Simple Summary
Mutations of the Boule gene, a gene that contributes to spermatogenesis, are a main cause of mammal infertility in reproduction. As a conserved gene, only some single nucleotide polymorphisms (SNPs) in the introns within it have been reported in humans with meiosis block, but not in goats, and especially its fundamental roles in female reproduction are still unknown. Therefore, this study detected the potential polymorphisms of the Boule gene in goats via the tetra-primer amplification refractory mutation system PCR (T-ARMS-PCR). Herein, one predicted SNP locus (rs661484476: g.7254T>C) of the Boule gene was first detected and displayed moderate polymorphism among all the studied groups. Notably, the polymorphisms of the goat Boule gene were significantly associated with the goat litter size in different groups, and female goats with the heterozygous genotype (CT) had more possibilities to produce multiple lambs than others, indicating that the Boule gene might underlie female reproductive capacities and that g.7254T>C could be a potential marker in the marker-assisted selection process for litter size in goats.
Abstract
As a gene contributing to spermatogenesis, the Boule gene (also called Boll), whose mutations result in azoospermia and sterility of flies and mice, was conserved in reductional maturation divisions. However, in goats, the polymorphisms of Boule, especially with regard to their fundamental roles in female reproduction traits, are still unknown. Therefore, the aims of this study were to detect a potential mutation (rs661484476: g.7254T>C) located in intron 2 of the Boule gene by tetra-primer amplification refractory mutation system PCR (T-ARMS-PCR) and to explore its potential association with the litter size of Shaanbei White-Cashmere goats (SBWGs). In this study, g.7254T>C was firstly detected. The TT genotype was the dominant genotype in the single-lamb group, and T was also the dominant allele in all tested groups. Additionally, the detected locus displayed moderate polymorphism with polymorphism information content (PIC) values among all studied goats ranging from 0.303 to 0.344. Notably, according to the χ2 test, the distribution differences for the genotypic frequencies between the single- and multi-lamb groups was significant (p = 0.014). Furthermore, the polymorphisms of the goat Boule gene were significantly associated with the goat litter size in SBWGs (p < 0.05), which indicated that g.7254T>C could be a potential marker in the marker-assisted selection process for potential litter size in goats. These results also indicated that the Boule gene might exercise important functions in female goat reproduction, which provided new insight for female goat breeding and could accelerate the process of goat breeding.
1. Introduction
With high economic value, litter size traits will always be the most vital concern of the global goat industry. To meet increasing profitability demands and considering the low heritability of the goat reproductive trait, marker-assisted selection (MAS), which is based on the genetic diversity in reproductive potential, can accelerate the breeding process and improve reproductive efficiency [1,2]. A few genetic markers have been identified and associated with goat litter size, and our group has focused on them. Our previous study demonstrated that a novel 14 bp duplicated deletion within goat GHR and a 14 bp functional deletion within the CMTM2 gene are significantly associated with litter size [3,4].
Similarly, the insertion/deletions (indels) in SPEF2 [1] and the 16 bp indel of the lysine demethylase 6A gene (KDM6A) [5] were identified as significantly related to the first-born litter size in the Shaanbei White-Cashmere goat (SBWG) population. Additionally, the novel 26 bp indel within the catenin beta 1 gene (CTNNB1) has a significant relationship with goat litter size [6].
Furthermore, except for indel mutation, the main type of genetic variation, some single nucleotide polymorphisms (SNP) are proposed to adversely affect mRNA stability or protein binding and eventually change the target traits [7,8]. Based on whole genome scanning for the litter size trait in dairy goats (Capra hircus), various associated genes and SNPs were specifically selected, such as SMAD2 and the aforementioned KDM6A [9]. Not only is it a candidate gene in goat reproduction, SMAD2 has also been validated as being strongly associated with sheep prolificacy [10]. Another highly used multiparous gene, goat GDF9, was significantly associated with the first-born litter size in goats, and the famous Q320P mutation was the major SNP affecting goat litter size rather than V397I [11,12]. Additionally, c.682G>T and c.837T>C loci and diplotypes of the caprine POU1F1 gene had significant effects on litter size (p < 0.05) [13]. Moreover, the missense mutation (L280V) within the POU1F1 gene was also identified as strongly affecting growth traits and litter size in goats [14]. Furthermore, SNPs existing around genome flanking regions surrounding the transcription start sites also contributed to enhancing goat litter size [15].
As a complicated reproductive trait, litter size is controlled by multiple genes and factors [16], such as oocyte maturation, fertilization, embryogenesis, and spermatogenesis. Meanwhile, female sterility or male infertility, to a large extent, results in restricted dairy goat breeding [17]. As a confirmed member of the encoding germ-cell-specific RNA binding proteins, the Boule gene has been identified as being involved in regulating the development and differentiation of germ cells, playing essential roles in arrhenotoky [18,19,20]. Nevertheless, several studies have also explored the role of the Boule gene in female reproduction. For instance, in flatworm Macrostomum lignano, the Boule orthologues’ RNAi resulted in aberrant egg maturation and led to female sterility [21]. Moreover, the RNA and protein of Boule exhibit mitotic and meiotic expression in female Chinese sturgeon [22]. The process of embryonic stem cell differentiation to form germ-cell-like cells in a cumulus cell-conditioned medium induced the highest expression of the buffalo Boule gene [23]. However, in female goats, whether the Boule gene plays an important role in female reproduction is unknown.
As a conserved gene, Boule is considered to be of very low frequency in genetic variation. Only some SNPs in introns are reported in humans with meiosis block, but not in goats, especially in regard to their fundamental roles in female reproduction traits. Additionally, among various methods to detect SNPs, tetra-primer amplification refractory mutation system PCR (T-ARMS-PCR) has been successfully used in many studies because of its high efficiency [24]. Therefore, the objectives of this study were to detect the potential SNP of the goat Boule gene via T-ARMS-PCR in Shaanbei White-Cashmere goats and to investigate its correlation with goat litter size traits, which could accelerate the progress of goat breeding via an marker-assisted selection (MAS) strategy.
2. Materials and Methods
All experimental processing in this study was approved by the International Animal Care and Use Committee (IACUC) of the Northwest A&F University (protocol number NWAFAC1008). Additionally, the treatment of the experimental animals was fully consistent with local animal welfare guidelines, laws, and policies.
2.1. Animal Samples and Genomic DNA Collection
A total of 357 ear samples of Shaanbei White-Cashmere goats (SBWGs, female) were randomly collected from the Shaanbei Cashmere goat breeding farm in Shaanxi Province. All the used individuals were in the same age group (2–3 years old) and healthy. Moreover, from the agricultural technical station of Hengshan county by production records, data of the first birth litter size of Shaanbei Cashmere goats were also collected. According to the production records, the 357 goats were divided into a single-lamb group (n = 176) and a multi-lamb group (n = 181).
DNA samples were isolated from the ear tissues (saved in 70% alcohol at −80 °C) through the method of high salt extraction [25]. The purity of the DNA samples was assayed by a NanoDrop 1000 (Thermo Scientific, Waltham, Massachusetts, MA, USA). Then, all the DNA samples were diluted to 10 ng/L and kept at 4 °C temporarily.
2.2. Primer Design, Genotyped for g.7254T>C by T-ARMS-PCR
Referencing the goat Boule gene sequence (GenBank No: NC 030809.1; GeneID: 102173977), primers were designed to detect SNPs in the coding regions and noncoding regions of the Boule gene (Table 1) and were synthesized by Sangon Biotech (Shanghai, China).

Table 1.
Single nucleotide polymorphism (SNP) primer information of the goat Boule gene.
Notably, the method of tetra-primer amplification refractory mutation system PCR (T-ARMS-PCR) was used in this study. With the use of specific inner and outer primers at exact proportion and amplification conditions, different alleles of the locus of interest were amplified. The specific inner and outer primers are shown in Table 1, and the specific steps are described below.
In the preliminary experiment, 30 SBWG individuals were randomly selected to construct a DNA pool for PCR amplification (pretest). Additionally, different groups were set up to explore the most suitable ratio of inner and outer primers and finally determine the most suitable reaction system (Table 2).

Table 2.
The tetra-primer amplification refractory mutation system PCR (T-ARMS-PCR) reaction system.
The pretest showed that the ratio of the inner and outer primers of the g.7254T>C was 1:4, which is the optimal ratio of inner and outer primers. Then the touchdown-PCR (TD-PCR) was performed as reported: initial denaturation for 5 min at 95 °C, followed by 18 cycles of denaturation for 30 s at 94 °C, annealing for 30 s at 68 °C (with a decrease of 1 °C per cycle), extension for 1000 bp/min at 72 °C, another 24 cycles of 30 s at 94 °C, 30 s at 50 °C, and 45 s at 72 °C, and a final extension of 10 min at 72 °C [26].
After that, the amplification products were detected by using 3.5% agarose gel electrophoresis. Different genotypes were distinguished according to the number and size of the fragments, and the two bands were homozygous, while the three bands were heterozygous. Furthermore, to confirm the mutation sites, DNA sequencing was performed only when products with different genotypes were first amplified. Subsequent genotyping of individuals was based on the results of the T-ARMS-PCR.
2.3. Statistical Analysis
After genotyping, analyses of the genetic polymorphisms, including gene frequencies, genotype frequencies, and polymorphism information content (PIC) were performed.
To test whether the polymorphisms were in Hardy–Weinberg equilibrium (HWE), the genotypic and allelic frequencies of the goat Boule gene were calculated directly using a chi-square (χ2) test [24]. Polymorphism information content (PIC) was calculated on the basis of Nei’s method, performed in the GDIcall Online Calculator (http://www.msrcall.com/Gdicall.aspx) [27]. Wherein, PIC > 0.5 means that the mutation is highly polymorphic, 0.25 < PIC ≤ 0.5 means moderate polymorphism, and PIC ≤ 0.25 means low polymorphism.
Distribution differences for genotypic and allelic frequencies between different groups were carried out with the χ2 test using SPSS software (Version 18.0) (IBM Corp., Armonk, NY, USA) [3].
Furthermore, the associations of the novel SNP of the Boule gene with goat litter size were inspected using the analysis of variance (ANOVA) available in SPSS (Version 18.0) [25], while different genotypes were considered as independent variables, and litter size traits were used as the dependent variable. The results were deemed to be statistically significant when p < 0.05, and all statistical tests were two-sided.
3. Results
3.1. Polymorphism Detection and Genotyping of Boule Gene via T-ARMS-PCR
One SNP locus (NC-030809.1, rs661484476: g.7254T>C), located at the second intron of goat Boule gene, was firstly detected in goat groups (Figure 1 and Figure 2). The agarose gel electrophoresis after genotyping showed that the CC genotype was 364 bp and 521 bp, the CT genotype was 208 bp, 364 bp, and 521 bp, and the TT genotype showed 208 bp and 521 bp (Figure 1).
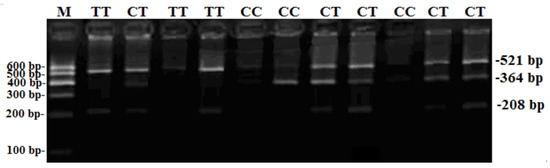
Figure 1.
The sequencing map for g.7254T>C locus within the Boule gene.
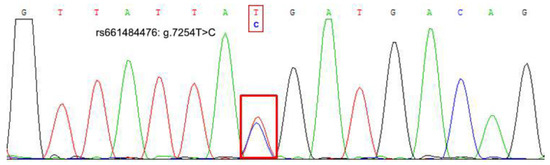
Figure 2.
The sequencing map of the Boule gene’s g.7254T>C locus.
3.2. Analysis of Population Genetics of g.7254T>C
The numbers of genotypes, genotype frequency, allele frequency, PIC value, and HWE of the g.7254T>C were calculated (Table 3). The results showed that the TT genotype was the dominant genotype in the single-lamb group and that T was also the dominant allele. In the single-lamb group, the frequencies of wild genotypes (TT) were higher than the CT/ homozygous mutant genotype, while the heterozygous genotype (CT) had a higher frequency than the others in the multi-lamb group. Additionally, the results of the population parameters demonstrated that this SNP marker displayed moderate polymorphism, with a PIC among all studied groups ranging from 0.303 to 0.344.

Table 3.
Genotypic and allelic frequencies and population indexes of Boule gene in the Shaanbei White-Cashmere goat (SBWG).
Nevertheless, as for the Hardy–Weinberg equilibrium (HWE), the g.7254T>C locus in the multi-lamb group was not at HWE (p < 0.05), while the single-lamb group had the opposite result.
3.3. Association of g.7254T>C with Goat Litter Size
After the χ2 test (Table 4), the value of Pearson chi-square for the genotypic frequencies between different groups was 8.510 (df = 2), and the distribution difference was significant (p = 0.014), while the allelic frequencies between the single- and multi-lamb groups had no statistical difference (χ2 = 2.892, df = 1, p = 0.089).

Table 4.
Chi-square (χ2) and p values from genotype and allele frequencies among different groups at the single nucleotide polymorphism (SNP) locus of the Boule gene in the SBWG.
To explore whether the polymorphisms of the goat Boule gene were related to the goat litter size, the associations between the g.7254T>C and litter size traits were investigated (Table 5). Consistent with the results of Table 4, individuals with the heterozygous genotype (CT) had higher frequencies of multi-lambs than goats with the TT genotype, indicating that the g.7254T>C had significant effects on litter size in the SBWG breed (p < 0.05) (Table 5). These results are also consistent with the distribution differences of the genotypic frequencies.

Table 5.
Relationship between the SNP of the Boule gene and the litter size of the SBWG.
4. Discussion
In recent years, to better meet the growing market demand, molecular genetic breeding techniques, especially marker-assisted selection (MAS) based on genetic diversity, have been widely used in livestock breeding for their convenience and high efficiency [28]. As the most representative of genomic variation, the potential SNPs of candidate genes and the most effective method of detecting mutations will always be the most vital concern of MAS [29]. Among various detection methods, the T-ARMS-PCR is inexpensive, convenient, rapid, and accurate for SNP genotyping [24,30]. Therefore, in this study, the novel mutations of the Boule gene, an essential contributor of spermatocyte meiosis in reproduction, were genotyped by T-ARMS-PCR in goats.
Herein, mutations in the coding region of the Boule gene were not found, but one SNP locus (g.7254T>C) was detected at intron 2. The mutation rate of the Boule gene is lower than that of other genes, which may be caused by the lack of polymorphism in its exon sequence. Three nucleotide mutations in the Boule gene intron regions were previously detected in 164 infertile males but were not found in the coding regions and the 3′ untranslated regions [31]. Luetjens et al. [32] detected mutations in the 2~11 exons of the Boule gene in 18 patients with meiosis block, but no mutations were found in detected exons, which is consistent with the results of Westerveld et al. [33]. Low variation of the human Boule gene is the most likely constraint of its strong function, suggesting that the Boule gene is conserved to a great degree for meiosis.
In recent years, the function of introns has been continuously revealed, especially in the intron-mediated splicing process [34,35,36]. SNP is the main cause of abnormal splicing of exon skipping and intron retention [8]. It has been reported that mutations at the intron will activate the recessive variable splice sites [37]. On the other hand, introns contain many transcriptional regulatory elements, such as the intron splicing enhancer (ISE) and the intron splicing silencer (ISS) [37]. The Boule gene was found to be expressed in a similar pattern with gene of DNA meiotic recombinase 1 and mutS homolog 4 between 49 and 94 days postcoitum in the sheep ovary, suggesting its possible participation in female reproductive capacities [38].
The Boule gene is largely known as a main cause of sperm deficiency, but very little data exists concerning its potential function in female reproductive traits. In this study, g.7254T>C had a significant effect on litter size in the SBWG breed, speculating that the Boule gene might underlie female reproductive capacities. Concerning female gametogenesis in mammals, does the Boule gene exist purely to promote germ-cell progression? A large body of study of the Boule gene in the male meiosis process has been identified, but its potential function in females is largely unknown and awaits further deep study.
5. Conclusions
Taken together, the g.7254T>C of the goat Boule gene was found and shown to have a significant effect in the SBWG breed litter size. This means that g.7254T>C is an informative molecular marker in the MAS process for optimal litter size in goats and provides insights into new concepts in female reproduction.
Author Contributions
Validation, P.F. and X.Z. formal analysis, J.L.; resources, X.S., P.F. and L.Q.; writing—original draft preparation, J.L. and X.S.; writing—review and editing, C.P., and H.C.; project administration, L.Q. and X.L.; funding acquisition, X.S., L.Q. and X.L.
Funding
This work was funded by the National Natural Science Foundation of China (No. 31172184), the Scientific Research Foundation of the Education Department of Shaanxi Province (No. 19JK1004), the Young Talent Fund of the University Association for Science and Technology in Shaanxi (No. 20190208), and the Research Foundation for High-Level Talents of Yulin University (No. 17GK22).
Acknowledgments
We greatly thank the staff of Shaanbei White-Cashmere goat breeding farm of Yunlin, Yulin, Shaanxi Province, China for collecting the samples. We also greatly thank the staff (Hailong Yan; Haijing Zhu; Jinwang Liu; Shuwei Dong; Longping Li and Lei Shi) of Shaanxi Provincial Engineering and Technology Research Center of Cashmere Goats, College of Life Science, Yulin University, Yulin, for helping to collect the samples.
Conflicts of Interest
We confirm that there is no conflict of interest with any financial organization for the material discussed in the manuscript and that the publication of the manuscript is approved by all authors.
References
- Chen, M.; Yan, H.; Wang, K.; Cui, Y.; Chen, R.; Liu, W.; Zhu, H.; Qu, L.; Pan, C. Goat SPEF2: Expression profile, indel variants identification and association analysis with litter size. Theriogenology 2019, 139, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, X.; Jiang, E.; Yan, H.; Zhu, H.; Chen, H.; Liu, J.; Qu, L.; Pan, C.; Lan, X. InDels within caprine IGF 2 BP 1 intron 2 and the 3′-untranslated regions are associated with goat growth traits. Anim. Genet. 2019. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, S.L.; Li, J.; Wang, X.Y.; Peng, K.; Lan, X.Y.; Pan, C.Y. Development of a touch down multiples PCR method for simultaneously rapidly detecting three novel insertion/deletions (indels) within one gene: an example for goat GHR gene. Anim. Biotech. 2019, 30, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Zhang, S.; He, L.; Zhu, H.; Wang, Z.; Yan, H.; Huang, Y.; Dang, R.; Lei, C.; Chen, H.; et al. A 14-bp functional deletion within the CMTM2 gene is significantly associated with litter size in goat. Theriogenology 2019, 139, 49–57. [Google Scholar] [CrossRef]
- Cui, Y.; Yan, H.; Wang, K.; Xu, H.; Zhang, X.; Zhu, H.; Liu, J.; Qü, L.; Lan, X.; Pan, C. Insertion/Deletion Within the KDM6A Gene Is Significantly Associated with Litter Size in Goat. Front. Genet. 2018, 9, 91. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, H.; Wang, K.; Zhou, T.; Chen, M.; Zhu, H.; Pan, C.; Zhang, E. Goat CTNNB1: mRNA expression profile of alternative splicing in testis and association analysis with litter size. Gene 2018, 679, 297–304. [Google Scholar] [CrossRef]
- Cartegni, L.; Krainer, A.R. Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat. Genet. 2002, 30, 377–384. [Google Scholar] [CrossRef]
- Montgomery, S.B.; Sammeth, M.; Gutierrez-Arcelus, M.; Lach, R.P.; Ingle, C.; Nisbett, J.; Guigo, R.; Dermitzakis, E.T. Transcriptome genetics using second generation sequencing in a Caucasian population. Nature 2010, 464, 773–777. [Google Scholar] [CrossRef]
- Lai, F.-N.; Zhai, H.-L.; Cheng, M.; Ma, J.-Y.; Cheng, S.-F.; Ge, W.; Zhang, G.-L.; Wang, J.-J.; Zhang, R.-Q.; Wang, X.; et al. Whole-genome scanning for the litter size trait associated genes and SNPs under selection in dairy goat (Capra hircus). Sci. Rep. 2016, 6, 38096. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, Z.; Yang, H.; Yao, X.; Yang, P.; Ren, C.; Wang, F.; Zhang, Y. Pituitary transcriptomic study reveals the differential regulation of lncRNAs and mRNAs related to prolificacy in different FecB genotyping sheep. Genes (Basel) 2019, 10, 157. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, S.; Yang, Q.; Pan, C.; Chen, H.; Qu, L.; Yan, H.; Lan, X. A novel 12-bp indel polymorphism within the GDF9 gene is significantly associated with litter size and growth traits in goats. Anim. Genet. 2017, 48, 735–736. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yang, Q.; Wang, K.; Yan, H.; Pan, C.; Chen, H.; Liu, J.; Zhu, H.; Qu, L.; Lan, X. Two strongly linked single nucleotide polymorphisms (Q320P and V397I) in GDF9 gene are associated with litter size in cashmere goats. Theriogenology 2019, 125, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, Y.; Bai, Y.; Yang, H.; Yan, H.; Liu, J.; Shi, L.; Song, X.; Li, L.; Dong, S.; et al. Relationship between SNPs of POU1F1 gene and litter size and growth traits in Shaanbei White Cashmere Goats. Animals (Basel) 2019, 9, 114. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, W.; Yang, H.; Wang, M.; Yan, H.; Zhu, H.; Liu, J.; Qu, L.; Lan, X.; Pan, C. A novel missense mutation (L280V) within POU1F1 gene strongly affects litter size and growth traits in goat. Theriogenology 2019, 135, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.-Q.; Lai, F.-N.; Wang, J.-J.; Zhai, H.-L.; Zhao, Y.; Sun, Y.-J.; Min, L.-J.; Shen, W. Analysis of the SNP loci around transcription start sites related to goat fecundity trait base on whole genome resequencing. Gene 2018, 643, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Feng, T.; Cao, G.-L.; Chu, M.-X.; Di, R.; Huang, D.-W.; Liu, Q.-Y.; Pan, Z.-Y.; Jin, M.; Zhang, Y.-J.; Li, N. Identification and verification of differentially expressed genes in the caprine hypothalamic-pituitary-gonadal axis that are associated with litter size. Mol. Reprod. Dev. 2015, 82, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Cai, H.; Yang, Q.; Shi, T.; Pan, C.; Lei, C.; Dang, R.; Chen, H.; Lan, X. Identification of novel alternative splicing transcript and expression analysis of bovine TMEM95 gene. Gene 2016, 575, 531–536. [Google Scholar] [CrossRef]
- Maines, J.Z.; Wasserman, S.A. Post-transcriptional regulation of the meiotic Cdc25 protein Twine by the Dazl orthologue Boule. Nat. Cell Biol. 1999, 1, 171–174. [Google Scholar] [CrossRef]
- Kim, B.; Rhee, K. BOULE, a Deleted in Azoospermia Homolog, Is Recruited to Stress Granules in the Mouse Male Germ Cells. PLoS ONE 2016, 11, e0163015. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, S.; Yang, Q.; Wang, K.; Zhang, S.; Pan, C.; Yan, H.; Dang, R.; Lei, C.; Chen, H.; et al. Goat Boule: Isoforms identification, mRNA expression in testis and functional study and promoter methylation profiles. Theriogenology 2018, 116, 53–63. [Google Scholar] [CrossRef]
- Kuales, G.; De Mulder, K.; Glashauser, J.; Salvenmoser, W.; Takashima, S.; Hartenstein, V.; Berezikov, E.; Salzburger, W.; Ladurner, P. Boule-like genes regulate male and female gametogenesis in the flatworm Macrostomum lignano. Dev. Boil. 2011, 357, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Li, C.-J.; Yue, H.-M.; Yang, X.-G.; Wei, Q.-W. Differential expression of fertility genes boule and dazl in Chinese sturgeon (Acipenser sinensis), a basal fish. Cell Tissue Res. 2015, 360, 413–425. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.M.; Saini, N.; Ashraf, S.; Singh, M.K.; Manik, R.S.; Singla, S.K.; Palta, P.; Chauhan, M.S. Cumulus cell-conditioned medium supports embryonic stem cell differentiation to germ cell-like cells. Reprod. Fertil. Dev. 2017, 29, 679. [Google Scholar] [CrossRef]
- Zhang, S.; Dang, Y.; Zhang, Q.; Qin, Q.; Lei, C.; Chen, H.; Lan, X. Tetra-primer amplification refractory mutation system PCR (T-ARMS-PCR) rapidly identified a critical missense mutation (P236T) of bovine ACADVL gene affecting growth traits. Gene 2015, 559, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, S.; Erdenee, S.; Sun, X.; Dang, R.; Huang, Y.; Lei, C.; Chen, H.; Xu, H.; Cai, Y.; et al. Nucleotide variants in prion-related protein (testis-specific) gene (PRNT) and effects on Chinese and Mongolian sheep phenotypes. Prion 2018, 12, 185–196. [Google Scholar] [CrossRef]
- Chen, M.; Wang, J.; Liu, N.; Cui, W.; Dong, W.; Xing, B.; Pan, C. Pig SOX9: Expression profiles of Sertoli cell (SCs) and a functional 18 bp indel affecting testis weight. Theriogenology 2019, 138, 94–101. [Google Scholar] [CrossRef]
- Li, Y.; Wang, K.; Jiang, Y.-Z.; Chang, X.-W.; Dai, C.-F.; Zheng, J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) inhibits human ovarian cancer cell proliferation. Cell. Oncol. (Dordr.) 2014, 37, 429–437. [Google Scholar] [CrossRef]
- Lande, R.; Thompson, R. Efficiency of Marker-Assisted Selection in the Improvement of Quantitative Traits. Genetics 1990, 124, 743–756. [Google Scholar]
- Nkrumah, J.D.; Sherman, E.L.; Bartusiak, R.; Murdoch, B.; Moore, S.S.; Li, C.; Marques, E.; Crews, D.H., Jr.; Wang, Z.; Basarab, J.A. Primary genome scan to identify putative quantitative trait loci for feedlot growth rate, feed intake, and feed efficiency of beef cattle1. J. Anim. Sci. 2007, 85, 3170–3181. [Google Scholar] [CrossRef]
- Medrano, R.F.V.; De Oliveira, C.A. Guidelines for the Tetra-Primer ARMS–PCR Technique Development. Mol. Biotechnol. 2014, 56, 599–608. [Google Scholar] [CrossRef]
- Lee, D.F.; Klebes, A.; Kornberg, T.B.; Xu, E.Y.; Turek, P.J.; Pera, R.A.R. Human BOULE gene rescues meiotic defects in infertile flies. Hum. Mol. Genet. 2003, 12, 169–175. [Google Scholar]
- Luetjens, C.M.; Xu, E.Y.; Pera, R.A.R.; Kamischke, A.; Nieschlag, E.; Gromoll, J. Association of Meiotic Arrest with Lack of BOULE Protein Expression in Infertile Men. J. Clin. Endocrinol. Metab. 2004, 89, 1926–1933. [Google Scholar] [CrossRef] [PubMed]
- Westerveld, G.H.; Repping, S.; Leschot, N.J.; Van Der Veen, F.; Lombardi, M.P. Mutations in the human BOULE gene are not a major cause of impaired spermatogenesis. Fertil. Steril. 2005, 83, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Fromm, M.; Walbot, V.; Callis, J. Introns increase gene expression in cultured maize cells. Genes Dev. 1987, 1, 1183–1200. [Google Scholar]
- Buchman, A.R.; Berg, P. Comparison of intron-dependent and intron-independent gene expression. Mol. Cell. Boil. 1988, 8, 4395–4405. [Google Scholar] [CrossRef] [PubMed]
- Walker, V.K.; Duncker, B.; Davies, P.L. Introns boost transgene expression in Drosophila melanogaster. Mol. Genet. Genom. 1997, 254, 291–296. [Google Scholar]
- Cáceres, J.F.; Kornblihtt, A.R. Alternative splicing: Multiple control mechanisms and involvement in human disease. Trends Genet. 2002, 18, 186–193. [Google Scholar] [CrossRef]
- Mandon-Pépin, B.; Oustry-Vaiman, A.; Vigier, B.; Piumi, F.; Cribiu, E.; Cotinot, C. Expression profiles and chromosomal localization of genes controlling meiosis and follicular development in the sheep ovary. Boil. Reprod. 2003, 68, 985–995. [Google Scholar]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).