87 |
| Crude ash, % of DM | 6.43 | 6.24 |
| Acid detergent fiber, % of DM | 13.08 | 10.21 |
| Neutral detergent fiber, % of DM | 21.78 | 13.52 |
| Free gossypol, mg/kg | 36.41 | - |
| Fatty Acid, % | ||
| C14:0 | 0.0069 | 0.0015 |
| C16:0 | 0.2304 | 0.1255 |
| C18:0 | 0.0341 | 0.0405 |
| C20:0 | 0.0048 | - |
| C22:0 | 0.0056 | 0.0074 |
| C24:0 | 0.0037 | 0.0027 |
| Saturated fatty acids | 0.2855 | 0.1775 |
| C16:1 | 0.0047 | 0.0007 |
| cis-C18:1n-9 | 0.2265 | 0.1211 |
| C20:1 | 0.0015 | - |
| C22:1n-9 | 0.0308 | 0.0268 |
| Monounsaturated fatty acids | 0.2636 | 0.1486 |
| cis-C18:2n-6 | 0.3280 | 0.2566 |
| C18:3n-3 | 0.0061 | 0.0339 |
| C22:2 | 0.0010 | 0.0009 |
| Polyunsaturated fatty acids | 0.3351 | 0.2914 |
| Amino Acid, % | ||
| Aspartic acid | 4.12 | 5.60 |
| Threonine | 1.55 | 2.05 |
| Serine | 2.07 | 2.59 |
| Glutamate | 9.63 | 9.26 |
| Glycine | 1.86 | 2.09 |
| Alanine | 1.84 | 2.25 |
| Cystine | 0.68 | 0.67 |
| Valine | 2.03 | 2.30 |
| Methionine | 0.35 | 0.54 |
| Isoleucine | 1.27 | 2.09 |
| Leucine | 2.63 | 3.87 |
| Tyrosine | 0.89 | 1.48 |
| Phenylalanine | 2.54 | 2.62 |
| Lysine | 2.05 | 3.12 |
| Histidine | 1.21 | 1.22 |
| Arginine | 4.99 | 3.55 |
| Proline | 1.91 | 2.66 |
| Items 1 | Treatment 2 | SEM 3 | p-Value | |
|---|---|---|---|---|
| CON | FCSM | |||
| Body weight, g | 596.67 | 581.53 | 14.53 | 0.63 |
| ADFI, g/d | 65.61 | 59.51 | 0.73 | 0.33 |
| ADG, g/d | 43.80 | 42.09 | 0.87 | 0.49 |
| F/G, g/g | 1.53 a | 1.41 b | 0.06 | 0.04 |
| Items 1 | Treatment 2 | SEM 3 | p-Value | |
|---|---|---|---|---|
| CON | FCSM | |||
| Serum | ||||
| Glu, mmol/L | 12.03 a | 10.03 b | 0.47 | 0.04 |
| TG, mmol/L | 0.49 a | 0.32 b | 0.05 | <0.01 |
| TC, mmol/L | 4.74 | 4.070 | 0.22 | 0.06 |
| NEFA, umol/L | 994.10 | 1006.50 | 49.67 | 0.90 |
| HDL-C, mmol/L | 1.53 | 1.25 | 0.15 | 0.36 |
| LDL-C, mmol/L | 1.35 a | 0.92 b | 0.11 | 0.04 |
| Liver | ||||
| TG, mmol/gprot | 39.01 a | 11.72 b | 4.34 | <0.01 |
| TC, mmol/gprot | 0.27 | 0.26 | 0.04 | 0.41 |
| NEFA, umol/gprot | 1961.70 | 1910.70 | 186.79 | 0.10 |
| Abdominal fat | ||||
| TG, mmol/gprot | 139.00 | 141.70 | 10.44 | 0.71 |
| TC, mmol/gprot | 0.20 | 0.19 | 0.01 | 0.61 |
| NEFA, umol/gprot | 9048.00 | 8921.22 | 383.11 | 0.88 |
| Items 1 | Treatment 2 | SEM 3 | p-Value | |
|---|---|---|---|---|
| CON | FCSM | |||
| Serum | ||||
| EPI, ng/mL | 11.26 b | 16.47 a | 0.89 | <0.01 |
| GH, ng/mL | 9.31 b | 12.21 a | 0.57 | <0.01 |
| INS, mU/L | 31.99 | 35.61 | 2.49 | 0.08 |
| Liver | ||||
| HSL, U/L | 1340.40 b | 1981.50 a | 110.13 | <.001 |
| ME, mIU/L | 2231.20 a | 1281.90 b | 196.23 | 0.01 |
| ACC, U/mL | 937.70 | 1069.70 | 98.51 | 0.07 |
| LPL, U/L | 483.60 b | 815.20 a | 71.12 | 0.01 |
| FAS, U/mL | 1544.90 | 1551.00 | 245.01 | 0.09 |
| EPI, ng/mL | 11.64 b | 20.18 a | 1.59 | <0.01 |
| GH, ng/mL | 7.88 b | 13.66 a | 1.18 | 0.01 |
| Abdominal fat | ||||
| HSL, U/L | 1310.20 b | 1736.70 a | 88.17 | 0.01 |
| ME, mIU/L | 1820.83 | 1832.10 | 89.99 | 0.09 |
| ACC, U/mL | 895.33 | 923.33 | 48.29 | 0.01 |
| LPL, U/L | 592.50 b | 773.67 a | 34.72 | <0.01 |
| FAS, U/mL | 1893.00 | 1916.34 | 28.52 | 0.07 |
| EPI, ng/mL | 11.26 b | 16.47 a | 0.891 | <0.01 |
| GH, ng/mL | 9.31 b | 12.21 a | 0.568 | <0.01 |
| Superclass | Metabolite Names | Identified by Positive- or Negative-Ion Mode | m/z 2 | RT 3 | VIP 4 | FC (FCSM/CON) 5 | p-Value |
|---|---|---|---|---|---|---|---|
| Phenylpropanoids | 2-Coumarate | negative | 163.04 | 321.99 | 1.82 | 0.27 | <0.01 |
| Organic acids | 2-Ethyl-2-Hydroxybutyric acid | negative | 131.07 | 313.56 | 1.31 | 0.74 | 0.03 |
| Organic acids | 2-Hexenal | positive | 99.08 | 4.19 | 1.94 | 0.70 | <0.01 |
| Organic acids | 3-Hydroxybutyric acid | positive | 105.05 | 113.94 | 1.73 | 3.38 | 0.01 |
| Organic acids | Azelaic acid | positive | 189.11 | 393.04 | 1.81 | 1.28 | <0.01 |
| Organic acids | 4-Oxododecanedioic acid | negative | 243.12 | 397.93 | 1.36 | 0.41 | 0.02 |
| Organic acids | Salicylic acid | negative | 137.02 | 405.03 | 1.47 | 0.48 | 0.01 |
| Organic acids | trans-Cinnamic acid | positive | 149.06 | 182.73 | 2.08 | 0.70 | 0.03 |
| amino acid | 5-Methylthioadenosine | positive | 298.10 | 230.92 | 1.53 | 1.89 | 0.02 |
| amino acid | N-acetyltryptophan | negative | 245.09 | 362.65 | 1.43 | 0.54 | 0.02 |
| Peptides | Phenylethylamine | positive | 122.10 | 216.64 | 2.05 | 0.22 | <0.01 |
| Lipids | Docosahexaenoic | positive | 329.25 | 858.32 | 1.68 | 1.67 | 0.01 |
| Pesticides | Choline | positive | 104.11 | 65.25 | 1.38 | 0.80 | 0.04 |
| Terpenoids | Costunolide | negative | 231.12 | 382.28 | 1.44 | 0.33 | 0.02 |
| Steroids | Taurocholic acid | positive | 516.30 | 527.94 | 1.78 | 0.25 | <0.01 |
| Alkaloids | Indoleacetic | positive | 176.07 | 397.17 | 1.97 | 0.31 | <0.01 |
| - | Biliverdin | negative | 581.24 | 625.68 | 1.26 | 1.64 | 0.04 |
| - | Dimethylglycine | positive | 104.07 | 133.43 | 1.73 | 2.00 | 0.01 |
| - | Prolylhydroxyproline | positive | 229.12 | 72.91 | 1.67 | 1.76 | 0.01 |
| - | 1-heptadecanoyl-sn-glycero-3-phosphocholine | positive | 510.35 | 810.16 | 1.39 | 1.69 | 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, J.-L.; Zhang, J.; Wei, L.-Q.; Zhang, W.-J.; Nie, C.-X. Effect of Fermented Cottonseed Meal on the Lipid-Related Indices and Serum Metabolic Profiles in Broiler Chickens. Animals 2019, 9, 930. https://doi.org/10.3390/ani9110930
Niu J-L, Zhang J, Wei L-Q, Zhang W-J, Nie C-X. Effect of Fermented Cottonseed Meal on the Lipid-Related Indices and Serum Metabolic Profiles in Broiler Chickens. Animals. 2019; 9(11):930. https://doi.org/10.3390/ani9110930
Chicago/Turabian StyleNiu, Jun-Li, Jun Zhang, Lian-Qing Wei, Wen-Ju Zhang, and Cun-Xi Nie. 2019. "Effect of Fermented Cottonseed Meal on the Lipid-Related Indices and Serum Metabolic Profiles in Broiler Chickens" Animals 9, no. 11: 930. https://doi.org/10.3390/ani9110930
APA StyleNiu, J.-L., Zhang, J., Wei, L.-Q., Zhang, W.-J., & Nie, C.-X. (2019). Effect of Fermented Cottonseed Meal on the Lipid-Related Indices and Serum Metabolic Profiles in Broiler Chickens. Animals, 9(11), 930. https://doi.org/10.3390/ani9110930





