The Effect of Substitution of Palm Fat with Linseed Oil on the Lipid Peroxidation, Antioxidative Capacity and Intestinal Morphology in Rabbits (Oryctolagus cuniculus)
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets
2.2. Animals
2.3. Tissue Sampling
2.4. Malondialdehyde (MDA) Determination
2.5. Antioxidative Capacity of Plasma and in Tissue and Content of Small Intestine
2.6. Histologic Measurements
2.7. Statistical Analysis
3. Results
3.1. Ingredients and Chemical Composition of the Diets
3.2. Oxidative Status of Rabbits
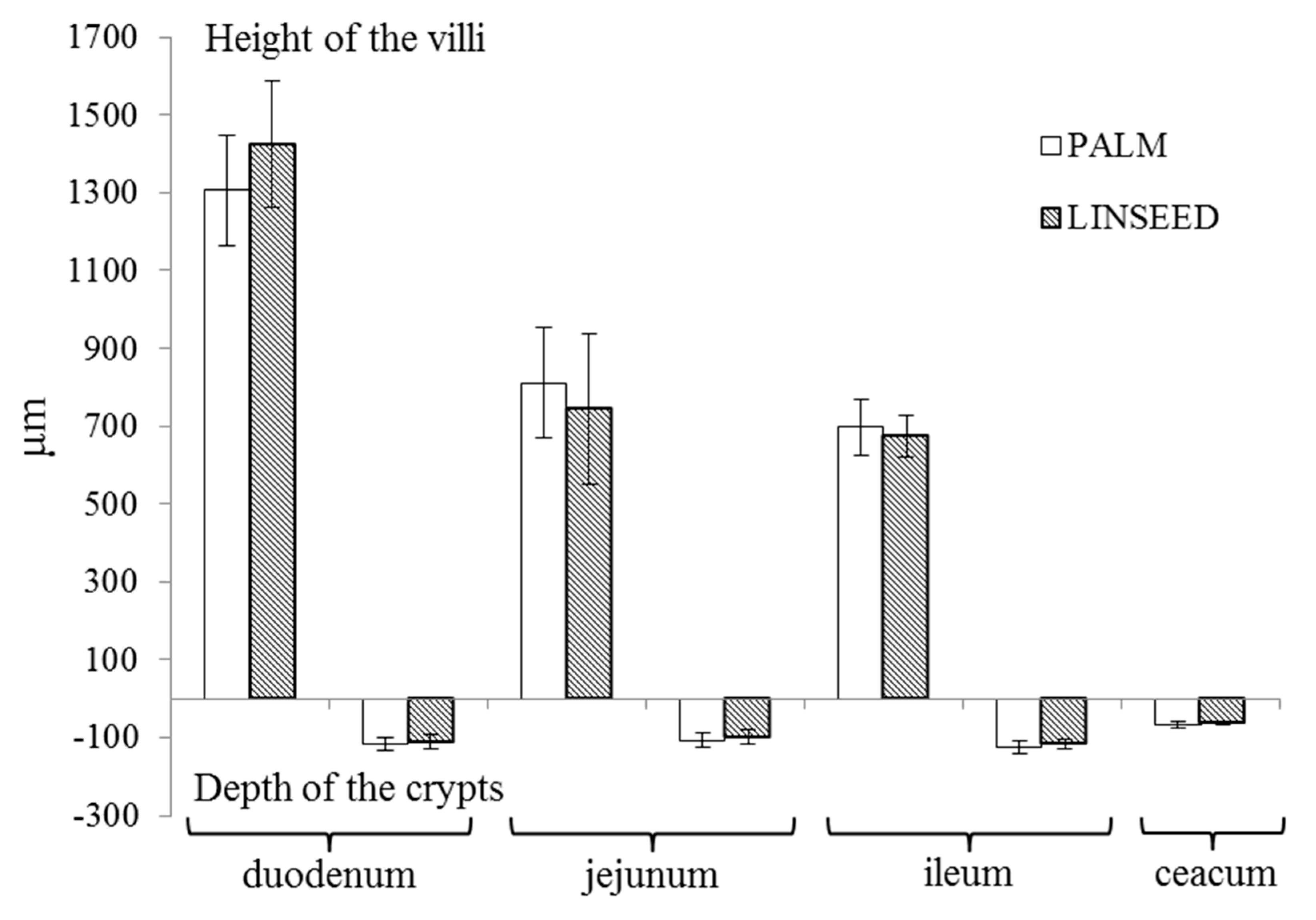
3.3. Intestinal Morphology
4. Discussion
4.1. Oxidative Stress and Antioxidant Capacity
4.2. Histology
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Williams, C.M. Dietary fatty acids and human health. Ann. Zootech. 2000, 49, 165–180. [Google Scholar] [CrossRef]
- Riediger, N.D.; Othman, R.A.; Suh, M.; Moghadasian, M.H. A systemic review of the roles of n-3 fatty acids in health and disease. J. Am. Diet. Assoc. 2009, 109, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002, 56, 365–379. [Google Scholar] [CrossRef]
- Kermauner, A.; Žgur, S. Nutrition and carcass quality in rabbits. In Proceedings of the 12th conference on nutrition of domestic animals “Zadravec-Erjavec days”, Radenci, Slovenia, 6–7 November 2003; pp. 193–204. [Google Scholar]
- Kermauner, A. Fiziologija prebave kuncev. Sodob. Kmet. 1994, 27, 358–366. [Google Scholar]
- Grün, P. Reja Kuncev; Kmečki Glas: Ljubljana, Slovenia, 2002. [Google Scholar]
- Maertens, L.; Huyghebaert, G.; De Groote, G. Digestibility and digestible energy content of various fats for growing rabbits. Cuni-Sciences 1986, 3, 7–14. [Google Scholar]
- Hernández, P. Enhancement of nutritional quality and safety in rabbit meat. In Proceedings of the 9th World Rabbit Congress, Verona, Italy, 10–13 June 2008; Xiccato, G., Trocina, A., Lukefahr, S.D., Eds.; Fondazione Iniziative Zooprofilattiche e Zootecniche: Brescia, Italy, 2008; pp. 1278–1299. [Google Scholar]
- Dalle Zotte, A. Perception of rabbit meat quality and major factors influencing the rabbit carcass and meat quality. Livest. Prod. Soc. 2002, 75, 11–32. [Google Scholar] [CrossRef]
- Trebušak, T.; Levart, A.; Salobir, J.; Pirman, T. Effect of Ganoderma lucidum (Reishi mushroom) or Olea europaea (olive) leaves on oxidative stability of rabbit meat fortified with n-3 fatty acids. Meat Sci. 2014, 96, 1275–1280. [Google Scholar] [CrossRef]
- Gray, J.I.; Gomaa, E.A.; Buckley, D.J. Oxidative quality and shelf life of meats. Meat Sci. 1996, 43, S111–S123. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, S.; Samaraweera, H.; Lee, E.J.; Ahn, D.U. Improving functional value of meat products. Meat Sci. 2010, 86, 15–31. [Google Scholar] [CrossRef]
- Voljč, M.; Frankič, T.; Levart, A.; Nemec, M.; Salobir, J. Evaluation of different vitamin E recommendations and bioactivity of α-tocopherol isomers in broiler nutrition by measuring oxidative stress in vivo and the oxidative stability of meat. Poult. Sci. 2011, 90, 1478–1488. [Google Scholar] [CrossRef]
- Castellini, C.; Dal Bosco, A.; Bernardini, M.; Cyril, H.W. Effect of dietary vitamin E on the oxidative stability of raw and cooked rabbit meat. Meat Sci. 1998, 50, 153–161. [Google Scholar] [CrossRef]
- Corino, C.; Pastorelli, G.; Pantaleo, L.; Oriani, G.; Salvatori, G. Improvement of color and lipid stability of rabbit meat by dietary supplement with vitamin E. Meat Sci. 1999, 52, 285–289. [Google Scholar] [CrossRef]
- Dal Bosco, A.; Castellini, C.; Bianchi, L.; Mugnai, C. Effect of dietary α-linolenic acid and vitamin E on the fatty acid composition, storage, stability and sensory traits of rabbit meat. Meat Sci. 2004, 66, 407–413. [Google Scholar] [CrossRef]
- Tres, A.; Bou, R.; Codony, R.; Guardiola, F. Dietary n-6- or n-3-rich vegetable fats and α-tocopheryl acetate: Effects on fatty acid composition and stability of rabbit plasma, liver and meat. Animal 2009, 3, 1408–1419. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiccato, G. Fat Digestion. In Nutrition of the Rabbit, 2nd ed.; de Blas, C., Wiseman, J., Eds.; CABI, Wallingford: Cambridge, MA, USA, 2010; pp. 56–64. [Google Scholar]
- Incharoen, T.; Yamauchi, K.; Erikawa, T.; Gotoh, H. Histology of intestinal villi and epithelial cell in shickene low-crude protein or low crude fat-diets. Ital. J. Anim. Sci. 2010, 9, 429–434. [Google Scholar] [CrossRef]
- Goda, T.; Takase, S. Effect of dietary fat content on microvillus in rat jejunum. J. Nutr. Sci. Vitaminol. 1994, 40, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Voljč, M.; Levart, A.; Žgur, S.; Salobir, J. The effect of α-tocopherol, sweet chestnut wood extract and their combination on oxidative stress in vivo and the oxidative stability of meat in broilers. Brit. Poult. Sci. 2013, 54, 144–156. [Google Scholar] [CrossRef]
- Frankič, T.; Salobir, J. In vivo antioxidant potential of Sweet chestnut (Castanea sativa Mill.) wood extract in young growing pigs exposed to n-3 PUFA-induced oxidative stress. J. Sci. Food Agric. 2011, 91, 1432–1439. [Google Scholar]
- Leskovec, J.; Rezar, V.; Nemec Svete, A.; Salobir, J.; Levart, A. Antioxidative Effects of Olive Polyphenols Compared to Vitamin E in Piglets Fed a Diet Rish in N-3 PUFA. Animals 2019, 9, 161. [Google Scholar] [CrossRef]
- AOAC. Official methods of Analysisi of AOAC International. In AOAC International; Horwith, W., Ed.; AOAC: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Park, P.W.; Goins, R.E. In situ preparation of fatty acid methyl esters for analysis of fatty acid composition in foods. J. Food Sci. 1994, 59, 1262–1266. [Google Scholar] [CrossRef]
- Wong, S.H.Y.; Knight, J.A.; Hopfer, S.M.; Zaharia, O.; Leach, C.N.; Sunderman, F.W. Lipoperoxides in plasma as measured by liquid-chromatographic separation of malondialdehyde-thiobarbituric acid adduct. Clin. Chem. 1987, 33, 214–220. [Google Scholar] [PubMed]
- Chirico, S. High-performance liquid chromatography-based thio-barbituric acid tests. Methods Enzymol. 1994, 233, 314–318. [Google Scholar] [PubMed]
- Fukunaga, K.; Takama, K.; Suzuki, T. High-performance liquid chromatography determination of plasma malondialdehyde level without a solvent extraction procedure. Anal. Biochem. 1995, 230, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Trebušak, T.; Levart, A.; Frankič, T.; Salobir, J.; Pirman, T. Effect of dietary linseed oil and Ganoderma lucidum or olive leaves supplementation on fatty acid composition and oxidative status of rabbits. World Rabbit Sci. 2014, 22, 71–81. [Google Scholar] [CrossRef][Green Version]
- Rodríguez, M.; Dolores Carro, M.D.; Valiente, V.; Formoso-Rafferty, N.; Rebollar, P.G. Supplementation with fish oil improves meat fatty acid profile although impairs growth performance of early weaned rabbits. Animals 2019, 9, 437. [Google Scholar] [CrossRef]
- Prola, L.; Mussa, P.P.; Strazzullo, G.; Minosi, A.; Radice, E.; Meineri, G. Oxidative status in rabbit supplemented with dietary falsa flax seed (Camelina sativa). J. Anim. Vet. Adv. 2011, 10, 1309–1312. [Google Scholar] [CrossRef][Green Version]
- Rezar, V.; Pajk, T.; Marinšek Logar, R.; Ješe Janežič, V.; Salobir, K.; Orešnik, A. Wheat bran and oat bran effectively reduce oxidative stress induced by high-fat diets in pig. Ann. Nutr. Metab. 2003, 47, 78–84. [Google Scholar] [CrossRef]
- De Moffarts, B.; Kirschvink, N.; Art, T.; Pincemail, J.; Lekeux, P. Effect of oral antioxidant supplementation on blood antioxidant status in trained thoroughbred horses. Vet. J. 2005, 169, 65–74. [Google Scholar] [CrossRef]
- Juśkiewicz, J.; Zduńczyk, Z.; Zary-Sikorska, E.; Król, B.; Milala, J.; Jurgoński, A. Effect of the dietary polyphenolic fraction on chicory root, peel, seed and leaf extracts on cereal fermentation and blood parametrs in rats fed diets containing prebiotic fructans. Br. J. Nutr. 2001, 105, 710–720. [Google Scholar] [CrossRef]
- Halliwell, B. Free radicals and antioxidants—Quo vadis? Trends Pharmacol. Sci. 2011, 32, 125–130. [Google Scholar] [CrossRef]
- Surai, P.F. Polyphenol compounds in the shicken/animal diet. From the past to the future. J. Anim. Physiol. Anim. Nutr. 2014, 98, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Bivolarski, B.L.; Vachkova, E.G. Morphological and functional events associated to weaning in rabbits. J. Anim. Physiol. Anim. Nutr. 2014, 98, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Makovicky, P.; Tumova, E.; Volek, Z.; Makovicky, P.; Vodicka, P. Histological aspects of the small intestine under variable feed restriction: The effects of short and intense restriction on growing rabbit model. Exp. Ther. Med. 2014, 8, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Fortun-Lamothe, L.; Gidenne, T. Recent advances in digestive physiology of growing rabbit. In Recent Advances in Rabbit Sciences; Maertens, L., Coudert, P., Eds.; ILVO: Melle, Belgium, 2006; pp. 201–210. [Google Scholar]
- Yu, B.; Chiou, P.W.S. The morphological changes of intestinal mucosa in growing rabbits. Lab. Anim. 1997, 31, 254–263. [Google Scholar] [CrossRef]
- Gallois, M.; Gidenne, T.; Fortun-Lamothe, L.; Le Huerou-Luron, I.; Lallès, J.P. An early stimulation of solid feed intake slightly influences the morphological gut maturation in the rabbit. Reprod. Nutr. Dev. 2005, 45, 109–122. [Google Scholar] [CrossRef]
- Tazzoli, M.; Trocino, A.; Birolo, M.; Radaelli, G.; Xiccato, G. Optimizing feed efficiency and nitrogen excretion in growing rabbits by increasing dietary energy with high-starch, high-soluble fibre, low-insoluble fibre supply at low protein levels. Livest. Sci. 2015, 172, 59–68. [Google Scholar] [CrossRef]
- Salas Montiel, R.; Torres Acosta, I.; Villarreal Delgado, E.; Juárez-Silva, M.E.; Azaola, A.; Pérez-Gil Romo, F. Inulin as a growth promotor in diets for rabbits. Rev. Bras. Zootec. 2013, 42, 885–891. [Google Scholar] [CrossRef]
- Kishawy, A.T.Y.; Arner, S.A.; Osman, A.; Elsayed, S.A.M.; Abd El-Hack, M.E.; Swelum, A.A.; Ba-Awadh, H.; Saadeldin, I.M. Impact of supplementing growing rabbit diets with whey powder and citric acid on growth performance, nutrition digestibility, meat and bone analysis, and gut health. AMB Express 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Delgado, R.; Abad-Guamán, R.; Nicodemus, N.; Diaz-Perales, A.; García, J.; Carabano, R.; Menoyo, D. Effect of pre- and post-weaning dietary supplementation with arginine and glutamine on rabbit performance and intestinal health. BMC Vet. Res. 2019, 15, 1–12. [Google Scholar] [CrossRef]
- Oso, A.O.; Idowu, O.M.O.; Haastrup, A.S.; Ajibade, A.J.; Olowonefa, K.O.; Aluko, A.O.; Ogunade, I.M.; Osho, S.O.; Bamgbose, A.M. Growth performance, apparent nutrient digestibility, caecal fermentation, ileal morphology and caecal microflora of growing rabbits fed diets containing probiotics and prebiotics. Livest. Sci. 2013, 157, 184–190. [Google Scholar] [CrossRef]
- Desantis, S.; Zizza, S.; Accogli, G.; Tufarelli, V.; Laudadio, V. Morphometric features and glycoconjugate pattern of rabbit intestine are affected by particle size of pelleted diets. Anat. Rec. 2011, 294, 1875–1889. [Google Scholar] [CrossRef] [PubMed]
- Tumová, E.; Volek, Z.; Chodova, D.; Härtlova, H.; Makovicky, P.; Svobodá, J.; Ebeid, T.A.; Uhlířová, L. The effect of 1-week feed restriction on performance, digestibility of nutrients and digestive system development in the growing rabbit. Animal 2016, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Montagne, L.; Pluske, J.R.; Hampson, D.J. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim. Feed Sci. Technol. 2003, 108, 95–117. [Google Scholar] [CrossRef]
- De Oliveira, M.C.; Da Silva, D.M.; Borges Dias, D.M. Effect of feed restriction on organs and intestinal mucosa of growing rabbits. Rev. Bras. Zootec. 2013, 42, 530–534. [Google Scholar] [CrossRef]
- Drozowski, L.; Thomson, A.B.R. Aging and the intestine. World J. Gastroenterol. 2006, 47, 7578–7584. [Google Scholar] [CrossRef]

| Ingredients | PALM | LINSEED |
|---|---|---|
| Alfalfa | 458.3 | 458.3 |
| Barley | 130.0 | 130.0 |
| Sunflower meal | 210.0 | 210.0 |
| Hay meal | 100.0 | 100.0 |
| Rapeseed oil | 10.0 | 10.0 |
| Palm fat | 60.0 | |
| Linseed oil | 60.0 | |
| Methionine | 0.5 | 0.5 |
| Lysine | 2.0 | 2.0 |
| Vitamin-mineral mix 1 | 5.0 | 5.0 |
| Lignobond 2 | 20.0 | 20.0 |
| Salt | 4.2 | 4.2 |
| Composition of Diets | PALM | LINSEED |
|---|---|---|
| Chemical composition (g/kg) | ||
| Dry matter (DM, g/kg) | 933 | 912 |
| Crude protein | 178 | 179 |
| Crude fat | 108 | 86 |
| Crude fiber | 227 | 228 |
| Crude ash | 69 | 70 |
| Main fatty acids (% of the total fatty acids) | ||
| C12:0 | 0.20 | 0.04 |
| C14:0 | 0.89 | 0.12 |
| C16:0 | 35.81 | 8.00 |
| C18:0 | 41.73 | 4.00 |
| ∑ C18:1 | 7.75 | 23.48 |
| C18:2 n-6 | 9.01 | 22.01 |
| C18:3 n-3 | 2.78 | 40.23 |
| ∑ SFA 1 | 80.08 | 13.48 |
| ∑ MUFA 2 | 8.05 | 24.14 |
| ∑ PUFA 3 | 11.86 | 62.38 |
| ∑ n-3 PUFA | 2.85 | 40.33 |
| ∑ n-6 PUFA | 9.01 | 22.05 |
| n-6/n-3 PUFA | 3.16 | 0.55 |
| Growth Performance | PALM | LINSEED | SEM | p-Value |
|---|---|---|---|---|
| Growth rate (g/day) | 28.8 | 33.6 | 5.23 | 0.213 |
| Diet intake (g/day) | 168.3 | 178.5 | 10.13 | 0.463 |
| Feed conversion ratio (g/g) 1 | 6.09 | 5.66 | 0.56 | 0.361 |
| MDA | PALM | LINSEED | SEM | p-Value |
|---|---|---|---|---|
| Urine (nmol/mL) | 8.22 | 8.77 | 0.77 | 0.632 |
| Urine (μmol/48 h) 1 | 2.87 | 2.63 | 0.26 | 0.511 |
| Plasma (nmol/mL) | 0.14 | 0.14 | 0.01 | 0.981 |
| Antioxidative Capacity | PALM | LINSEED | SEM | p-Value | |
|---|---|---|---|---|---|
| Tissue | ACL (nmol/g) | 20.9 | 18.9 | 2.00 | 0.501 |
| ACW (μmol/g) | 2.81 | 2.83 | 0.28 | 0.970 | |
| Content | ACL (nmol/g) | 94.0 | 79.0 | 5.90 | 0.089 |
| ACW (μmol/g) | 5.28 | 6.38 | 1.67 | 0.643 | |
| Plasma | ACL (nmol/mL) | 273.5 | 253.2 | 9.85 | 0.155 |
| ACW (nmol/mL) | 44.5 | 40.7 | 5.21 | 0.612 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trebušak, T.; Vrecl Fazarinc, M.; Salobir, J.; Pirman, T. The Effect of Substitution of Palm Fat with Linseed Oil on the Lipid Peroxidation, Antioxidative Capacity and Intestinal Morphology in Rabbits (Oryctolagus cuniculus). Animals 2019, 9, 830. https://doi.org/10.3390/ani9100830
Trebušak T, Vrecl Fazarinc M, Salobir J, Pirman T. The Effect of Substitution of Palm Fat with Linseed Oil on the Lipid Peroxidation, Antioxidative Capacity and Intestinal Morphology in Rabbits (Oryctolagus cuniculus). Animals. 2019; 9(10):830. https://doi.org/10.3390/ani9100830
Chicago/Turabian StyleTrebušak, Tina, Milka Vrecl Fazarinc, Janez Salobir, and Tatjana Pirman. 2019. "The Effect of Substitution of Palm Fat with Linseed Oil on the Lipid Peroxidation, Antioxidative Capacity and Intestinal Morphology in Rabbits (Oryctolagus cuniculus)" Animals 9, no. 10: 830. https://doi.org/10.3390/ani9100830
APA StyleTrebušak, T., Vrecl Fazarinc, M., Salobir, J., & Pirman, T. (2019). The Effect of Substitution of Palm Fat with Linseed Oil on the Lipid Peroxidation, Antioxidative Capacity and Intestinal Morphology in Rabbits (Oryctolagus cuniculus). Animals, 9(10), 830. https://doi.org/10.3390/ani9100830






