A Study of Population Size and Activity Patterns and Their Relationship to the Prey Species of the Eurasian Lynx Using a Camera Trapping Approach
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
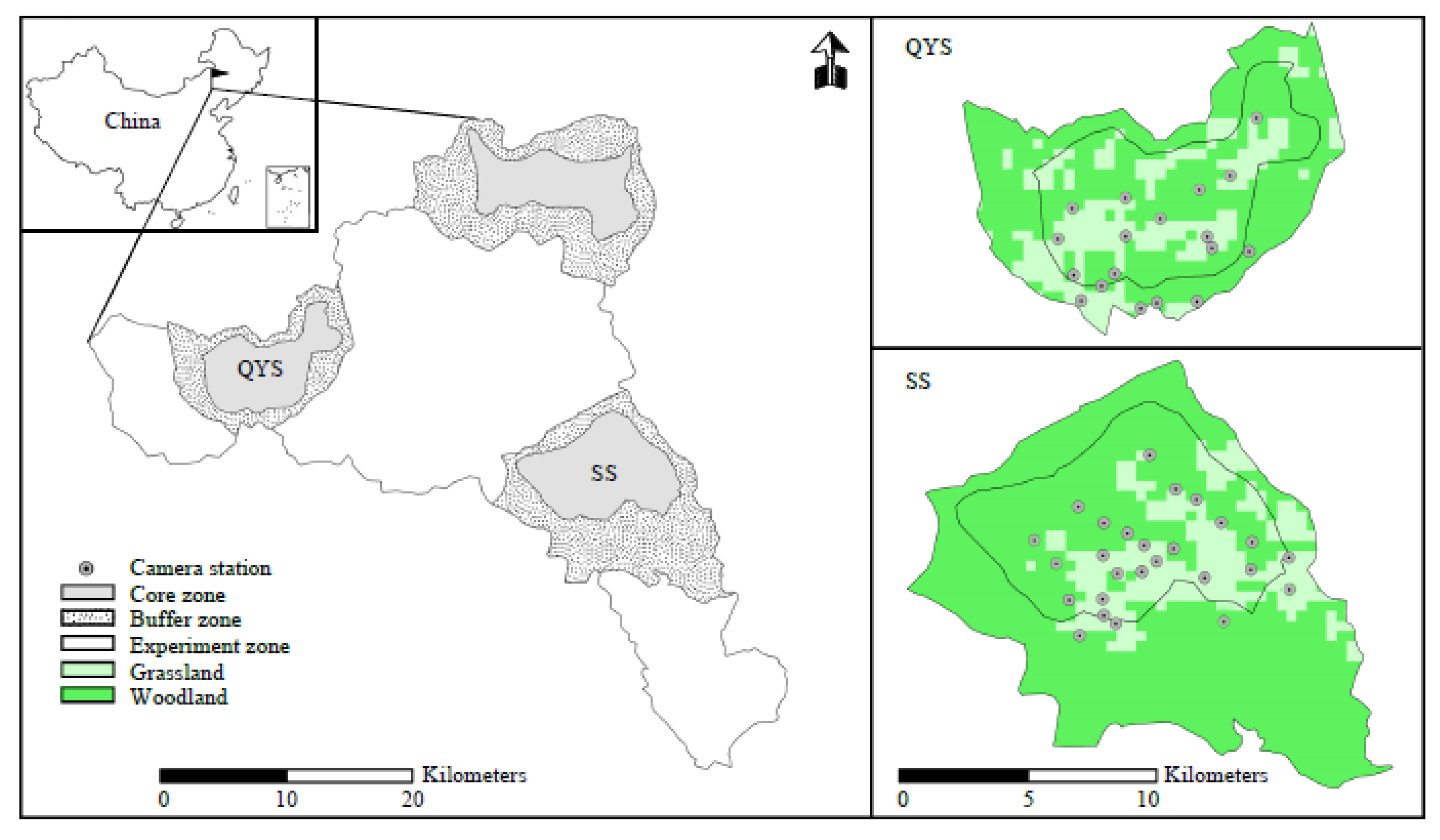
2.1. Study Area
2.2. Camera Trapping Deployment and Data Analyses
3. Results
3.1. Population Abundance
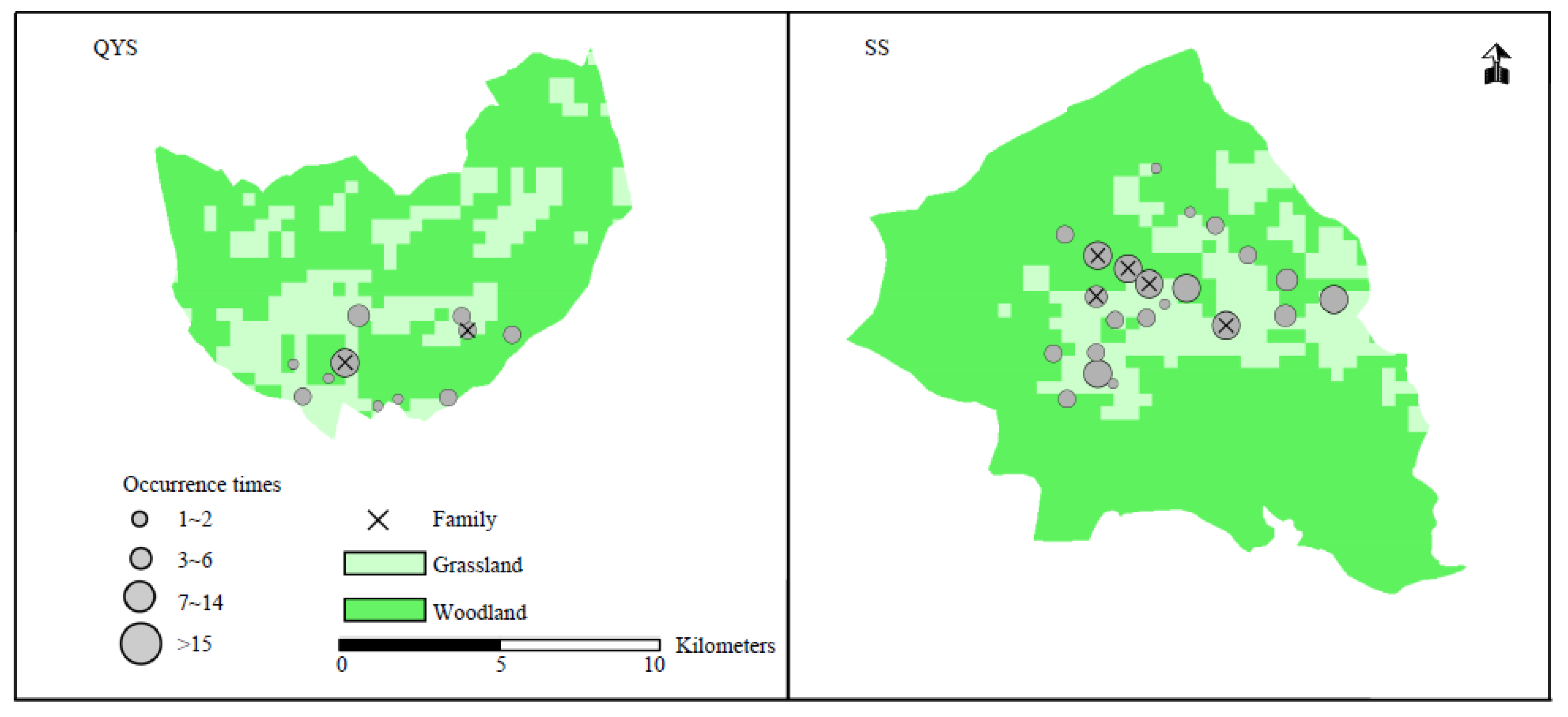
3.2. Distribution of Lynx
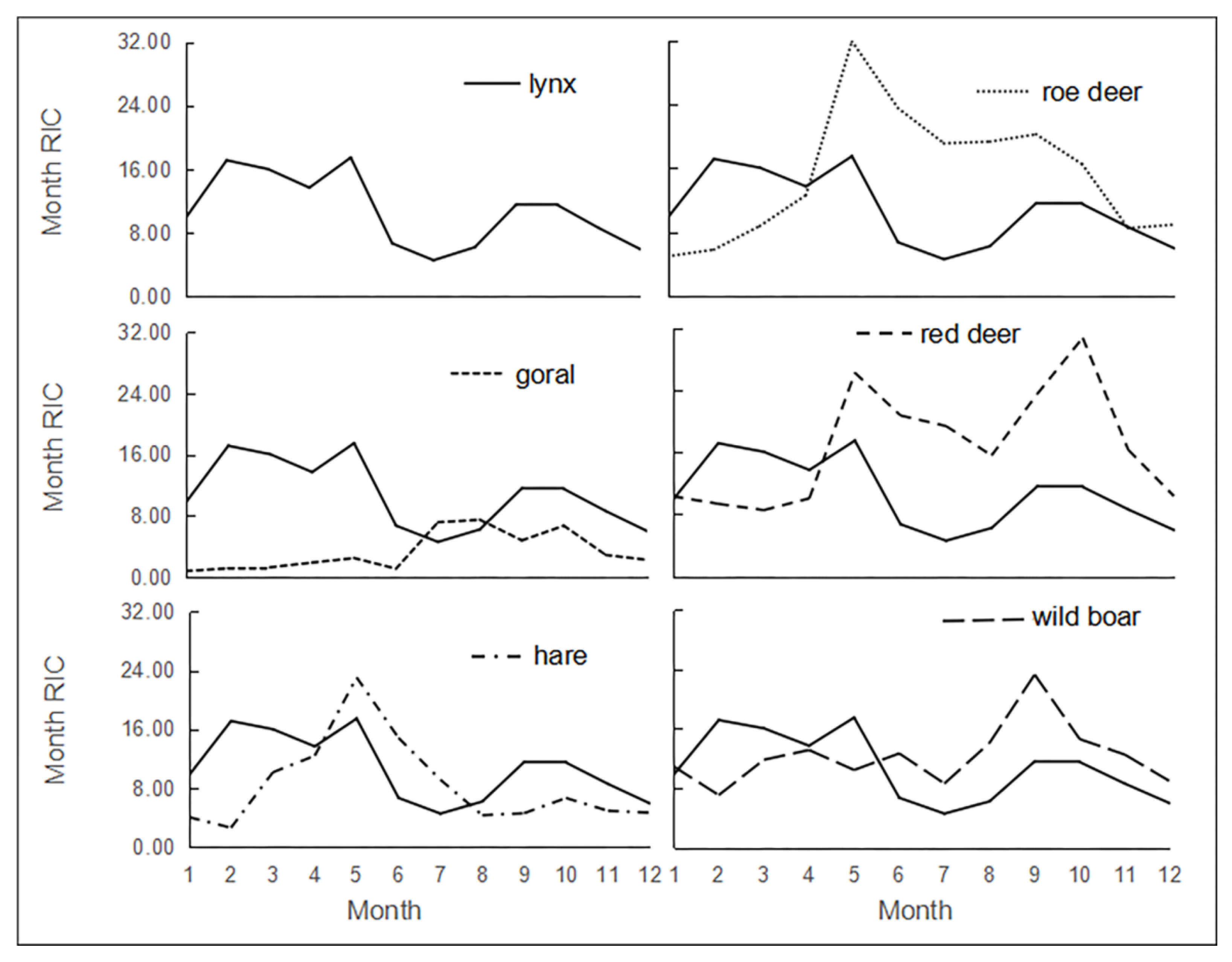
3.3. Activity Rhythm of Lynx
3.3.1. Monthly Activity Rhythm
3.3.2. Daily Activity Rhythm
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harmsen, B.J.; Foster, R.J.; Silver, S.C.; Ostro, L.E.T.; Doncaster, C.P. Jaguar and puma activity patterns in relation to their main prey. Mamm. Biol. 2011, 76, 320–324. [Google Scholar] [CrossRef]
- Sunquist, M.; Sunquist, F. Wild Cats of the World; University of Chicago Press: Chicago, IL, USA, 2002; p. 452. [Google Scholar]
- Griffiths, M.; Van Schaik, C.P. Camera-trapping: A new tool for the study of elusive rain forest animals. Trop. Biodivers. 1993, 1, 131–135. [Google Scholar]
- Karanth, K.U.; Chundawat, R.S.; Nichols, J.D.; Kumar, N.S. Estimation of tiger densities in the tropical dry forests of Panna, Central India, using photographic capture–recapture sampling. Anim. Conserv. 2004, 7, 285–290. [Google Scholar] [CrossRef]
- Eléanor, B.; Daniel, P. Trapping Elusive Cats: Using Intensive Camera Trapping to Estimate the Density of a Rare African Felid. PLoS ONE 2015, 10, e0142508. [Google Scholar]
- Buzzard, P.J.; MaMing, R.; Turghan, M.; Xiong, J.; Zhang, T. Presence of the snow leopard Panthera uncia confirmed at four sites in the Chinese Tianshan Mountains. Oryx 2017, 51, 594–596. [Google Scholar] [CrossRef]
- Defeng, B.; Pengju, C.; Luciano, A.; Lhaba, C.; Qian, L.; Kun, S. Assessment of habitat suitability of the snow leopard (Panthera uncia) in Qomolangma National Nature Reserve based on MaxEnt modeling. Zool. Res. 2018, 39, 373–386. [Google Scholar] [CrossRef]
- Buzzard, P.J.; Xueyon, L.; Bleisch, W.V. The status of snow leopards Panthera uncia, and high altitude use by common leopard P. pardus, in north-west Yunnan, China. Oryx 2017, 51, 587–589. [Google Scholar] [CrossRef]
- Chen, P.; Gao, Y.; Wang, J.; Pu, Q.; Lhaba, C.; Hu, H.; Xu, J.; Shi, K. Status and conservation of the Endangered snow leopard Panthera uncia in Qomolangma National Nature Reserve, Tibet. Oryx 2016, 51, 590–593. [Google Scholar] [CrossRef]
- Li, Z.; Wang, T.; Smith, J.L.D.; Feng, R.; Feng, L.; Mou, P.; Ge, J. Coexistence of two sympatric flagship carnivores in the human-dominated forest landscapes of Northeast Asia. Landsc. Ecol. 2019, 34, 291–305. [Google Scholar] [CrossRef]
- Yang, H.; Han, S.; Xie, B.; Mou, P.; Kou, X.; Wang, T.; Ge, J.; Feng, L. Do prey availability, human disturbance and habitat structure drive the daily activity patterns of Amur tigers (Panthera tigris altaica)? J. Zool. 2018, 307, 131–140. [Google Scholar] [CrossRef]
- Boutros, D.; Breitenmoser-Würsten, C.; Zimmermann, F.; Ryser, A.; Molinari-Jobin, A.; Capt, S.; Güntert, M.; Breitenmoser, U. Characterisation of Eurasian lynx Lynx lynx den sites and kitten survival. Wildl. Biol. 2007, 13, 417–429. [Google Scholar] [CrossRef]
- Podgorski, T.; Schmidt, K.; Kowalczyk, R.; Gulczynska, A. Microhabitat selection by Eurasian lynx and its implications for species conservation. Acta Theriol. 2008, 53, 97–110. [Google Scholar] [CrossRef]
- Kramer-Schadt, S.; Revilla, E.T.; Breitenmoser, U. Fragmented landscapes, road mortality and patch connectivity: Modelling influences on the dispersal of Eurasian lynx. J. Appl. Ecol. 2004, 41, 711–723. [Google Scholar] [CrossRef]
- Schadt, S.; Knauer, F.; Kaczensky, P.; Revilla, E.; Wiegand, T.; Trepl, L. Rule-Based Assessment of Suitable Habitat and Patch Connectivity for the Eurasian Lynx. Ecol. Appl. 2002, 12, 1469. [Google Scholar] [CrossRef]
- Stenseth, N.C.; Falck, W.; Bjornstad, O.N.; Krebs, C.J. Population regulation in snowshoe hare and Canadian lynx: Asymmetric food web configurations between hare and lynx. Proc. Natl. Acad. Sci. USA 1997, 94, 5147–5152. [Google Scholar] [CrossRef] [PubMed]
- Okarma, H.; Jędrzejewski, W.; Schimidt, K. Predation of Eurasian lynx on roe deer and red deer in Białowieża Primeval Forest, Poland. Acta Theriol. 1997, 42, 203–224. [Google Scholar] [CrossRef]
- Molinari-Jobin, A.; Zimmermann, F.; Ryser, A.; Breitenmoser-Würsten, C.; Capt, S.; Breitenmoser, U.; Molinari, P.; Haller, H.; Eyholzer, R. Variation in diet, prey selectivity and home-range size of Eurasian lynx Lynx lynx in Switzerland. Wildl. Biol. 2007, 13, 393–405. [Google Scholar] [CrossRef]
- Schmidt, K. Behavioural and spatial adaptation of the Eurasian lynx to a decline in prey availability. Acta Theriol. 2008, 53, 1–16. [Google Scholar] [CrossRef]
- Vik, J.O.; Brinch, C.N.; Boutin, S.; Stenseth, N.C. Interlinking hare and lynx dynamics using a century’s worth of annual data. Popul. Ecol. 2008, 50, 267–274. [Google Scholar] [CrossRef]
- Breitenmoser, U.; Breitenmoser-Würsten, C.; Okarma, H. Action Plan for the Conservation of the Eurasian Lynx (Lynx lynx) in Europe; Council of Europe: Strasbourg, France, 2000. [Google Scholar]
- Chinese State Forestry Administration. The Resource Survey of State Key Terrestrial Wildlife in China; China Forestry Publishing House: Beijing, China, 2008; pp. 255–257.
- Gao, Y. Fauna Sinica, Mammalia, Volune 8: Carnivora; A.S. Editorial Committee of Fauna Sinica; Science Press: Beijing, China, 1987; pp. 335–336. [Google Scholar]
- Smith, A.T.; Xie, Y. A Guide to the Mammals of China Guide to the Mammals of China; Hunan Education Press: Changsha, China, 2009; p. 375. [Google Scholar]
- Zhou, X.; Bao, Q.; Song, J.; Xia, W.; Menhedaila; Zhang, S.; Yang, Y.; Bao, W. Individual identification and behavior study of Eurasian lynx(Lynx lynx) based on camera trapping technique. J. Biol. 2015, 32, 20–23. [Google Scholar] [CrossRef]
- Tan, S.; Jiang, X.; Zhang, G.; Zhang, J.; Men, H.; Han, Y.; Bao, W. Preliminary Analysis on the Diet Composition of Eurasian Lynx at Saihanwula National Nature Reserve. Chin. J. Zool. 2019, 54, 151–158. [Google Scholar] [CrossRef]
- Zhang, L. Population Ecology of Eurasian (Lynx lynx) in Saihanwual Nature Reserve, Inner Mongolia. Master’s Thesis, Beijing Forestry University, Beijing, China, 2010. [Google Scholar]
- Zhou, X.; Song, J.; Wang, F.; Xia, W.; Wu, L.; Zhang, Z.; Bao, W. The application of extracting DNA from noninvasive sample in feline species identification. Acta Theriol. Sin. 2015, 35, 110–118. [Google Scholar] [CrossRef]
- Abilimit, A. Study on ecology, distribution, resources and protection strategies of Lynx lynx in Xinjiang. Arid Zone Res. 1998, 15, 38–43. [Google Scholar]
- Tian, J.; Li, G.; Sun, H. Current Status and Protection of Felis Lynx Population in the Northeast of China. Spec. Wild Econ. Anim. Plant Res. 2002, 1, 59–61. [Google Scholar] [CrossRef]
- Piao, Z.; Sui, Y.; Cui, Z.; Zhang, G.; Wang, Q.; Fu, X. The History and Current Status of Felid Population in Changbai Mountain Nature Reserve. Chin. J. Zool. 2011, 46, 78–84. [Google Scholar] [CrossRef]
- Yong, S.; Xing, L.; Li, G. Biodivistity of Saihanwula National Natrure Reserve; Inner Mongolia University Press: Huhhot, China, 2011; pp. 1–506. [Google Scholar]
- Azlan, J.M.; Sharma, D.S. The diversity and activity patterns of wild felids in a secondary forest in Peninsular Malaysia. Oryx 2006, 40, 36–41. [Google Scholar] [CrossRef]
- Schmidt, K.; Nakanishi, N.; Izawa, M.; Okamura, M.; Watanabe, S.; Tanaka, S.; Doi, T. The reproductive tactics and activity patterns of solitary carnivores: The Iriomote cat. J. Ethol. 2008, 27, 165. [Google Scholar] [CrossRef]
- Heurich, M.; Hilger, A.; Küchenhoff, H.; Andrén, H.; Bufka, L.; Krofel, M.; Mattisson, J.; Odden, J.; Persson, J.; Rauset, G.R. Activity patterns of Eurasian lynx are modulated by light regime and individual traits over a wide latitudinal range. PLoS ONE 2014, 9, e114143. [Google Scholar] [CrossRef]
- Podolski, I.; Belotti, E.; Bufka, L.; Reulen, H.; Heurich, M. Seasonal and daily activity patterns of free-living Eurasian lynx Lynx lynx in relation to availability of kills. Wildl. Biol. 2013, 19, 69–77. [Google Scholar] [CrossRef]
- Kolbe, J.A.; Squires, J.R. Circadian activity patterns of canada lynx in western Montana. J. Wildl. Manag. 2007, 71, 1607–1611. [Google Scholar] [CrossRef]
- Parker, G.R.; Maxwell, J.W.; Morton, L.D.; Smith, G.E. The ecology of the Lynx (Lynx canadensis) on Cape Breton Island. Can. J. Zool. 1983, 61, 770–786. [Google Scholar] [CrossRef]
- Fedriani, J.M.; Palomares, F.; Delibes, M. Niche relations among three sympatric Mediterranean carnivores. Oecologia 1999, 121, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Bitetti, M.S.D.; Paviolo, A.; De Angelo, C.D. Density, habitat use and activity patterns of ocelots (Leopardus pardalis) in the Atlantic Forest of Misiones, Argentina. Proc. Zool. Soc. Lond. 2010, 270, 153–163. [Google Scholar] [CrossRef]
- Hayward, M.W.; Slotow, R. Temporal partitioning of activity in large african carnivores: Tests of multiple hypotheses. S. Afr. J. Wildl. Res. 2009, 39, 109–125. [Google Scholar] [CrossRef]
- Odden, M.; Wegge, P. Spacing and activity patterns of leopards Panthera pardus in the Royal Bardia National Park, Nepal. Wildl. Biol. 2007, 11, 145–152. [Google Scholar] [CrossRef]
- Zielinski, W.J.; Spencer, W.D.; Barrett, R.H. Relationship between Food Habits and Activity Patterns of Pine Martens. J. Mammal. 1983, 64, 387–396. [Google Scholar] [CrossRef]
- Ferguson, J.W.H.; Galpin, J.S.; Wet, M.J.D. Factors affecting the activity patterns of black-backed jackals Canis mesomelas. J. Zool. 1988, 214, 55–69. [Google Scholar] [CrossRef]
- Keim, J.L.; Lele, D.W.R. Predators choose prey over prey habitats: Evidence from a lynx-hare system. Ecol. Appl. 2011, 21, 1011–1016. [Google Scholar] [CrossRef]
- Sidorovich, V.E. Relationship between prey availability and population dynamics of the Eurasian lynx and its diet in northern Belarus. Acta Theriol. 2006, 51, 265–274. [Google Scholar] [CrossRef]
- Kauhala, K.; Helle, P. The interactions of predator and hare populations in Finland—A study based on wildlife monitoring counts. Ann. Zool. Fenn. 2000, 37, 151–160. [Google Scholar]
- Remmen, J. Risk of Lynx Predation in Roe Deer Depends on Habitat Structure. Master’s Thesis, Norwegian University of Life Sciences, Oslo, Norway, 2012. [Google Scholar]
- Krofel, M.; Jerina, K.; Kljun, F.; Kos, I.; Potočnik, H.; Ražen, N.; Zor, P.; Žagar, A. Comparing patterns of human harvest and predation by Eurasian lynx Lynx lynx on European roe deer Capreolus capreolus in a temperate forest. Eur. J. Wildl. Res. 2014, 60, 11–21. [Google Scholar] [CrossRef]
- Odden, J.; Linnell, J.D.C.; Andersen, R. Diet of Eurasian lynx, Lynx lynx, in the boreal forest of southeastern Norway: The relative importance of livestock and hares at low roe deer density. Eur. J. Wildl. Res. 2006, 52, 237–244. [Google Scholar] [CrossRef]
- Krofel, M.; Huber, D.; Kos, I. Diet of Eurasian lynx Lynx lynx in the northern Dinaric Mountains (Slovenia and Croatia). Acta Theriol. 2011, 56, 315–322. [Google Scholar] [CrossRef]
- Beltran, J.; Delibes, M. Environmental determinants of cireadian activity of free-ranging Iberian lynxes. J. Mammal. 1994, 75, 382–393. [Google Scholar] [CrossRef]
- Schmidt, K. Variation in daily activity of the free-living Eurasian lynx (Lynx lynx) in Białowieża Primeval Forest, Poland. Proc. Zool. Soc. Lond. 1999, 249, 417–425. [Google Scholar]
- Greenville, A.C.; Wardle, G.M.; Tamay, B.; Dickman, C.R. Bottom-up and top-down processes interact to modify intraguild interactions in resource-pulse environments. Oecologia 2014, 175, 1349–1358. [Google Scholar] [CrossRef]
- Helldin, J.O.; Liberg, O.; Glöersen, G. Lynx (Lynx lynx) killing red foxes (Vulpes vulpes) in boreal Sweden ? frequency and population effects. J. Zool. 2006, 270, 657–663. [Google Scholar] [CrossRef]
- Jobin, A.; Molinari, P.; Breitenmoser, U. Prey spectrum, prey preference and consumption rates of Eurasian lynx in the Swiss Jura Mountains. Acta Theriol. 2000, 45, 243–252. [Google Scholar] [CrossRef]
- Zhang, Y. Food Habits and Habitat Selection of Five Canivoras in Saihanwula Nature Reserve. Master’s Thesis, Beijing Forestry University, Beijing, China, 2008. [Google Scholar]
- Palomares, F.; Ferreras, P.; Fedriani, J.M.; Delibes, M. Spatial relationships between Iberian lynx and other carnivores in an area of south-western Spain. J. Appl. Ecol. 1996, 33, 5–13. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, A.; Yuan, L.; Bao, W.; Yang, Y. Preliminary comparison of diet composition of four small sized carnivores at Saihanwula nature reserve, Inner Mongolia. Acta Theriol. Sin. 2011, 31, 1–8. [Google Scholar] [CrossRef]

| Year | Trap Days | IC | Yearly RIC | Min. Numbers in SS | Min. Numbers in QYS | No. of Groups |
|---|---|---|---|---|---|---|
| 2014 | 8357 | 94 | 11.25 | ≥5 | ≥7 | 5 |
| 2015 | 7606 | 61 | 8.02 | ≥7 | ≥1 | 0 |
| 2016 | 5395 | 68 | 12.60 | ≥5 | ≥3 | 1 |
| 2017 | 8534 | 120 | 14.06 | ≥5 | ≥6 | 4 |
| Total | 29,892 | 343 | 11.47 | ≥10 | ≥10 | 10 |
| Seasons | Roe Deer | Goral | Red Deer | Hare | Wild Boar | Badger | Raccoon Dog | Red Fox | |
|---|---|---|---|---|---|---|---|---|---|
| Spring | r | −0.311 | −0.229 | 0.588 ** | 0.847 ** | 0.620 ** | |||
| p | 0.139 | 0.281 | 0.002 | 0.000 | 0.001 | ||||
| Summer | r | 0.212 | 0.383 | 0.212 | 0.135 | 0.256 | |||
| p | 0.321 | 0.065 | 0.321 | 0.528 | 0.227 | ||||
| Autumn | r | 0.507 * | 0.109 | 0.507 * | 0.400 | 0.856 ** | |||
| p | 0.011 | 0.611 | 0.011 | 0.053 | 0.000 | ||||
| Winter | r | −0.421 * | −0.180 | 0.132 | 0.460 * | 0.098 | |||
| p | 0.041 | 0.400 | 0.537 | 0.024 | 0.648 | ||||
| Total | r | −0.220 | 0.128 | 0.644 ** | 0.712 ** | 0.563 ** | 0.669 ** | 0.730 ** | −0.059 |
| p | 0.302 | 0.552 | 0.001 | 0.000 | 0.004 | 0.000 | 0.000 | 0.784 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, X.; Tang, S.; Li, X.; Menghe, D.; Bao, W.; Xiang, C.; Gao, F.; Bao, W. A Study of Population Size and Activity Patterns and Their Relationship to the Prey Species of the Eurasian Lynx Using a Camera Trapping Approach. Animals 2019, 9, 864. https://doi.org/10.3390/ani9110864
Tang X, Tang S, Li X, Menghe D, Bao W, Xiang C, Gao F, Bao W. A Study of Population Size and Activity Patterns and Their Relationship to the Prey Species of the Eurasian Lynx Using a Camera Trapping Approach. Animals. 2019; 9(11):864. https://doi.org/10.3390/ani9110864
Chicago/Turabian StyleTang, Xiaoming, Shupei Tang, Xiaoyu Li, Dalai Menghe, Wuliji Bao, Changlin Xiang, Fuli Gao, and Weidong Bao. 2019. "A Study of Population Size and Activity Patterns and Their Relationship to the Prey Species of the Eurasian Lynx Using a Camera Trapping Approach" Animals 9, no. 11: 864. https://doi.org/10.3390/ani9110864
APA StyleTang, X., Tang, S., Li, X., Menghe, D., Bao, W., Xiang, C., Gao, F., & Bao, W. (2019). A Study of Population Size and Activity Patterns and Their Relationship to the Prey Species of the Eurasian Lynx Using a Camera Trapping Approach. Animals, 9(11), 864. https://doi.org/10.3390/ani9110864





