Effects of Breed and Stage of Lactation on Milk Fatty Acid Composition of Italian Goat Breeds
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Management Conditions
2.2. Sample Collection and Chemical Analysis
2.3. Statistical Analysis
3. Results
3.1. Breed Effect
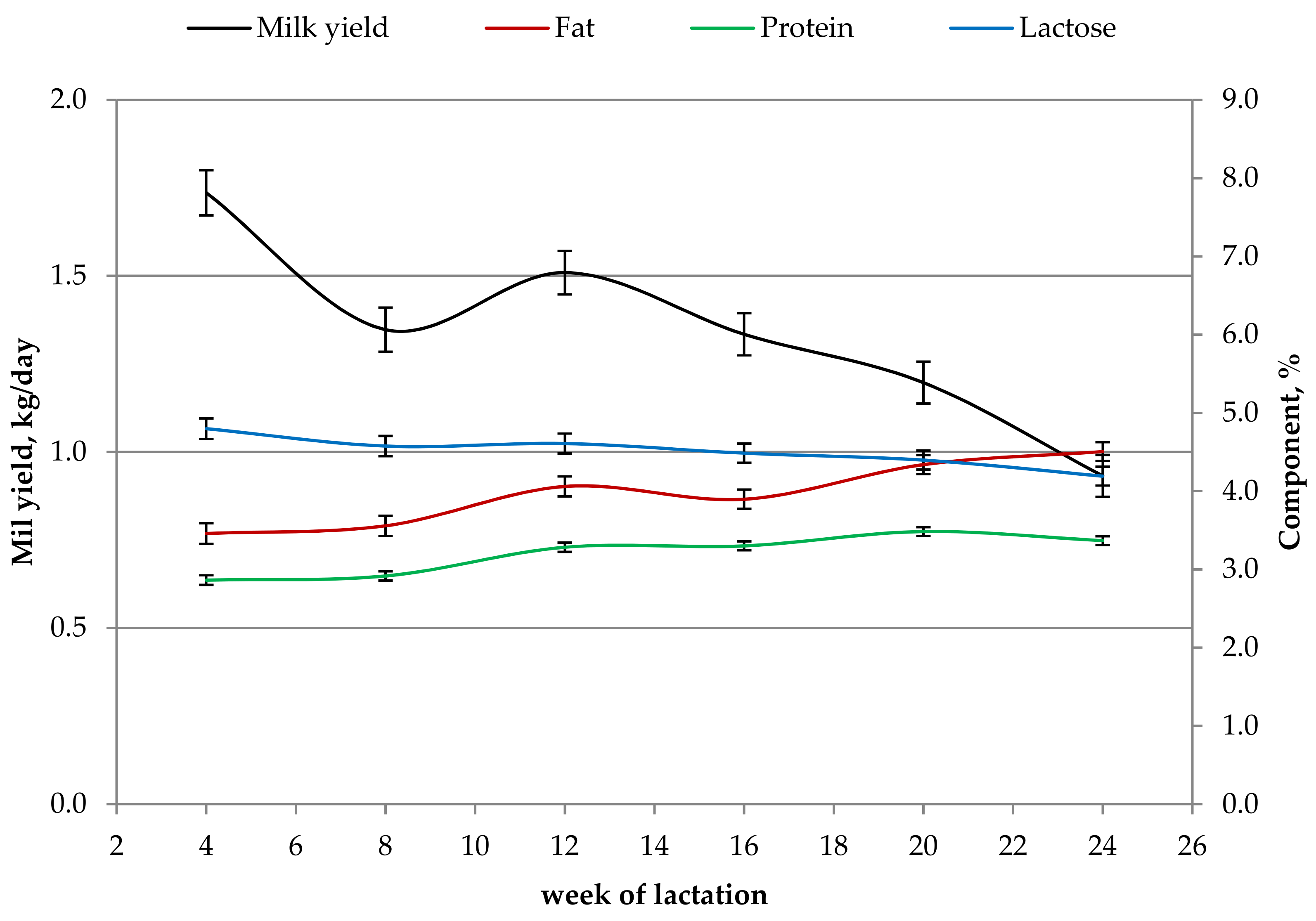
3.2. Effect of Stage of Lactation
4. Discussion
4.1. Breed Effect
4.2. Effect of Stage of Lactation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT; The Food and Agriculture Organization Corporate Statistical Database. Available online: www.fao.org/faostat/en/ (accessed on 5 March 2019).
- Boyazoglu, J.; Morand-Fehr, P. Mediterranean dairy sheep and goat products and their quality A critical review. Small Rumin. Res. 2001, 40, 1–11. [Google Scholar] [CrossRef]
- Lordan, R.; Tsoupras, A.; Mitra, B.; Zabetakis, I. Dairy fats and cardiovascular disease: Do we really need to be concerned? Foods 2018, 7, 29. [Google Scholar] [CrossRef] [PubMed]
- Jirillo, F.; Jirillo, E.; Magrone, T. Donkey’s and Goat’s Milk Consumption and Benefits to Human Health with Special Reference to the Inflammatory Status. Curr. Pharm. Des. 2010, 16, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Faye, B.; Konuspayeva, G. The sustainability challenge to the dairy sector—The growing importance of non-cattle milk production worldwide. Int. Dairy J. 2012, 24, 50–56. [Google Scholar] [CrossRef]
- Park, Y.W. Hypo-allergenic and therapeutic significance of goat milk. Small Rumin. Res. 1994, 14, 151–159. [Google Scholar] [CrossRef]
- Manuelian, C.L.; Currò, S.; Penasa, M.; Cassandro, M.; De Marchi, M. Characterization of major and trace minerals, fatty acid composition, and cholesterol content of Protected Designation of Origin cheeses. J. Dairy Sci. 2017, 100, 3384–3385. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Walther, B.; Schmid, A.; Sieber, R.; Wehrmüller, K. Cheese in nutrition and health. Dairy Sci. Technol. 2008, 88, 389–405. [Google Scholar] [CrossRef]
- Poppitt, S.D.; Koegh, G.F.; Mulvey, T.B.; McArdle, B.H.; MacGibbon, A.K.H.; Cooper, G.J.S. Lipid-loweing effects of a modified butter-fat: A controlled intervention trial in healthy men. Eur. J. Clin. Nutr. 2002, 56, 64–71. [Google Scholar] [CrossRef]
- Schönfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Van Schalkwijk, D.B.; Pasman, W.J.; Hendriks, H.F.J.; Verheij, E.R.; Rubingh, C.M.; Van Bochove, K.; Vaes, W.H.J.; Adiels, M.; Freidig, A.P.; De Graaf, A.A. Dietary medium chain fatty acid supplementation leads to reduced VLDL lipolysis and uptake rates in comparison to linoleic acid supplementation. PLoS ONE 2014, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chilliard, Y.; Ferlay, A.; Mansbridge, R.M.; Doreau, M. Ruminant milk fat plasticity: Nutritional control of saturated, polyunsaturated, trans and conjugated fatty acids. Ann. Zootech. 2000, 49, 181–205. [Google Scholar] [CrossRef]
- Palmquist, D.L. Milk fat: Origin of fatty acids and influence of nutritional factors thereon. Adv. Dairy Chem. 2009, 2, 43–92. [Google Scholar]
- Benedet, A.; Manuelian, C.L.; Zidi, A.; Penasa, M.; De Marchi, M. Invited review: β-hydroxybutyrate concentration in blood and milk and its associations with cow performance. Animal 2019, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Griinari, J.M.; Dwyer, D.A.; McGuire, M.A.; Bauman, D.E.; Palmquist, D.L.; Nurmela, K.V.V. Trans-Octadecenoic Acids and Milk Fat Depression in Lactating Dairy Cows. J. Dairy Sci. 1998, 81, 1251–1261. [Google Scholar] [CrossRef]
- Gama, M.A.S.; Garnsworthy, P.C.; Griinari, J.M.; Leme, P.R.; Rodrigues, P.H.M.; Souza, L.W.O.; Lanna, D.P.D. Diet-induced milk fat depression: Association with changes in milk fatty acid composition and fluidity of milk fat. Livest. Sci. 2008, 115, 319–331. [Google Scholar] [CrossRef]
- Nantapo, C.T.W.; Muchenje, V.; Hugo, A. Atherogenicity index and health-related fatty acids in different stages of lactation from Friesian, Jersey and Friesian × Jersey cross cow milk under a pasture-based dairy system. Food Chem. 2014, 146, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Solaiman, S.G. Goat science and production, 1st ed.; Wiley-Blackwell: Ames, IA, USA, 2010; pp. 193–216. [Google Scholar]
- Strzalkowska, N.; Jóźwik, A.; Bagnicka, E.; Krzyzewski, J.; Horbańczuk, K.; Pyzel, B.; Horbańczuk, J.O. Chemical composition, physical traits and fatty acid profile of goat milk as related to the stage of lactation. Anim. Sci. Pap. Rep. 2009, 27, 311–320. [Google Scholar]
- Yurchenko, S.; Sats, A.; Tatar, V.; Kaart, T.; Mootse, H.; Jõudu, I. Fatty acid profile of milk from Saanen and Swedish Landrace goats. Food Chem. 2018, 254, 326–332. [Google Scholar] [CrossRef]
- Di Trana, A.; Sepe, L.; Di Gregorio, P.; Di Napoli, M.A.; Giorgio, D.; Caputo, A.R.; Claps, S. The Role of Local Sheep and Goat Breeds and Their Products as a Tool for Sustainability and Safeguard of the Mediterranean Environment. In The Sustainability of Agro-Food and Natural Resource Systems in the Mediterranean Basin; Vastola, A., Ed.; Springer: Potenza, Italy, 2015. [Google Scholar]
- Malhado, C.H.M.; Carneiro, P.L.S.; Affonso, P.R.A.M.; Souza, A.A.O.; Sarmento, J.L.R. Growth curves in Dorper sheep crossed with the local Brazilian breeds, Morada Nova, Rabo Largo, and Santa Inês. Small Rumin. Res. 2009, 84, 16–21. [Google Scholar] [CrossRef]
- Benjelloun, B.; Alberto, F.J.; Streeter, I.; Boyer, F.; Coissac, E.; Stucki, S.; BenBati, M.; Ibnelbachyr, M.; Chentouf, M.; Bechchari, A.; et al. Characterizing neutral genomic diversity and selection signatures in indigenous populations of Moroccan goats (Capra hircus) using WGS data. Front. Genet. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed]
- DAD-IS Domestic Animal Diversity Information System (DAD-IS). Available online: http://dad.fao.org/.
- Gandini, G.C.; Villa, E. Analysis of the cultural value of local livestock breeds: A methodology. J. Anim. Breed. Genet. 2003, 120, 1–11. [Google Scholar] [CrossRef]
- Currò, S.; Manuelian, C.L.; De Marchi, M.; De Palo, P.; Claps, S.; Maggiolino, A.; Campanile, G.; Rufrano, D.; Fontana, A.; Pedota, G.; et al. Autochthonous dairy goat breeds showed better milk quality than Saanen under the same environmental conditions. Arch. Anim. Breed. 2019, 62, 83–89. [Google Scholar] [CrossRef]
- Tufarelli, V.; Dario, M.; Laudadio, V. Forage to concentrate ratio in Jonica breed goats: Influence on lactation curve and milk composition. J. Dairy Res. 2009, 76, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Albenzio, M.; Caroprese, M.; Marino, R.; Muscio, A.; Santillo, A.; Sevi, A. Characteristics of Garganica goat milk and Cacioricotta cheese. Small Rumin. Res. 2006, 64, 35–44. [Google Scholar] [CrossRef]
- Pizzillo, M.; Claps, S.; Cifuni, G.F.; Fedele, V.; Rubino, R. Effect of goat breed on the sensory, chemical and nutritional characteristics of ricotta cheese. Livest. Prod. Sci. 2004, 94, 33–40. [Google Scholar] [CrossRef]
- Sacchi, P.; Chessa, S.; Budelli, E.; Bolla, P.; Ceriotti, G.; Soglia, D.; Rasero, R.; Cauvin, E.; Caroli, A. Casein Haplotype Structure in Five Italian Goat Breeds. J. Dairy Sci. 2005, 88, 1561–1568. [Google Scholar] [CrossRef]
- Currò, S.; De Marchi, M.; Claps, S.; Salzano, A.; De Palo, P.; Manuelian, C.L.; Neglia, G. Differences in the Detailed Milk Mineral Composition of Italian Local and Saanen Goat Breeds. Animals 2019, 9, 412. [Google Scholar] [CrossRef]
- Villaquiran, M.; Gipson, T.A.; Merkel, R.; Goetsch, A.; Sahlu, T. Body Condition Scoring for Improved Management. Proceeding of the 20th Annual Goat Field Day, Langston University, Langston, OK, USA; 30 April 2005. [Google Scholar]
- National Research Council. Nutrient Requirements of Small Ruminants: Sheep, Goats, Cervids, and New World Camelids, 6th ed.; The National Academies Press: Washington DC, USA, 2007. [Google Scholar]
- Pulina, G.; Cannas, A.; Serra, A.; Vallebella, R. Determination and estimation of the energy value in Sardinian goat milk. In Proceedings of the Congress of Società Italiana Scienze Veterinarie (SISVet), Altavilla Milicia (PA), Italy, 25–28 September 1991; pp. 1779–1781. [Google Scholar]
- Wiggans, G.R.; Shook, G.E. A Lactation Measure of Somatic Cell Count. J. Dairy Sci. 1987, 70, 2666–2672. [Google Scholar] [CrossRef]
- Christie, W.W. (Ed.) Preparation of Ester Derivatives of Fatty Acids for Chromatographic Analysis. In Advanes Lipid Methodoly II; Oily Press: Dundee, Scotland, UK, 1993; pp. 69–111. [Google Scholar]
- Čejna, V.; Chládek, G. The importance of monitoring changes in milk fat to milk protein ratio in Holstein cows during lactation. J. Cent. Eur. Agric. 2005, 6, 539–546. [Google Scholar]
- Paura, L.; Jonkus, D.; Ruska, D. Evaluation of the milk fat to protein ratio and fertility traits in Latvian brown and Holstein dairy cows. Acta Agric. Slov. Suppl. 2012, 3, 155–159. [Google Scholar]
- Roca Fernandez, A.I.; Gonzalez Rodriguez, A. Effect of Dietary and Animal Factors on Milk Fatty Acids Composition of Grazing Dairy Cows: A Review. Iran. J. Appl. Anim. Sci. 2012, 2, 97–109. [Google Scholar]
- Claps, S.; Roberta, R.; Di Trana, A.; di Napoli, M.A.; Giorgio, D.; Sepe, L. Bioactive compounds in goat milk and cheese: The role of feeding system and breed. In Goat Science; InTech open science/open minds in press: London, UK, 2018; pp. 233–263. [Google Scholar]
- Food and Agriculture Organization of the United Nations (FAO). Milk and dairy products in human nutrition. In Milk and Dairy Products in Human Nutrition; FAO: Rome, Italy, 2013; pp. 1–376. [Google Scholar]
- Tripaldi, C.; Martillotti, F.; Terramoccia, S. Content of taurine and other free amino acids in milk of goats bred in Italy. Small Rumin. Res. 1998, 30, 127–136. [Google Scholar] [CrossRef]
- Sung, Y.Y.; Wu, T.I.; Wang, P.H. Evaluation of milk quality of Alpine, Nubian, Saanen and Toggenburg breeds in Taiwan. Small Rumin. Res. 1999, 33, 17–23. [Google Scholar] [CrossRef]
- Raynal-Ljutovac, K.; Pirisi, A.; de Crémoux, R.; Gonzalo, C. Somatic cells of goat and sheep milk: Analytical, sanitary, productive and technological aspects. Small Rumin. Res. 2007, 68, 126–144. [Google Scholar] [CrossRef]
- Barrón-Bravo, O.G.; Gutiérrez-Chávez, A.J.; Ángel-Sahagún, C.A.; Montaldo, H.H.; Shepard, L.; Valencia-Posadas, M. Losses in milk yield, fat and protein contents according to different levels of somatic cell count in dairy goats. Small Rumin. Res. 2013, 113, 421–431. [Google Scholar] [CrossRef]
- Jiménez-Granado, R.; Sánchez-Rodríguez, M.; Arce, C.; Rodríguez-Estévez, V. Factors affecting somatic cell count in dairy goats: A review. Spanish J. Agric. Res. 2014, 12, 133–150. [Google Scholar] [CrossRef]
- Trancoso, I.M.; Trancoso, M.A.; Martins, A.P.L.; Roseiro, L.B. Chemical composition and mineral content of goat milk from four indigenous Portuguese breeds in relation to one foreign breed. Int. J. Dairy Technol. 2010, 63, 516–522. [Google Scholar] [CrossRef]
- Vlaeminck, B.; Fievez, V.; Cabrita, A.R.J.; Fonseca, A.J.M.; Dewhurst, R.J. Factors affecting odd- and branched-chain fatty acids in milk: A review. Anim. Feed Sci. Technol. 2006, 131, 389–417. [Google Scholar] [CrossRef]
- Hanus, O.; Samkova, E.; Křížova, L.; Hasoňova, L.; Kala, R. Role of fatty acids in milk fat and the influence of selected factors on their variability—a review. Molecules 2018, 23, 1636. [Google Scholar] [CrossRef]
- Bainbridge, M.L.; Cersosimo, L.M.; Wright, A.D.G.; Kraft, J. Rumen bacterial communities shift across a lactation in Holstein, Jersey and Holstein × Jersey dairy cows and correlate to rumen function, bacterial fatty acid composition and production parameters. FEMS Microbiol. Ecol. 2016, 92, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, B.; West, J.A.; Koulman, A. A review of odd-chain fatty acid metabolism and the role of pentadecanoic acid (C15:0) and heptadecanoic acid (C17:0) in health and disease. Molecules 2015, 20, 2425–2444. [Google Scholar] [CrossRef] [PubMed]
- Talpur, F.N.; Bhanger, M.I.; Memon, N.N. Milk fatty acid composition of indigenous goat and ewe breeds from Sindh, Pakistan. J. Food Compos. Anal. 2009, 22, 59–64. [Google Scholar] [CrossRef]
- Albenzio, M.; Santillo, A.; Caroprese, M.; Ciliberti, M.G.; Marino, R.; Sevi, A. Effect of stage of lactation on the immune competence of goat mammary gland. J. Dairy Sci 2016, 99, 3889–3895. [Google Scholar] [CrossRef] [PubMed]
- Sitzia, M.; Bonanno, A.; Todaro, M.; Cannas, A.; Atzori, A.S.; Francesconi, A.H.D.; Trabalza-Marinucci, M. Feeding and management techniques to favour summer sheep milk and cheese production in the Mediterranean environment. Small Rumin. Res. 2015, 126, 43–58. [Google Scholar] [CrossRef]
- Goetsch, A.L.; Zeng, S.S.; Gipson, T.A. Factors affecting goat milk production and quality. Small Rumin. Res. 2011, 101, 55–63. [Google Scholar] [CrossRef]
- Kawas, J.R.; Lopes, J.; Danelon, D.L.; Lu, C.D. Influence of forage-to-concentrate ratios on intake, digestibility, chewing and milk production of dairy goats. Small Rumin. Res. 1991, 4, 11–18. [Google Scholar] [CrossRef]
- Desnoyers, M.; Duvaux-Ponter, C.; Rigalma, K.; Roussel, S.; Martin, O.; Giger-Reverdin, S. Effect of concentrate percentage on ruminal pH and time-budget in dairy goats. Animal 2008, 2, 1802–1808. [Google Scholar] [CrossRef]
- Vlček, M.; Žitný, J.; Kasarda, R. Changes of fat-to-protein ratio from start to the mid- lactation and the impact on milk yield. J. Cent. Eur. Agric. 2016, 17, 1194–1203. [Google Scholar] [CrossRef]
- Craninx, M.; Steen, A.; Van Laar, H.; Van Nespen, T.; Martín-Tereso, J.; De Baets, B.; Fievez, V. Effect of Lactation Stage on the Odd- and Branched-Chain Milk Fatty Acids of Dairy Cattle Under Grazing and Indoor Conditions. J. Dairy Sci. 2008, 91, 2662–2677. [Google Scholar] [CrossRef]
- Kuchtík, J.; KrálÍčková, Š.; Zapletal, D.; Węglarzy, K.; Šustová, K.; Skrzyżala, I. Changes in physico-chemical characteristics, somatic cell count and fatty acid profile of brown short-haired goat milk during lactation. Anim. Sci. Pap. Reports 2015, 33, 71–83. [Google Scholar]
- Tsiplakou, E.; Zervas, G. Comparative study between sheep and goats on rumenic acid and vaccenic acid in milk fat under the same dietary treatments. Livest. Sci. 2008, 119, 87–94. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Denise Beaulieu, A.; Barbano, D.M. Feed and Animal Factors Influencing Milk Fat Composition. J. Dairy Sci. 1993, 76, 1753–1771. [Google Scholar] [CrossRef]
- Cossignani, L.; Giua, L.; Urbani, E.; Simonetti, M.S.; Blasi, F. Fatty acid composition and CLA content in goat milk and cheese samples from Umbrian market. Eur. Food Res. Technol. 2014, 239, 905–911. [Google Scholar] [CrossRef]
- Ghaeni, M.; Ghahfarokhi, K.N. Fatty Acids Profile, Atherogenic (IA) and Thrombogenic (IT) Health Lipid Indices in Leiognathusbindus and Upeneussulphureus. J. Mar. Sci. Res. Dev. 2015, 3, 3–5. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. The Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Clark, S.; García, M.B.M. A 100-Year Review: Advances in goat milk research1. J. Dairy Sci. 2017, 100, 10026–10044. [Google Scholar] [CrossRef]
- Salari, F.; Altomonte, I.; Ribeiro, N.L.; Ribeiro, M.N.; Bozzi, R.; Martini, M. Effects of season on the quality of Garfagnina goat milk. Ital. J. Anim. Sci. 2016, 15, 568–575. [Google Scholar] [CrossRef][Green Version]
- Fekadu, B.; Soryal, K.; Zeng, S.; Van Hekken, D.; Bah, B.; Villaquiran, M. Changes in goat milk composition during lactation and their effect on yield and quality of hard and semi-hard cheeses. Small Rumin. Res. 2005, 59, 55–63. [Google Scholar] [CrossRef]
| Trait 1 | Hay | Commercial Concentrate |
|---|---|---|
| DM (%) | 89.1 | 88.2 |
| CP (% of DM) | 15.1 | 21.7 |
| Fat (% of DM) | 1.9 | 3.5 |
| NSC (% of DM) | 20.7 | 42.7 |
| Cellulose (% of DM) | 29.5 | 10.5 |
| Ash (% of DM) | 9.5 | 9.1 |
| NDF (% of DM) | 52.6 | 23.0 |
| ADF (% of DM) | 36.6 | 9.0 |
| ADL (% of DM) | 3.9 | 3.3 |
| NEL (Mcal/kg DM) | 1.10 | 1.77 |
| Traits | Breed | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GA | GI | JO | MA | MR | SA | Mean | SEM | p | |
| Milk yield (kg/day) | 1.26 ab | 1.06 b | 1.48 ab | 1.39 ab | 1.13 b | 1.73 a | 1.34 | 0.11 | <0.001 |
| FCM3.5% (kg/day) | 1.32 ab | 1.14 b | 1.45 ab | 1.43 ab | 1.16 b | 1.67 a | 1.36 | 0.10 | <0.001 |
| Fat (%) | 4.15 | 4.20 | 4.01 | 3.92 | 3.92 | 3.61 | 3.97 | 0.18 | 0.310 |
| Protein (%) | 3.52 a | 3.02 b | 3.20 ab | 3.02 b | 3.27 ab | 3.20 ab | 3.20 | 0.10 | 0.012 |
| Lactose (%) | 4.31 b | 4.55 ab | 4.58 a | 4.59 a | 4.69 a | 4.34 b | 4.51 | 0.05 | <0.001 |
| F/P | 1.15 b | 1.37 a | 1.25 ab | 1.30 ab | 1.21 ab | 1.14 b | 1.22 | 0.03 | 0.005 |
| SCS (units) | 5.77 a | 4.20 b | 5.34 ab | 4.93 b | 5.10 b | 6.79 a | 5.35 | 0.34 | <0.001 |
| Fatty Acid | Breed | Overall | |||||||
|---|---|---|---|---|---|---|---|---|---|
| GA | GI | JO | MA | MR | SA | Mean | SEM | P | |
| C4:0 | 1.89 b | 2.03 ab | 2.01 ab | 2.00 ab | 2.09 ab | 2.17 a | 2.03 | 0.06 | 0.043 |
| C6:0 | 2.19 | 2.22 | 2.35 | 2.24 | 2.37 | 2.26 | 2.27 | 0.07 | 0.330 |
| C8:0 | 2.65 | 2.58 | 2.90 | 2.66 | 2.79 | 2.58 | 2.69 | 0.10 | 0.189 |
| C10:0 | 8.44 | 7.84 | 9.01 | 8.24 | 8.73 | 8.22 | 8.41 | 0.34 | 0.186 |
| C12:0 | 3.43 | 3.14 | 3.85 | 3.44 | 3.46 | 3.31 | 3.44 | 0.16 | 0.065 |
| C14:0 | 8.57 ab | 7.69 b | 8.47 ab | 8.50 ab | 8.67 ab | 9.25 a | 8.52 | 0.30 | 0.029 |
| C15:0 | 1.72 ab | 1.86 a | 1.82 ab | 1.85 a | 1.73 ab | 1.58 b | 1.76 | 0.05 | 0.013 |
| C15:0 iso | 0.42 a | 0.40 a | 0.41 a | 0.43 a | 0.42 a | 0.31 b | 0.40 | 0.02 | 0.009 |
| C15:0 anteiso | 0.35 ab | 0.35 ab | 0.40 a | 0.37 ab | 0.35 ab | 0.28 b | 0.35 | 0.02 | 0.018 |
| C16:0 | 23.74 ab | 22.48 b | 22.14 b | 23.46 ab | 24.19 ab | 25.85 a | 23.54 | 0.71 | 0.014 |
| C16:1 | 1.01 a | 0.98 a | 0.90 ab | 0.86 ab | 0.71 b | 0.87 ab | 0.89 | 0.05 | 0.002 |
| C17:0 | 1.87 ab | 2.02 a | 1.95 a | 2.00 a | 1.89 ab | 1.72 b | 1.91 | 0.04 | 0.002 |
| C17:0 iso | 0.63 a | 0.68 a | 0.67 a | 0.66 a | 0.63 a | 0.56 b | 0.64 | 0.01 | <0.001 |
| C17:0 anteiso | 0.49 ab | 0.52 a | 0.50 a | 0.51 a | 0.48 ab | 0.43 b | 0.49 | 0.01 | 0.003 |
| C18:0 | 13.85 ab | 15.60 a | 14.16 ab | 14.29 a | 13.65 ab | 11.85 b | 13.90 | 0.54 | 0.002 |
| C18:1 | 23.32 | 24.59 | 22.95 | 23.06 | 22.68 | 22.78 | 23.23 | 0.69 | 0.359 |
| C18:1trans-11 | 1.84 | 2.09 | 2.03 | 2.00 | 1.92 | 2.03 | 1.98 | 0.10 | 0.593 |
| CLAc9 trans-11 | 0.73 | 0.73 | 0.73 | 0.73 | 0.72 | 0.85 | 0.75 | 0.03 | 0.119 |
| C18:2 | 3.00 | 3.17 | 3.20 | 3.18 | 2.87 | 3.31 | 3.12 | 0.10 | 0.083 |
| n3 | 0.86 | 1.00 | 0.96 | 0.99 | 0.86 | 0.92 | 0.93 | 0.04 | 0.069 |
| n6 | 4.30 | 4.36 | 4.37 | 4.41 | 4.07 | 4.72 | 4.37 | 0.13 | 0.063 |
| CLA | 0.91 | 0.88 | 0.88 | 0.91 | 0.88 | 1.05 | 0.92 | 0.04 | 0.068 |
| SFA | 70.23 | 69.14 | 70.46 | 71.00 | 70.91 | 70.35 | 70.35 | 0.65 | 0.346 |
| UFA | 30.16 | 31.59 | 29.86 | 29.97 | 28.97 | 29.93 | 30.08 | 0.77 | 0.280 |
| MUFA | 24.84 | 25.53 | 24.21 | 23.56 | 24.11 | 24.03 | 24.38 | 0.55 | 0.147 |
| PUFA | 5.15 | 5.36 | 5.33 | 5.40 | 4.97 | 5.65 | 5.31 | 0.15 | 0.102 |
| SCFA | 15.28 | 14.79 | 16.39 | 15.25 | 16.12 | 15.34 | 15.53 | 0.50 | 0.194 |
| MCFA | 38.96 | 36.34 | 37.67 | 38.52 | 38.87 | 40.34 | 38.45 | 0.87 | 0.059 |
| LCFA | 45.77 | 48.64 | 45.84 | 46.13 | 44.64 | 43.36 | 45.73 | 1.14 | 0.057 |
| n6/n3 | 5.20 | 4.56 | 4.67 | 4.63 | 4.93 | 5.36 | 4.89 | 0.19 | 0.070 |
| SFA/UFA | 2.36 | 2.21 | 2.39 | 2.40 | 2.51 | 2.43 | 2.38 | 0.08 | 0.207 |
| DI C16:0 | 4.09 a | 4.18 a | 3.93 a | 3.51 ab | 2.82 b | 3.32 ab | 3.64 | 0.22 | <0.001 |
| DI C18:0 | 62.93 | 61.41 | 62.10 | 61.83 | 62.51 | 65.73 | 62.75 | 1.00 | 0.073 |
| AI | 2.08 ab | 1.82 b | 2.05 ab | 2.10 ab | 2.22 ab | 2.32 a | 2.10 | 0.10 | 0.035 |
| EI | 5.63 | 5.00 | 5.59 | 5.37 | 5.29 | 5.30 | 5.36 | 0.22 | 0.342 |
| TI | 2.71 | 2.55 | 2.62 | 2.73 | 2.80 | 2.80 | 2.71 | 0.09 | 0.258 |
| Fatty Acid | Week of Lactation | Overall | ||||||
|---|---|---|---|---|---|---|---|---|
| 4 | 8 | 12 | 16 | 20 | 24 | Mean | SEM | |
| C4:0 | 1.96 bc | 1.92 c | 2.16 a | 2.01 bc | 2.09 ab | 2.05 ab | 2.03 | 0.04 |
| C6:0 | 2.38 ab | 2.24 bc | 2.44 a | 2.14 c | 2.33 bc | 2.09 c | 2.27 | 0.05 |
| C8:0 | 2.93 a | 2.73 ab | 2.92 a | 2.48 c | 2.79 ab | 2.31 c | 2.69 | 0.07 |
| C10:0 | 9.47 a | 8.89 a | 8.81 a | 7.37 b | 8.96 a | 6.98 b | 8.41 | 0.26 |
| C12:0 | 3.75 a | 3.73 a | 3.46 ab | 3.11 bc | 3.76 a | 2.83 c | 3.44 | 0.12 |
| C14:0 | 9.51 a | 9.31 a | 8.11 b | 7.52 c | 8.81 a | 7.88 bc | 8.52 | 0.19 |
| C15:0 | 1.69 c | 2.10 a | 1.60 c | 1.90 b | 1.61 c | 1.64 c | 1.76 | 0.04 |
| C15:0 iso | 0.37 b | 0.50 a | 0.38 b | 0.45 a | 0.33 b | 0.36 b | 0.40 | 0.02 |
| C15:0 anteiso | 0.32 b | 0.44 a | 0.33 b | 0.40 a | 0.28 b | 0.33 b | 0.35 | 0.01 |
| C16:0 | 24.63 ab | 23.75 b | 22.48 c | 21.52 c | 25.61 a | 23.88 b | 23.60 | 0.44 |
| C16:1 | 0.82 b | 0.90 ab | 0.77 b | 0.88 ab | 0.92 ab | 1.04 a | 0.89 | 0.04 |
| C17:0 | 1.95 bc | 2.14 a | 1.80 cd | 2.00 ab | 1.72 d | 1.84 cd | 1.91 | 0.04 |
| C17:0 iso | 0.59 b | 0.67 a | 0.63 b | 0.67 a | 0.62 b | 0.64 b | 0.64 | 0.01 |
| C17:0 anteiso | 0.51 b | 0.59 a | 0.46 bc | 0.52 b | 0.41 c | 0.44 c | 0.49 | 0.01 |
| C18:0 | 12.43 cd | 13.05 cd | 15.75 b | 17.13 a | 11.75 d | 13.28 c | 13.90 | 0.35 |
| C18:1 | 21.61 c | 21.98 c | 22.38 b | 25.50 a | 22.00 b | 25.91 a | 23.23 | 0.52 |
| C18:1trans-11 | 1.75 c | 1.61 c | 2.03 b | 2.28 a | 1.94 b | 2.29 a | 1.98 | 0.07 |
| CLAc9 trans-11 | 0.57 c | 0.52 c | 0.67 c | 0.83 b | 0.86 ab | 1.06 a | 0.75 | 0.03 |
| C18:2 | 2.94 bc | 3.20 ab | 3.04 bc | 2.88 c | 3.21 ab | 3.46 a | 3.12 | 0.08 |
| n3 | 0.82 b | 0.70 c | 1.10 a | 0.88 b | 1.06 a | 1.04 a | 0.93 | 0.03 |
| n6 | 4.02 c | 4.25 bc | 4.16 c | 4.19 c | 4.55 b | 5.06 a | 4.37 | 0.09 |
| CLA | 0.74 cd | 0.68 d | 0.83 c | 1.00 b | 1.03 b | 1.23 a | 0.92 | 0.03 |
| SFA | 72.79 a | 71.64 a | 71.30 a | 68.17 b | 71.01 a | 67.17 b | 70.35 | 0.49 |
| UFA | 27.95 b | 28.55 b | 29.03 b | 32.02 a | 29.19 b | 33.74 a | 30.08 | 0.60 |
| MUFA | 22.43 b | 23.41 b | 23.43 b | 26.67 a | 23.38 b | 26.97 a | 24.38 | 0.43 |
| PUFA | 4.86 c | 4.96 c | 5.26 bc | 5.07 c | 5.61 ab | 6.10 a | 5.31 | 0.11 |
| SCFA | 16.86 a | 15.90 a | 16.44 a | 14.13 b | 16.33 a | 13.52 b | 15.53 | 0.37 |
| MCFA | 40.44 a | 40.27 a | 36.91 b | 35.10 c | 40.56 a | 37.43 b | 38.45 | 0.54 |
| LCFA | 42.26 c | 43.85 c | 46.63 b | 50.51 a | 42.36 c | 48.76 ab | 45.73 | 0.82 |
| n6/n3 | 5.04 bc | 6.16 a | 3.86 d | 4.81 bc | 4.49 c | 4.99 bc | 4.89 | 0.14 |
| SFA/UFA | 2.67 a | 2.54 a | 2.48 a | 2.15 b | 2.47 a | 1.99 b | 2.38 | 0.07 |
| DI C16:0 | 3.27c | 3.65 bc | 3.27 c | 3.96 ab | 3.49 bc | 4.21 a | 3.64 | 0.18 |
| DI C18:0 | 63.45bc | 62.79 c | 58.72 d | 60.06 d | 65.28 ab | 66.20 a | 62.75 | 0.65 |
| AI | 2.48a | 2.30 a | 2.06 b | 1.72 c | 2.26 ab | 1.75 c | 2.10 | 0.07 |
| EI | 5.95a | 5.90 a | 5.04 b | 4.90 b | 5.55 a | 4.83 b | 5.36 | 0.14 |
| TI | 3.02a | 2.88 ab | 2.71 bc | 2.53 cd | 2.71 bc | 2.38 d | 2.71 | 0.06 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Currò, S.; Manuelian, C.L.; De Marchi, M.; Claps, S.; Rufrano, D.; Neglia, G. Effects of Breed and Stage of Lactation on Milk Fatty Acid Composition of Italian Goat Breeds. Animals 2019, 9, 764. https://doi.org/10.3390/ani9100764
Currò S, Manuelian CL, De Marchi M, Claps S, Rufrano D, Neglia G. Effects of Breed and Stage of Lactation on Milk Fatty Acid Composition of Italian Goat Breeds. Animals. 2019; 9(10):764. https://doi.org/10.3390/ani9100764
Chicago/Turabian StyleCurrò, Sarah, Carmen L. Manuelian, Massimo De Marchi, Salvatore Claps, Domenico Rufrano, and Gianluca Neglia. 2019. "Effects of Breed and Stage of Lactation on Milk Fatty Acid Composition of Italian Goat Breeds" Animals 9, no. 10: 764. https://doi.org/10.3390/ani9100764
APA StyleCurrò, S., Manuelian, C. L., De Marchi, M., Claps, S., Rufrano, D., & Neglia, G. (2019). Effects of Breed and Stage of Lactation on Milk Fatty Acid Composition of Italian Goat Breeds. Animals, 9(10), 764. https://doi.org/10.3390/ani9100764





