Single-Dose Pharmacokinetics and Preliminary Safety Assessment with Use of CBD-Rich Hemp Nutraceutical in Healthy Dogs and Cats
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Dogs
2.2. Cats
2.3. Serum Cannabidiol (CBD) Extraction and Mass Spectrometry Analysis
2.4. CBD Serum Concentration Data Analysis
2.5. Physical Examination
2.6. Data Analysis
3. Results
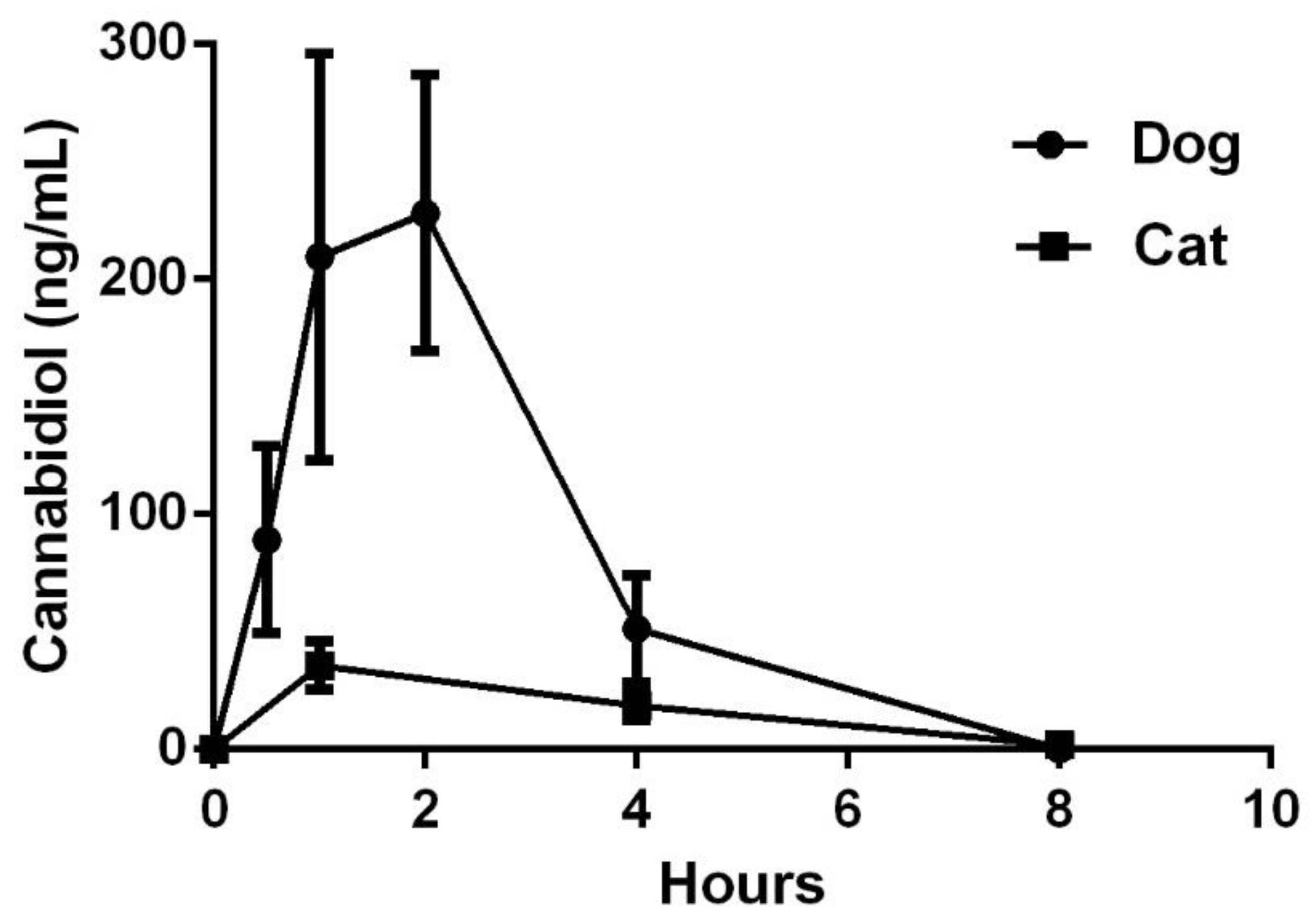
3.1. Pharmacokinetics
3.2. Complete Blood Counts (CBC) and Chemistry
3.3. Physical Examination and Treatment Acceptance
4. Discussions
Author Contributions
Funding
Conflicts of Interest
References
- Kogan, L.R.; Hellyer, P.W.; Robinson, N.G. Consumers perception fo hemp products for animals. J. Am. Holist. Vet. Med. Assoc. 2017, 42, 40–48. [Google Scholar]
- Landa, L.; Sulcova, A.; Gbelec, P. The use of cannabinoids in animals and therapeutic implications for veterinary medicine: A review. Vet. Med. 2016, 61, 111–122. [Google Scholar] [CrossRef]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharm. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703. [Google Scholar] [CrossRef]
- Zhornitsky, S.; Potvin, S. Cannabidiol in humans—The quest for therapeutic targets. Pharmaceuticals 2012, 5, 529–552. [Google Scholar] [CrossRef]
- Yamamoto, I.; Watanabe, K.; Narimatsu, S.; Yoshimura, H. Recent advances in the metabolism of cannabinoids. Int. J. Biochem. Cell Biol. 1995, 27, 741–746. [Google Scholar] [CrossRef]
- Brutlag, A.; Hommerding, H. Toxicology of Marijuana, Synthetic Cannabinoids, and Cannabidiol in Dogs and Cats. Vet. Clin. Small Anim. 2018, 48, 1087–1102. [Google Scholar] [CrossRef]
- Bergamaschi, M.M.; Costa-Querioz, R.H.; Crippa, J.A.S.; Zuardi, A.W. Safety and Side Effects of Cannabidiol, a Cannabis sativa Constituent. Curr. Drug Saf. 2011, 6, 237–249. [Google Scholar] [CrossRef]
- Russo, E.B. The Case for the Entourage Effect and Conventional Breeding of Clinical Cannabis: No Strain, No Gain. Front. Plant Sci. 2019, 9, 1969. [Google Scholar] [CrossRef]
- Ruhaak, L.R.; Felth, J.; Karlsson, P.C.; Rafter, J.J.; Verpoorte, R.; Bohlin, L. Evaluation of the cyclooxygenase inhibiting effects of six major cannabinoids isolated from Cannabis sativa. Biol. Pharm. Bull. 2011, 34, 774–778. [Google Scholar] [CrossRef]
- McGrath, S.; Bartner, L.R.; Rao, S.; Packer, R.A.; Gustafson, D.L. Randomized blinded controlled clinical trial to assess the effect of oral cannabidiol administration in addition to conventional antiepileptic treatment on seizure frequency in dogs with intractable idiopathic epilepsy. J. Am. Vet. Med. Assoc. 2019, 254, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Gamble, L.J.; Boesch, J.M.; Frye, C.W.; Schwark, W.S.; Mann, S.; Wolfe, L.; Brown, H.; Berthelsen, E.S.; Wakshlag, J.J. Pharmacokinetics, safety, and clinical efficacy of cannabidiol treatment in osteoarthritic dogs. Front. Vet. Sci. 2018, 5, 165–172. [Google Scholar] [CrossRef] [PubMed]
- McGrath, S.; Bartner, L.R.; Rao, S.; Kogan, L.R.; Hellyer, P.W. A report of adverse effects associated with the administration of cannabidiol in healthy dogs. J. Am. Holist. Vet. Med. Assoc. 2018, 52, 34–38. [Google Scholar]
- Jiang, R.; Yamaori, S.; Takeda, S.; Yamamoto, I.; Wantanabe, K. Identification of cytochrome P450 enzymes responsible for metabolism of cannabidiol by human liver microsomes. Life Sci. 2011, 89, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Bornheim, L.M.; Correia, M.A. Effect of cannabidiol on cytochrome P-450 isozymes. Biochem. Pharm. 1989, 38, 2789–2794. [Google Scholar] [CrossRef]
- Harvey, D.J.; Samara, E.; Mechoulam, R. Comparative metabolism of cannabidiol in dog, rat and man. Pharmacol. Biochem. Behav. 1991, 40, 523–532. [Google Scholar] [CrossRef]
- Samara, E.; Bialer, M.; Harvey, D.J. Pharmacokinetics of urinary metabolites of cannabidiol in the dog. Biopharm. Drug Dispos. 1990, 11, 785–795. [Google Scholar] [CrossRef]
- Bartner, L.R.; McGrath, S.; Rao, S.; Hyatt, L.K.; Wittenburg, L.A. Pharmacokinetics of cannabidiol administered by 3 delivery methods at 2 different dosages to healthy dogs. Can. J. Vet. Res. 2018, 82, 178–183. [Google Scholar]
- Zgair, A.; Wong, J.C.M.; Sabri, A.; Fischer, P.M.; Barrett, D.A.; Constantinescu, C.S.; Gershkovich, P. Development of a simple and sensitive HPLC-UV method for the simultaneous determination of cannabidiol and Δ(9)-tetrahydrocannabinol in rat plasma. J. Pharm. Biomed. Anal. 2015, 114, 145–151. [Google Scholar] [CrossRef]
- Kirkwood, J.S.; Broeckling, C.D.; Donahue, S.; Prenni, J.E. A novel microflow LC-MS method for the quantitation of endocannabinoids in serum. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1033, 271–277. [Google Scholar] [CrossRef]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef] [PubMed]
- Broccardo, C.J.; Schauer, K.L.; Kohrt, W.M.; Schwartz, R.S.; Murphy, J.P.; Prenni, J.E. Multiplexed analysis of steroid hormones in human serum using novel microflow tile technology and LC–MS/MS. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 934, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Eichler, M.; Spinedi, L.; Unfer-Grauwiler, S.; Bodmer, M.; Surber, C.; Luedi, M.; Drewe, J. Heat exposure of Cannabis sativa extracts affects the pharmacokinetic and metabolic profile in healthy male subjects. Planta Med. 2012, 78, 686–691. [Google Scholar] [CrossRef]
- Millar, S.A.; Stone, N.L.; Yates, A.S.; O’Sullivan, S.E. A Systematic Review on the Pharmacokinetics of Cannabidiol in Humans. Front. Pharmacol. 2018, 9, 1365. [Google Scholar] [CrossRef]
- Taylor, L.; Gidal, B.; Blakey, G.; Tayo, B.; Morrison, G. A Phase I, Randomized, Double-Blind, Placebo-Controlled, Single Ascending Dose, Multiple Dose, and Food Effect Trial of the Safety, Tolerability and Pharmacokinetics of Highly Purified Cannabidiol in Healthy Subjects. CNS Drugs 2018, 32, 1053–1067. [Google Scholar] [CrossRef]
- Perlin, E.; Smith, C.G.; Nichols, A.I.; Almirez, R.; Flora, K.P.; Cradock, J.C.; Peck, C.C. Disposition and bioavailability of various formulation of tetrahydrocannabinol in the Rhesus Monkey. J. Pharm. Sci. 1985, 74, 171–174. [Google Scholar] [CrossRef]
- Huntsman, R.J.; Tang-Wai, R.; Alcorn, J.; Vuong, S.; Acton, B.; Corley, S.; Laprairie, R.; Lyon, A.W.; Meier, S.; Mousseau, D.D.; et al. Dosage Related Efficacy and Tolerability of Cannabidiol in Children with Treatment-Resistant Epileptic Encephalopathy: Preliminary Results of the CARE-E Study. Front. Neurol. 2019, 10, 716. [Google Scholar] [CrossRef]
- Birnbaum, A.K.; Karanam, A.; Marino, S.E.; Barkley, C.M.; Remmel, R.P.; Roslawski, M.; Gramling-Aden, M.; Leppik, I.E. Food effect on pharmacokinetics of cannabidiol oral capsules in adult patients with refractory epilepsy. Epilepsia 2019, 60, 1586–1592. [Google Scholar] [CrossRef]
- Pamplona, F.A.; Da Silva, L.R.; Coan, A.C. Potential Clinical Benefits of CBD-Rich Cannabis Extracts Over Purified CBD in Treatment-Resistant Epilepsy: Observational Data Meta-analysis. Front. Neurol. 2018, 9, 759. [Google Scholar] [CrossRef]
- De Godoy, M.R.C.; McLeod, K.R.; Harmon, D.L. Influence of feeding a fish oil-containing diet to mature, overweight dogs: Effects on lipid metabolites, postprandial glycaemia and body weight. J. Anim. Physiol. Anim. Nutr. 2018, 102, e155–e165. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, C.; Paris, D.; Martella, A.; Melck, D.; Guadagnino, I.; Cawthorne, M.; Motta, A.; Di Marzo, V. Two non-psychoactive cannabinoids reduce intracellular lipid levels and inhibit hepatosteatosis. J. Hepatol. 2015, 62, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Iannotti, F.A.; Pagano, E.; Moriello, A.S.; Alvino, F.G.; Sorrentino, N.C.; D’Orsi, L.; Gazzerro, E.; Capasso, R.; De Leonibus, E.; De Petrocellis, L.; et al. Effects of non-euphoric plant cannabinoids on muscle quality and performance of dystrophic mdx mice. Br. J. Pharmacol. 2018, 176, 1568–1584. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.G.; Santos, V.C.; Levada-Pires, A.C.; Jacintho, T.M.; Gorjão, R.; Pithon-Curi, T.C.; Cury-Boaventura, M.F. Effects of DHA-rich fish oil supplementation on the lipid profile, markers of muscle damage, and neutrophil function in wheelchair basketball athletes before and after acute exercise. Appl. Physiol. Nutr. Metab. 2015, 40, 596–604. [Google Scholar] [CrossRef]
- Nie, B.; Henion, J.; Wakshlag, J. Analysis of Veterinary Hemp-Based Oils for Product Integrity by LC/MS. Cannabis Sci. Technol. 2019, 2, 36–45. [Google Scholar]

| Dog | Cmax (ng/mL) | Tmax (h) | T1/2 el. (h) | AUC 0-t (ng-h/mL) | MRT (h) |
|---|---|---|---|---|---|
| 1 | 266 | 2 | 1.8 | 1494 | 2.6 |
| 2 | 315 | 1 | 0.5 | 1431 | 0.7 |
| 3 | 242 | 1 | 0.8 | 803 | 1.1 |
| 4 | 151 | 2 | 1.1 | 845 | 1.6 |
| 5 | 531 | 1 | 0.8 | 1912 | 1.2 |
| Mean ± SEM | 301 ± 63 | 1.4 ± 0.2 | 1.0 ± 0.2 | 1297 ± 210 | 1.4 ± 0.3 |
| Cat | Cmax (ng/mL) | Tmax (h) | T1/2 elim (h) | AUC 0-t (ng-h/mL) | MRT (h) |
|---|---|---|---|---|---|
| 1 | 75 | 1 | 1.2 | 212 | 2.1 |
| 2 | 41 | 1 | 1.3 | 125 | 2.4 |
| 3 | 53 | 1 | 1.7 | 194 | 2.9 |
| 4 | 21 | 4 | 1.7 | 134 | 5.4 |
| 5 | 20 | 1 | 1.7 | 60 | 2.7 |
| 6 | 48 | 4 | 1.2 | 256 | 5.7 |
| Mean + SE | 43 ± 9 | 2.0 ± 0.6 | 1.5 ± 0.1 | 164 ± 29 | 3.5 ± 1.4 |
| Comp. Blood Count (Ref. Range) ** | Week 0 | Week 4 | Week 8 | Week 12 | p-Value |
|---|---|---|---|---|---|
| WBC (4.0–15.5 × 103/mm3) | 8.4 ± 0.7 | 7.7 ± 0.5 | 7.3 ± 1 | 7.7 ± 0.7 | 0.22 |
| RBC (4.8–9.3 × 106/mm3) | 7.5 ± 0.3 | 7.3 ± 0.2 | 7.9 ± 0.1 | 7.5 ± 0.1 | 0.35 |
| Hb (12.1–20.3 g/dL) | 17.5 ± 0.6 | 17.1 ± 0.5 | 17.9 ± 0.2 | 17.8 ± 0.3 | 0.32 |
| Hct (36–60%) | 54 ± 1 | 53 ± 1 | 57 ± 2 | 51 ± 1 | 0.73 |
| MCV (58–79 μm3) | 73 ± 1 | 72 ± 2 | 73 ± 1 | 69 ± 1 * | <0.01 |
| MCH (19–28 μg) | 24 ± 0 | 23 ± 1 | 23 ± 0 | 24 ± 0 | 0.92 |
| MCHC (30–38 g/dL) | 33 ± 1 | 33 ± 1 | 32 ± 1 | 35 ± 1 | 0.34 |
| Platelets (170–400 × 103/mm3) | 318 ± 18 | 311 ± 15 | 304 ± 11 | 347 ± 19 | 0.16 |
| Neutrophils (2060–10,600/μL) | 5508 ± 567 | 5216 ± 388 | 4889 ± 740 | 5221 ± 586 | 0.64 |
| Lymphocytes (690–4500/μL) | 2347 ± 59 | 1912 ± 65 | 1960 ± 131 | 1904 ± 160 | 0.07 |
| Monocytes (0–840/μL) | 361 ± 20 | 297 ± 36 | 335 ± 66 | 359 ± 70 | 0.88 |
| Eosinophils (0–1200/μL) | 198 ± 11 | 238 ± 36 | 154 ± 23 | 181 ± 22 | 0.28 |
| Serum Chemistry (Ref Range) ** | Week 0 | Week 4 | Week 8 | Week 12 | p-Value |
|---|---|---|---|---|---|
| TP (5.0–7.4 g/dL) | 6.1 ± 0.1 | 5.9 ± 0.2 | 6.3 ± 0.2 | 6.0 ± 0.2 | 0.65 |
| Albumin (2.7–4.4 g/dL) | 3.5 ± 0.1 | 3.5 ± 0.1 | 3.5 ± 0.1 | 3.4 ± 0.1 | 0.22 |
| Globulin (1.6–3.6 g/dL) | 2.6 ± 0.1 | 2.5 ± 0.1 | 2.9 ± 0.1 | 2.6 ± 0.2 | 0.18 |
| AST (15–66 U/L) | 27 ± 2 | 25 ± 2 | 23 ± 2 | 25 ± 1 | 0.45 |
| ALT (12–118 U/L) | 34 ± 3 | 27 ± 2 | 35 ± 10 | 28 ± 3 | 0.57 |
| ALP (5–131 U/L) | 39 ± 6 | 46 ± 7 | 56 ± 10 | 61 ± 13 | 0.09 |
| GGT (1–12 U/L) | 4 ± 0 | 3 ± 0 | 4 ± 0 | 4 ± 0 | 0.72 |
| BUN (6–31 mg/dL) | 11 ± 1 | 10 ± 1 | 11 ± 1 | 11 ± 0 | 0.82 |
| Creatinine (0.5–1.6 mg/dL) | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.5 ± 0.0 | 0.36 |
| Phosphorous (2.5–6.0 mg/dL) | 4.3 ± 0.2 | 4.1 ± 0.2 | 4.2 ± 0.3 | 4.0 ± 0.2 | 0.11 |
| Glucose (70–138 mg/dL) | 97 ± 3 | 92 ± 2 | 102 ± 3 | 99 ± 2 | 0.16 |
| Calcium (8.9–11.4 mg/dL) | 10.4 ± 0.1 | 10.0 ± 0.1 | 10.2 ± 0.1 | 10.1 ± 0.1 | 0.16 |
| Magnesium (1.5–2.5 mEq/L) | 1.6 ± 0.0 | 1.6 ± 0.0 | 1.6 ± 0.0 | 1.6 ± 0.0 | 0.11 |
| Sodium (139–154 mEq/L) | 148 ± 0 | 148 ± 0 | 146 ± 1 | 148 ± 0 | 0.58 |
| Potassium (3.6–5.5 mEq/L) | 4.3 ± 0.1 | 4.4 ± 0.1 | 4.3 ± 0.1 | 4.2 ± 0.0 | 0.23 |
| Chloride (102–120 mEq/L) | 113 ± 0 | 113 ± 1 | 111 ± 1 | 113 ± 1 | 0.74 |
| Cholesterol (92–324 mg/dL) | 182 ± 13 | 203 ± 12 | 211 ± 12 | 212 ± 17 | 0.06 |
| Triglycerides (29–291 mg/dL) | 48 ± 4 | 44 ± 4 | 43 ± 5 | 46 ± 6 | 0.44 |
| Creatine Kinase (59–895 U/L) | 130 ± 16 | 142 ± 43 | 83 ± 5 | 97 ± 5 | 0.10 |
| Comp. Blood Count (Ref. Range) ** | Week 0 | Week 4 | Week 8 | Week 12 | p-Value |
|---|---|---|---|---|---|
| WBC (3.5–16.0 × 103/μL) | 14.0 ± 1.6 | 13.6 ± 1.5 | 12.9 ± 1.3 | 12.5 ± 1.6 | 0.10 |
| RBC (5.9–15.9 × 106/μL) | 8.8 ± 0.2 | 8.0 ± 0.3 | 9.0 ± 0.2 | 9.0 ± 0.3 | 0.22 |
| Hb (9.3–15.9 g/dL) | 11.4 ± 0.4 | 10.7 ± 0.4 | 12.4 ± 0.4 | 11.8 ± 0.5 | 0.06 |
| Hct (29–48%) | 39 ± 1 | 34 ± 1 | 40 ± 1 | 39 ± 2 | 0.48 |
| MCV (37–61 fL) | 44 ± 1 | 42 ± 1 | 44 ±1 | 43 ± 1 | 0.86 |
| MCH (11–21 pg) | 13 ± 0 | 13 ± 1 | 13 ± 1 | 13 ± 1 | 0.16 |
| MCHC (30–38 g/dL) | 30 ± 2 | 32 ± 0 | 31 ± 1 | 31 ± 0 | 0.12 |
| Platelets (200–500 × 103/μL) | 333 ± 31 | 374 ± 30 | 361 ± 20 | 289 ± 19 | 0.08 |
| Neutrophils (2500–8500/μL) | 7980 ± 1081 | 8993 ± 1124 | 7394 ± 1082 | 7847 ± 1349 | 0.70 |
| Lymphocytes (1200–8000/μL) | 4481 ± 518 | 3314 ± 782 | 3856 ± 803 | 3614 ± 1052 | 0.24 |
| Monocytes (0–600/μL) | 428 ± 138 | 416 ± 66 | 545 ± 36 | 364 ± 73 | 0.08 |
| Eosinophils (0–1000/μL) | 1149 ± 148 | 876 ± 139 | 1026 ± 212 | 650 ± 91 * | 0.02 |
| Serum Chemistry (Ref. Range) ** | Week 0 | Week 4 | Week 8 | Week 12 | p-Value |
|---|---|---|---|---|---|
| TP (5.2–8.8 g/dL) | 7.2 ± 0.2 | 6.7 ± 0.2 | 7.1 ± 0.2 | 7.1 ± 0.2 | 0.94 |
| Albumin (2.5–3.9 g/dL) | 3.2 ± 0.1 | 3.2 ± 0.1 | 3.4 ± 0.1 | 3.2 ± 0.1 | 0.65 |
| Globulin (2.3–5.3 g/dL) | 4.0 ± 0.2 | 3.5 ± 0.2 | 3.8 ± 0.2 | 3.9 ± 0.2 | 0.72 |
| AST (10–100 U/L) | 21 ± 2 | 24 ± 4 | 24 ± 3 | 24 ± 3 | 0.17 |
| ALT (10–100 U/L) | 51 ± 5 | 90 ± 30 | 76 ± 17 | 75 ± 15 | 0.29 |
| ALP (6–102 U/L) | 30 ± 5 | 30 ± 6 | 29 ± 5 | 28 ± 6 | 0.53 |
| GGT (1–10 U/L) | 1 ± 0 | 1 ± 0 | 2 ± 0 | 1 ± 0 | 0.91 |
| BUN (14–36 mg/dL) | 23 ± 1 | 22 ± 1 | 20 ± 1 * | 19 ± 1 * | <0.01 |
| Creatinine (0.6–2.4 mg/dL) | 1.3 ± 0.1 | 1.3 ± 0.0 | 1.3 ± 0.1 | 1.3 ± 0.1 | 0.84 |
| Phosphorous (2.4–8.2 mg/dL) | 4.5 ± 0.4 | 4.6 ± 0.4 | 4.3 ± 0.4 | 4.1 ± 0.2 | 0.06 |
| Glucose (64–170 mg/dL) | 90 ± 2 | 85 ± 2 | 88 ± 3 | 86 ± 3 | 0.25 |
| Calcium (8.2–10.8 mg/dL) | 9.6 ± 0.1 | 9.0 ± 0.1 | 9.5 ± 0.2 | 9.3 ± 0.1 | 0.72 |
| Magnesium (1.5–2.5 mEq/L) | 1.9 ± 0.1 | 1.8 ± 0.0 | 1.8 ± 0.0 | 1.8 ± 0.0 | 0.65 |
| Sodium (145–158 mEq/L) | 151 ± 1 | 153 ± 1 | 154 ± 1 | 151 ± 1 | 0.39 |
| Potassium (3.4–5.6 mEq/L) | 4.7 ± 0.2 | 4.7 ± 0.2 | 4.7 ± 0.3 | 4.5 ± 0.1 | 0.30 |
| Chloride (104–128 mEq/L) | 119 ± 1 | 121 ± 1 | 122 ± 1 | 119 ± 1 | 0.33 |
| Cholesterol (75–220 mg/dL) | 139 ± 9 | 123 ± 6 | 128 ± 6 | 123 ± 7 | 0.09 |
| Triglycerides (25–160 mg/dL) | 32 ± 1 | 28 ± 2 | 26 ± 2 * | 25 ± 2 * | 0.02 |
| Creatine Kinase (59–529 U/L) | 197 ± 31 | 113 ± 15 * | 106 ± 12 * | 126 ± 14 * | <0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deabold, K.A.; Schwark, W.S.; Wolf, L.; Wakshlag, J.J. Single-Dose Pharmacokinetics and Preliminary Safety Assessment with Use of CBD-Rich Hemp Nutraceutical in Healthy Dogs and Cats. Animals 2019, 9, 832. https://doi.org/10.3390/ani9100832
Deabold KA, Schwark WS, Wolf L, Wakshlag JJ. Single-Dose Pharmacokinetics and Preliminary Safety Assessment with Use of CBD-Rich Hemp Nutraceutical in Healthy Dogs and Cats. Animals. 2019; 9(10):832. https://doi.org/10.3390/ani9100832
Chicago/Turabian StyleDeabold, Kelly A., Wayne S. Schwark, Lisa Wolf, and Joseph J. Wakshlag. 2019. "Single-Dose Pharmacokinetics and Preliminary Safety Assessment with Use of CBD-Rich Hemp Nutraceutical in Healthy Dogs and Cats" Animals 9, no. 10: 832. https://doi.org/10.3390/ani9100832
APA StyleDeabold, K. A., Schwark, W. S., Wolf, L., & Wakshlag, J. J. (2019). Single-Dose Pharmacokinetics and Preliminary Safety Assessment with Use of CBD-Rich Hemp Nutraceutical in Healthy Dogs and Cats. Animals, 9(10), 832. https://doi.org/10.3390/ani9100832




