The Effect of Behaviour and Diet on the Rumen Temperature of Holstein Bulls
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study one: Bulls at Grass
2.1.1. Animals and Rumen Temperature
2.1.2. Observations
2.1.3. Activity Monitoring
2.2. Study Two: Bulls Housed
2.2.1. Animals and Rumen Temperature
2.2.2. Observations
2.3. Statistical Analysis
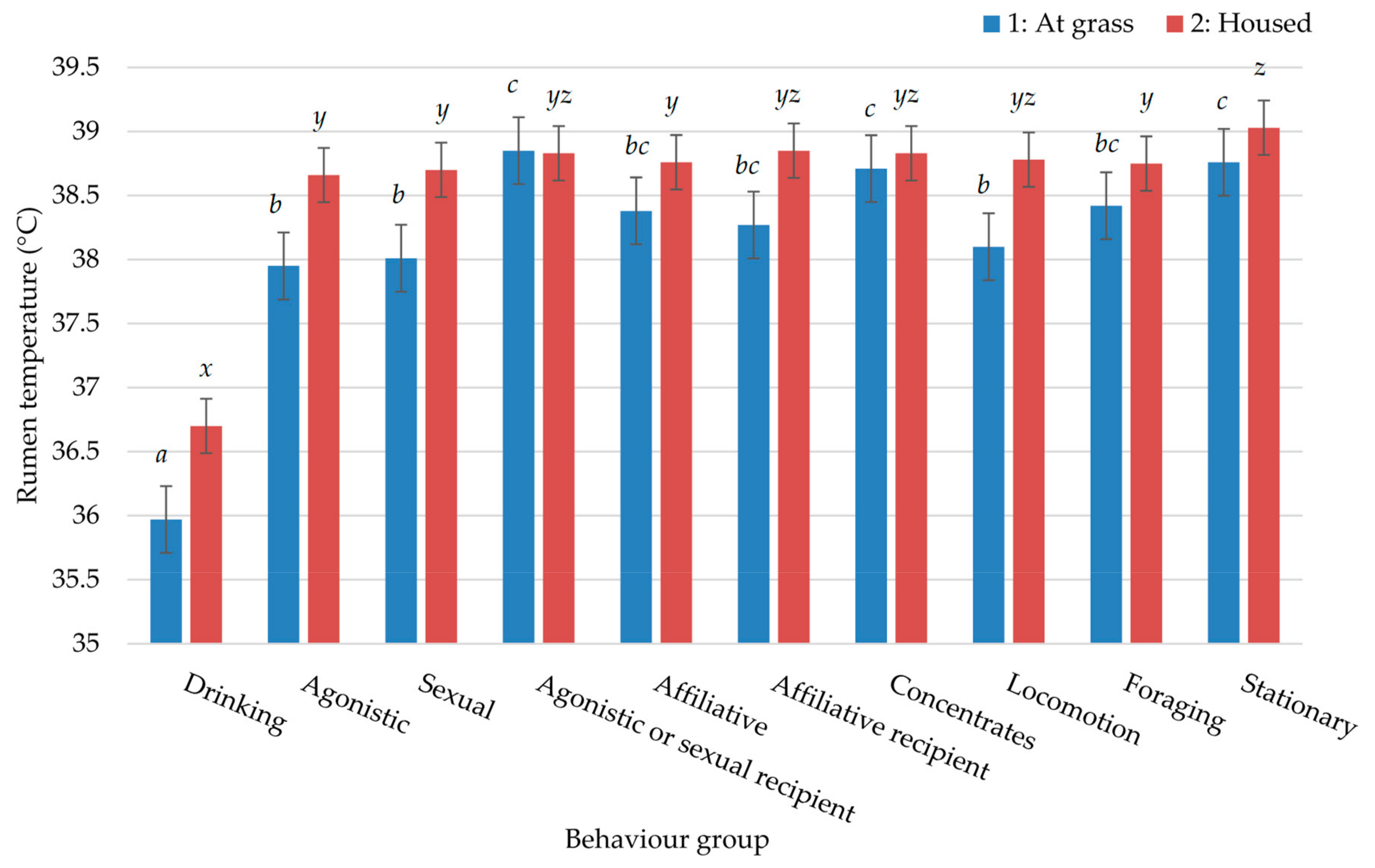
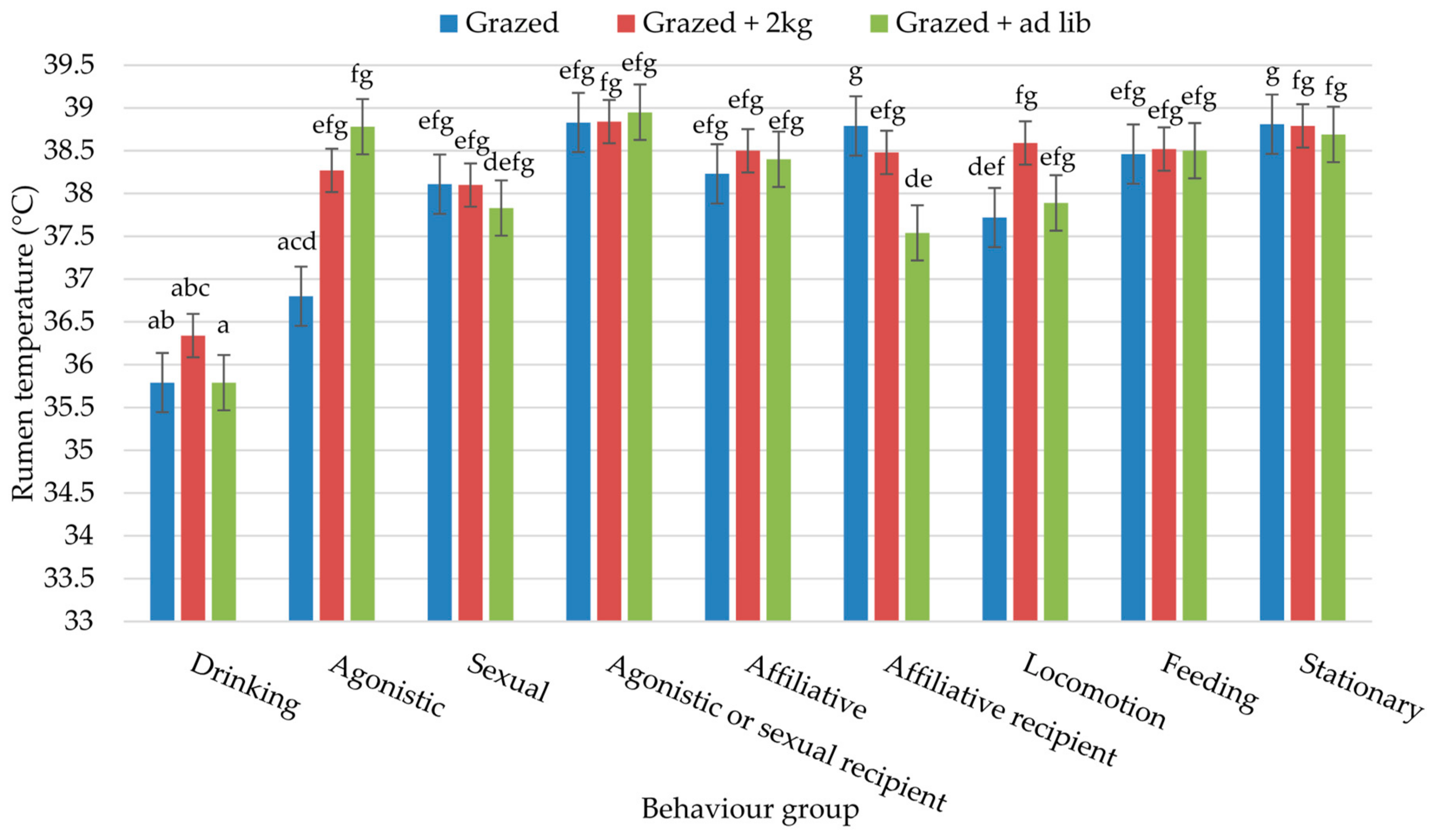
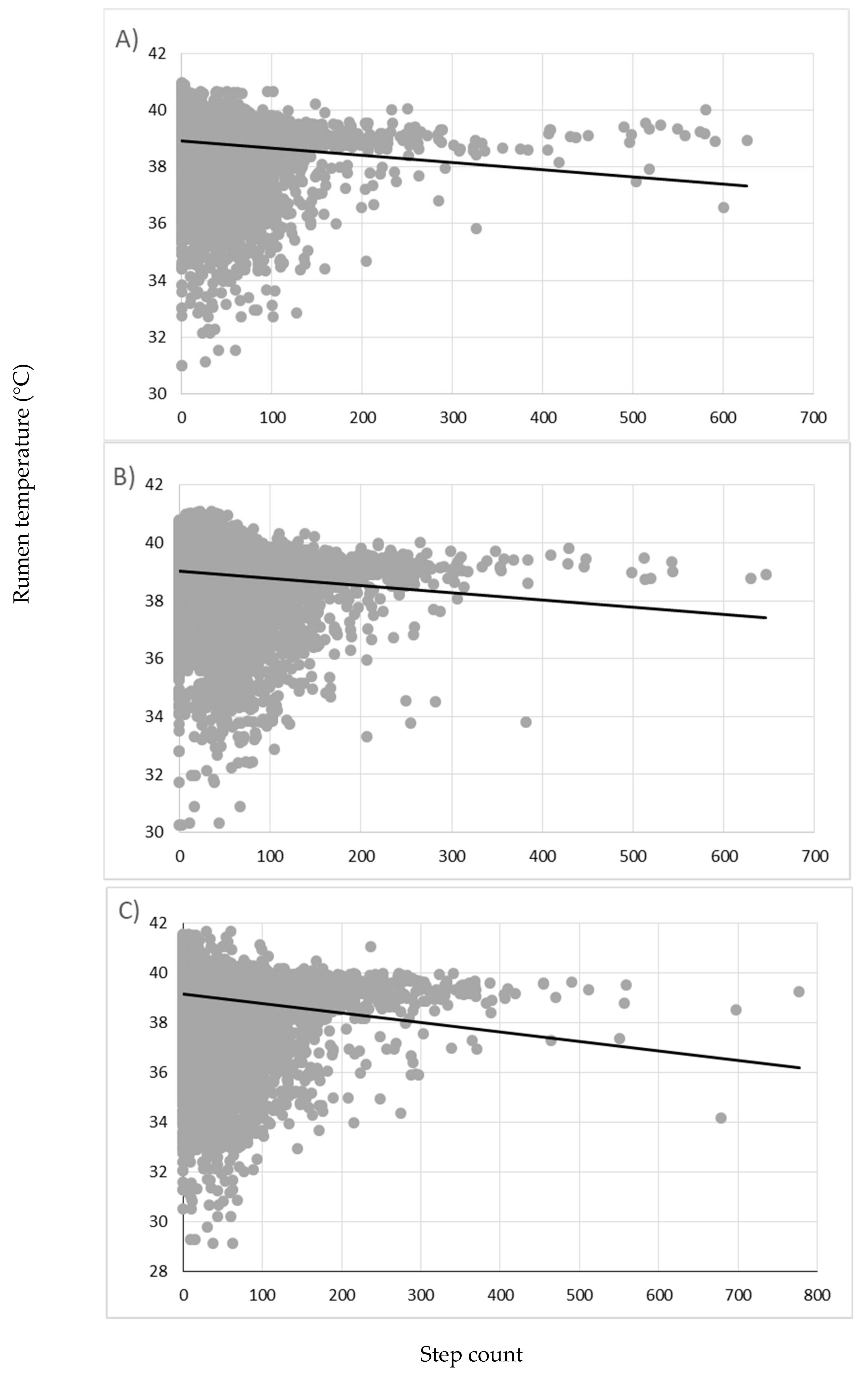
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Burfeind, O.; Keyserlingk, M.A.G.V.; Weary, D.M.; Veira, D.M.; Heuwieser, W. Short communication: Repeatability of measures of rectal temperature in dairy cows. J. Dairy Sci. 2010, 93, 624–627. [Google Scholar] [CrossRef]
- Rose-Dye, T.K.; Burciaga-Robles, L.O.; Krehbiel, C.R.; Step, D.L.; Fulton, R.W.; Confer, A.W.; Richards, C.J. Rumen temperature change monitored with remote rumen temperature boluses after challenges with bovine viral diarrhea virus and Mannheimia haemolytica. J. Anim. Sci. 2011, 89, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Buckham Sporer, K.R.; Weber, P.S.D.; Burton, J.L.; Earley, B.; Crowe, M.A. Transportation of young beef bulls alters circulating physiological parameters that may be effective biomarkers of stress. J. Anim. Sci. 2008, 86, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Timsit, E.; Assié, S.; Quiniou, R.; Seegers, H.; Bareille, N. Early detection of bovine respiratory disease in young bulls using reticulo-rumen temperature boluses. Vet. J. 2011, 190, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, N.H.; Lively, F.O.; Arnott, G. Evaluation of rumen temperature as a novel indicator of meat quality: Rumen temperature, and haematological indicators of stress during the pre-slaughter period as predictors of instrumental meat quality in bulls. Meat Sci. 2019, 158, 107913. [Google Scholar] [CrossRef]
- Cooper-Prado, M.J.; Long, N.M.; Wright, E.C.; Goad, C.L.; Wettemann, R.P. Relationship of ruminal temperature with parturition and estrus of beef cows. J. Anim. Sci. 2011, 89, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.B.G.; Ahola, J.K.; Weller, Z.D.; Peel, R.K.; Whittier, J.C.; Barcellos, J.O.J. Reticulo-rumen temperature as a predictor of calving time in primiparous and parous Holstein females. J. Dairy Sci. 2016, 99, 4839–4850. [Google Scholar] [CrossRef]
- Bewley, J.M.; Einstein, M.E.; Grott, M.W.; Schutz, M.M. Comparison of Reticular and Rectal Core Body Temperatures in Lactating Dairy Cows. J. Dairy Sci. 2008, 91, 4661–4672. [Google Scholar] [CrossRef]
- Kleen, J.L.; Hooijer, G.A.; Rehage, J.; Noordhuizen, J. Subacute ruminal acidosis (SARA): A review. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2003, 50, 406–414. [Google Scholar] [CrossRef]
- Bewley, J.M.; Grott, M.W.; Einstein, M.E.; Schutz, M.M. Impact of Intake Water Temperatures on Reticular Temperatures of Lactating Dairy Cows. J. Dairy Sci. 2008, 91, 3880–3887. [Google Scholar] [CrossRef]
- Cantor, M.C.; Costa, J.B.G.; Bewley, J.M. Impact of Observed and Controlled Water Intake on Reticulorumen Temperature in Lactating Dairy Cattle. Animals 2018, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- Kilgour, R.J. In pursuit of “normal”: A review of the behaviour of cattle at pasture. Appl. Anim. Behav. Sci. 2012, 138, 1–11. [Google Scholar] [CrossRef]
- Estevez, I.; Andersen, I.-L.; Nævdal, E. Group size, density and social dynamics in farm animals. Appl. Anim. Behav. Sci. 2007, 103, 185–204. [Google Scholar] [CrossRef]
- Jago, J.G.; Cox, N.R.; Bass, J.J.; Matthews, L.R. The Effect of Prepubertal Immunization Against Gonadotropin-Releasing Hormone on the Development of Sexual and Social Behavior of Bulls. J. Anim. Sci. 1997, 75, 2609–2619. [Google Scholar] [CrossRef]
- Galindo, F.; Newberry, R.C.; Mendl, M. Social Conditions. In Animal Welfare; Appleby, M.C., Olsson, J.M., Hughes, B.O., Eds.; CABI International: Wallingford, UK, 2011; pp. 228–245. [Google Scholar]
- Partida, J.A.; Olleta, J.L.; Campo, M.M.; Sanudo, C.; Maria, G.A. Effect of social dominance on the meat quality of young Friesian bulls. Meat Sci. 2007, 76, 266–273. [Google Scholar] [CrossRef]
- Mohan Raj, A.B.; Moss, B.W.; McCaughey, W.J.; McLaughlan, W.; Kilpatrick, D.J.; McCaughey, S.J. Behavioural response to mixing of entire bulls, vasectomised bulls and steers. Appl. Anim. Behav. Sci. 1991, 31, 157–168. [Google Scholar] [CrossRef]
- Gutmann, A.K.; Špinka, M.; Winckler, C. Long-term familiarity creates preferred social partners in dairy cows. Appl. Anim. Behav. Sci. 2015, 169, 1–8. [Google Scholar] [CrossRef]
- Val-Laillet, D.; Guesdon, V.; von Keyserlingk, M.A.G.; de Passillé, A.M.; Rushen, J. Allogrooming in cattle: Relationships between social preferences, feedingdisplacements and social dominance. Appl. Anim. Behav. Sci. 2009, 116, 141–149. [Google Scholar] [CrossRef]
- Baldock, N.M.; Sibly, R.M. Effects of handling and transportation on the heart rate and behaviour of sheep. Appl. Anim. Behav. Sci. 1990, 28, 15–39. [Google Scholar] [CrossRef]
- Price, S.; Sibly, R.M.; Davies, M.H. Effects of behaviour and handling on heart rate in farmed red deer. Appl. Anim. Behav. Sci. 1993, 37, 111–123. [Google Scholar] [CrossRef]
- Oka, T.; Oka, K.; Hori, T. Mechanisms and mediators of psychological stress-induced rise in core temperature. Psychosom. Med. 2001, 63, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Wahrmund, J.L.; Ronchesel, J.R.; Krehbiel, C.R.; Goad, C.L.; Trost, S.M.; Richards, C.J. Ruminal acidosis challenge impact on ruminal temperature in feedlot cattle. J. Anim. Sci. 2012, 90, 2794–2801. [Google Scholar] [CrossRef] [PubMed]
- Jansen, A.; Nguyen, X.V. Central command neurons of the sympathetic nervous system: Basis of the fight-or-flight response. Science 1995, 270, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Mounier, L.; Veissier, I.; Boissy, A. Behavior, physiology, and performance of bulls mixed at the onset of finishing to form uniform body weight groups. J. Anim. Sci. 2005, 83, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Miranda-de la Lama, G.C.; Villarroel, M.; María, G.A. Behavioural and physiological profiles following exposure to novel environment and social mixing in lambs. Small Rumin. Res. 2012, 103, 158–163. [Google Scholar] [CrossRef]
- MacKay, J.R.D.; Turner, S.P.; Hyslop, J.; Deag, J.M.; Haskell, M.J. Short-term temperament tests in beef cattle relate to long-term measures of behavior recorded in the home pen. J. Anim. Sci. 2013, 91, 4917–4924. [Google Scholar] [CrossRef]
- Haskell, M.J.; Simm, G.; Turner, S.P. Genetic selection for temperament traits in dairy and beef cattle. Front. Genet. 2014, 5, 18. [Google Scholar] [CrossRef]
- MacKay, J.R.D.; Haskell, M.J. Consistent Individual Behavioral Variation: The Difference between Temperament, Personality and Behavioral Syndromes. Animals 2015, 5, 455–478. [Google Scholar] [CrossRef]
- Voisinet, B.D.; Grandin, T.; Oconnor, S.F.; Tatum, J.D.; Deesing, M.J. Bos indicus cross feedlot cattle with excitable temperaments have tougher meat and a higher incidence of borderline dark cutters. Meat Sci. 1997, 46, 367–377. [Google Scholar] [CrossRef]
- Voisinet, B.D.; Grandin, T.; Tatum, J.D.; O’Connor, S.F.; Struthers, J.J. Feedlot cattle with calm temperaments have higher average daily gains than cattle with excitable temperaments. J. Anim. Sci. 1997, 75, 892–896. [Google Scholar] [CrossRef]
- Fell, L.R.; Colditz, I.G.; Walker, K.H.; Watson, D.L. Associations between temperament, performance and immune function in cattle entering a commercial feedlot. Aust. J. Exp. Agric. 1999, 39, 795–802. [Google Scholar] [CrossRef]
- Burdick, N.C.; Carroll, J.A.; Hulbert, L.E.; Dailey, J.W.; Ballou, M.A.; Randel, R.D.; Willard, S.T.; Vann, R.C.; Welsh, T.H. Temperament influences endotoxininduced changes in rectal temperature, sickness behavior, and plasma epinephrine concentrations in bulls. Innate Immun. 2011, 17, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Burdick, N.C.; Randel, R.D.; Carroll, J.A.; Welsh, T.H. Interactions between temperament, stress, and immune function in cattle. Int. J. Zool. 2011, 2011, 373197. [Google Scholar] [CrossRef]
- Burdick, N.C.; Carroll, J.A.; Hulbert, L.E.; Dailey, J.W.; Willard, S.T.; Vann, R.C.; Welsh, T.H., Jr.; Randel, R.D. Relationships between temperament and transportation with rectal temperature and serum concentrations of cortisol and epinephrine in bulls. Livest. Sci. 2010, 129, 166–172. [Google Scholar] [CrossRef]
- Miranda-de la Lama, G.C.; Pascual-Alonso, M.; Guerrero, A.; Alberti, P.; Alierta, S.; Sans, P.; Gajan, J.P.; Villarroel, M.; Dalmau, A.; Velarde, A.; et al. Influence of social dominance on production, welfare and the quality of meat from beef bulls. Meat Sci. 2013, 94, 432–437. [Google Scholar] [CrossRef]
- Hahn, G.L.; Gaughan, J.B.; Mader, T.L.; Eigenberg, R.A. Thermal Indices and Their Applications for Livestock Environments. In Livestock Energetics and Thermal Environmental Management American Society of Agricultural and Biological Engineer; DeShazer, J.A., Ed.; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2009; pp. 113–130. [Google Scholar]
- Finney, G.; Gordon, A.; Scoley, G.; Morrison, S.J. Validating the IceRobotics IceQube tri-axial accelerometer for measuring daily lying duration in dairy calves. Livest. Sci. 2018, 214, 83–87. [Google Scholar] [CrossRef]
- Ammer, S.; Lambertz, C.; Gauly, M. Is reticular temperature a useful indicator of heat stress in dairy cattle? J. Dairy Sci. 2016, 99, 10067–10076. [Google Scholar] [CrossRef]
- AlZahal, O.; Kebreab, E.; France, J.; Froetschel, M.; McBride, B.W. Ruminal temperature may aid in the detection of subacute ruminal acidosis. J. Dairy Sci. 2008, 91, 202–207. [Google Scholar] [CrossRef]
- Petzold, M.; Meyer, U.; Spilke, J.; Dänicke, S. Using rumen probes to examine effects of conjugated linoleic acids and dietary concentrate proportion on rumen pH and rumen temperature of periparturient dairy cows. J. Anim. Physiol. Anim. Nutr. 2014, 98, 785–796. [Google Scholar] [CrossRef]
- Castro-Costa, A.; Salama, A.A.K.; Moll, X.; Aguiló, J.; Caja, G. Using wireless rumen sensors for evaluating the effects of diet and ambient temperature in nonlactating dairy goats. J. Dairy Sci. 2015, 98, 4646–4658. [Google Scholar] [CrossRef]
- Loholter, M.; Rehage, R.; Meyer, U.; Lebzien, P.; Rehage, J.; Danicke, S. Evaluation of a device for continuous measurement of rumen pH and temperature considering localization of measurement and dietary concentrate proportion. Landbauforschung 2013, 63, 61–68. [Google Scholar]
- Tafaj, M.; Junck, B.; Maulbetsch, A.; Steingass, H.; Piepho, H.P.; Drochner, W. Digesta characteristics of dorsal, middle and ventral rumen of cows fed with different hay qualities and concentrate levels. Arch. Anim. Nutr. 2004, 58, 325–342. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Devillard, E.; Michalet-Doreau, B. Influence of Sampling Site on Concentrations and Carbohydrate-Degrading Enzyme Activities of Protozoa and Bacteria in the Rumen. Am. Soc. Anim. Sci. 1999, 77, 979–987. [Google Scholar] [CrossRef][Green Version]
- Abijaoude, J.A.; Morand-Fehr, P.; Tessier, J.; Schmidely, P.; Sauvant, D. Diet effect on the daily feeding behaviour, frequency and characteristics of meals in dairy goats. Livest. Prod. Sci. 2000, 64, 29–37. [Google Scholar] [CrossRef]
- Jarvis, S.; D’Eath, R.B.; Robson, S.K.; Lawrence, A.B. The effect of confinement during lactation on the hypothalamic-pituitary-adrenal axis and behaviour of primiparous sows. Physiol. Behav. 2006, 87, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Rind, M.I.; Phillips, C.J.C. The effects of group size on the ingestive and social behaviour of grazing dairy cows. Anim. Sci. 1999, 68, 589–596. [Google Scholar] [CrossRef]
- Laister, S.; Stockinger, B.; Regner, A.M.; Zenger, K.; Knierim, U.; Winckler, C. Social licking in dairy cattle—Effects on heart rate in performers and receivers. Appl. Anim. Behav. Sci. 2011, 130, 81–90. [Google Scholar] [CrossRef]
- Winckler, C.; Tucker, C.B.; Weary, D.M. Effects of under- and overstocking freestalls on dairy cattle behaviour. Appl. Anim. Behav. Sci. 2015, 170, 14–19. [Google Scholar] [CrossRef]
- Kenny, F.J.; Tarrant, P.V. The Physiological and Behavioural Responses of crossbred Friesian Steers to Short-haul Transport by Road. Livest. Prod. Sci. 1987, 17, 63–75. [Google Scholar] [CrossRef]
- Nelson, R. Aggression and Social Behavior. In An Introduction to Behavioral Endocrinology; Sinauer Associates Inc.: Sunderland, MA, USA, 1995; Volume 10, pp. 443–484. [Google Scholar]
- Beilharz, R.G.; Zeeb, K. Social dominance in dairy cattle. Appl. Anim. Ethol. 1982, 8, 79–97. [Google Scholar] [CrossRef]
- Gordon, C.J. A review of terms for regulated vs. forced, neurochemical-induced changes in body temperature. Life Sci. 1983, 32, 1285–1295. [Google Scholar] [CrossRef]
- Reith, S.; Brandt, H.; Hoy, S. Simultaneous analysis of activity and rumination time, based on collar-mounted sensor technology, of dairy cows over the peri-estrus period. Livest. Sci. 2014, 170, 219–227. [Google Scholar] [CrossRef]
- Minegishi, K.; Heins, B.J.; Pereira, G.M. Peri-estrus activity and rumination time and its application to estrus prediction: Evidence from dairy herds under organic grazing and low-input conventional production. Livest. Sci. 2019, 221, 144–154. [Google Scholar] [CrossRef]
- Johnson, R.W. The concept of sickness behavior: A brief chronological account of four key discoveries. Vet. Immunol. Immunopathol. 2002, 87, 443–450. [Google Scholar] [CrossRef]

| Rank | Behaviour Group | Behaviour | Description |
|---|---|---|---|
| 1 | Drinking | Drinking | Drinking |
| 2 | Agonistic | Bunt | Lowers its head, then uses the head to sharply strike another animal |
| Head to head pushing | Pushes its head against the head of another individual | ||
| Fight | Continued forceful head to head pushing, results in animals pushing each other off-balance or across the ground | ||
| Pushing at the feed face y | Pushing another animal at the feed face in order to gain access to feed | ||
| 3 | Sexual | Mount intention | Head and shoulders are raised, and weight is shifted to the rear, at least one front hoof remains on the ground |
| Attempt to mount | Both front feet simultaneously leave the ground, but the animal does not become positioned on the mountee’s body | ||
| Mounting | Lifts its forelegs off the ground and rests the chest on the body of another animal | ||
| Sucking | Cross-sucking /drinking urine from another animal | ||
| 4 | Agonistic or sexual recipient | Avoids slowly | Moves away to avoid the aggressor slowly, does not turn towards the aggressor |
| Moves away with speed | Moves away from the aggressor quickly | ||
| Retaliates | Retaliates with an attack (bunt or push) towards the aggressor. No more than two physical responses | ||
| Fights | Retaliates with continued aggressive behaviour. Behaviour is then scored based on agonistic behaviour group | ||
| 5 | Affiliative | Licking | Licking another animal |
| Sniffing | Sniffing another animal | ||
| Touching/rubbing | Touching or rubbing its head against the body of another animal | ||
| Grooming/scratching | Animal grooming itself or scratching on bars in the pen | ||
| 6 | Affiliative recipient | Recipient of an affiliative behaviour | Being sniffed, touched or licked by another animal |
| 7 | Concentrates | Eating concentrates | Eating concentrates |
| 8 | Locomotion | Out of pen y | Cattle out of pen to be weighed |
| Walking | Walking | ||
| Trotting | Trotting | ||
| 9 | Forage | Cantering x | Cantering |
| Grazing x | Eating grass | ||
| Eating silage y | Eating silage | ||
| 10 | Stationary | Environmental exploring | Standing while sniffing, liking and biting an object in the environment |
| Standing | Standing—appears to be doing nothing | ||
| Lying | Lying down—either sleeping or resting |
| Study | 1:At Grass | 2: Housed | ||
|---|---|---|---|---|
| Feedstuff | Grass | Concentrates | Grass Silage | Concentrates |
| DM (g/kg F) | 128 | 946 | 281 | 949 |
| CP (g/kg DM) | 200.0 | - | - | - |
| Water soluble sugars (g/kg DM) | 69.6 | - | - | - |
| ME (MJ/kg DM) | 11.0 | - | - | - |
| ADF (g/kg DM) | 299.0 | 114.6 | 316.4 | 130.5 |
| NDF (g/kg DM) | - | 308.2 | 541.2 | 298.0 |
| Ash (g/kg DM) | - | 63.8 | 113.0 | 83.2 |
| Nitrogen (g/kg DM) | - | 29.5 | 18.8 | 28.2 |
| Behaviour Group | 1: At Grass | 2: Housed | ||
|---|---|---|---|---|
| IR (%) | SED | IR (%) | SED | |
| Drinking | 3.45 | 0.261 | 3.86 | 0.626 |
| Agonistic | 0.80 | 0.093 | 2.61 | 0.595 |
| Sexual | 0.83 | 0.103 | 3.20 | 0.700 |
| Agonistic or sexual recipient | 0.18 | 0.027 | 0.92 | 0.186 |
| Affiliative | 0.94 | 0.100 | 5.84 | 0.569 |
| Affiliative recipient | 0.72 | 0.088 | 1.01 | 0.140 |
| Stationary | 52.25 | 0.859 | 65.11 | 1.809 |
| Locomotion | 0.46 | 0.034 | 1.66 | 0.343 |
| Concentrates | - | - | 6.19 | 0.488 |
| Foraging | - | - | 10.91 | 0.611 |
| Feeding * | 40.63 | 0.896 | - | - |
| Behaviour Group | Study 1: At Grass | Study 2: Housed | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Grazed | Grazed + 2 kg | Grazed + ad Lib | SED | p-Value | 2 kg conc. | 4 kg conc. | ad Lib conc | Housed + ad Lib | SED | p-Value | |
| Drinking | 2.63 | 3.93 | 3.77 | 0.639 | ns | 1.58 | 2.40 | 6.09 | 5.36 | 1.770 | ns |
| Agonistic | 0.57 | 0.70 | 1.13 | 0.229 | ns | 2.69 | 4.38 | 2.25 | 1.12 | 1.682 | ns |
| Sexual | 0.81 | 0.70 | 0.98 | 0.253 | ns | 1.39 | 3.16 | 3.32 | 4.92 | 1.980 | ns |
| Agonistic or sexual recipient | 0.15 | 0.19 | 0.19 | 0.065 | ns | 0.45 | 0.85 | 1.12 | 1.25 | 0.520 | ns |
| Affiliative | 0.88 | 0.69 | 1.24 | 0.244 | ns | 2.95 a | 4.00 a | 3.94 a | 12.47 b | 1.610 | <0.001 |
| Affiliative recipient | 0.57 | 0.55 | 1.02 | 0.216 | ns | 1.51 | 1.28 | 0.40 | 0.85 | 0.395 | ns |
| Stationary | 42.94 a | 46.75 a | 67.05 b | 2.105 | <0.001 | 68.17 | 63.69 | 67.89 | 60.69 | 5.115 | ns |
| Locomotion | 0.23 a | 0.63 b | 0.51 b | 0.083 | <0.001 | 2.22 | 2.48 | 0.65 | 1.29 | 0.969 | ns |
| Concentrates | - | - | - | - | - | 3.04 a | 5.34 ab | 8.40 b | 7.97 b | 1.381 | <0.05 |
| Foraging | - | - | - | - | - | 17.43 b | 13.26 b | 7.58 a | 5.38 a | 1.726 | <0.01 |
| Feeding * | 51.61 c | 46.05 b | 24.24 a | 2.196 | <0.001 | - | - | - | - | - | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutherford, N.H.; Gordon, A.W.; Lively, F.O.; Arnott, G. The Effect of Behaviour and Diet on the Rumen Temperature of Holstein Bulls. Animals 2019, 9, 1000. https://doi.org/10.3390/ani9111000
Rutherford NH, Gordon AW, Lively FO, Arnott G. The Effect of Behaviour and Diet on the Rumen Temperature of Holstein Bulls. Animals. 2019; 9(11):1000. https://doi.org/10.3390/ani9111000
Chicago/Turabian StyleRutherford, Naomi H., Alan W. Gordon, Francis O. Lively, and Gareth Arnott. 2019. "The Effect of Behaviour and Diet on the Rumen Temperature of Holstein Bulls" Animals 9, no. 11: 1000. https://doi.org/10.3390/ani9111000
APA StyleRutherford, N. H., Gordon, A. W., Lively, F. O., & Arnott, G. (2019). The Effect of Behaviour and Diet on the Rumen Temperature of Holstein Bulls. Animals, 9(11), 1000. https://doi.org/10.3390/ani9111000




