Expression of Myoepithelial Markers in Mammary Carcinomas of 119 Pet Rabbits
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Histopathological Features
2.2. Immunohistochemistry
2.3. Image Analysis
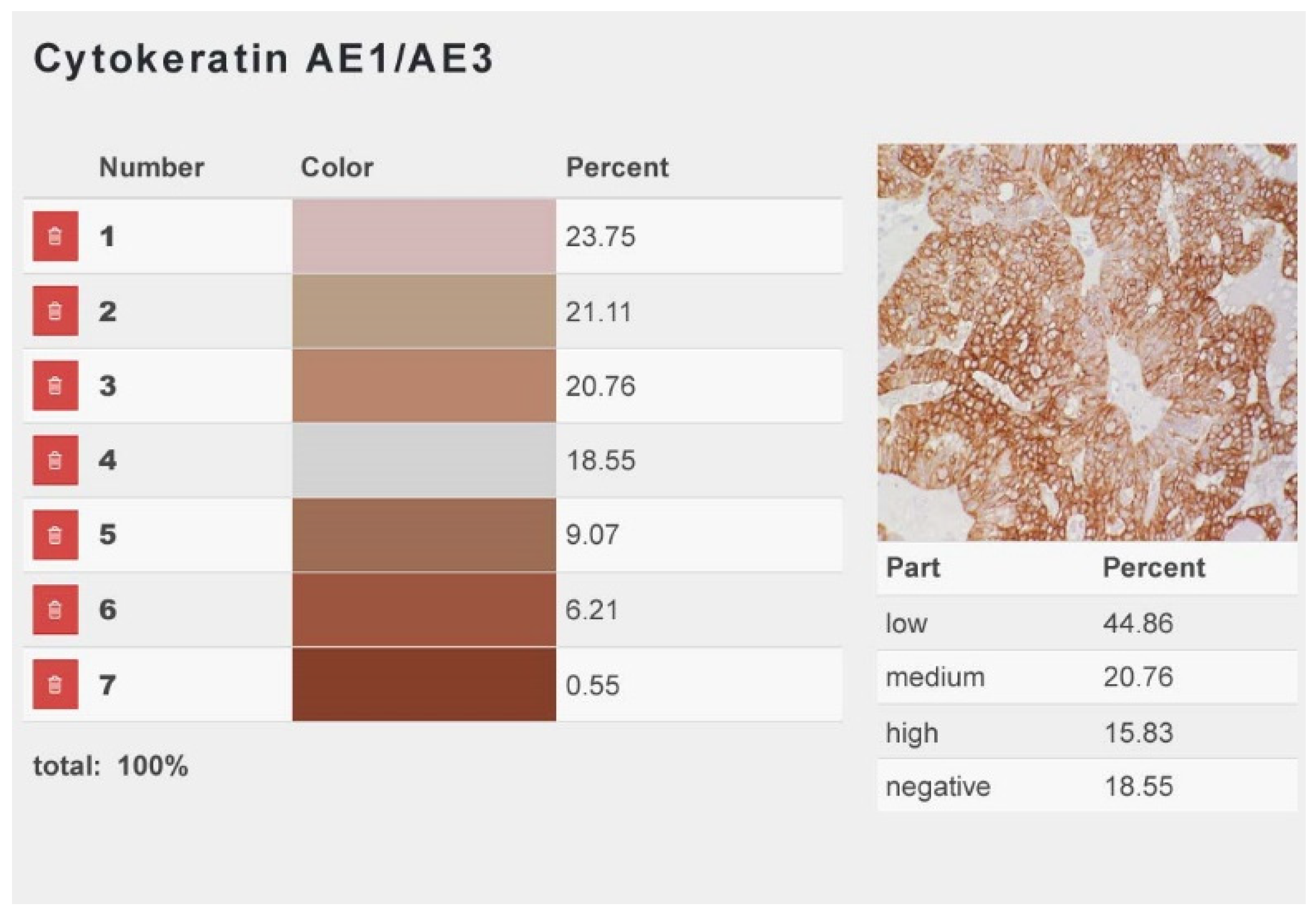
2.4. Measurement of the Immunopositive Tumor Area
2.5. Determination of Immunopositive Cells
2.6. Statistical Evaluation
3. Results
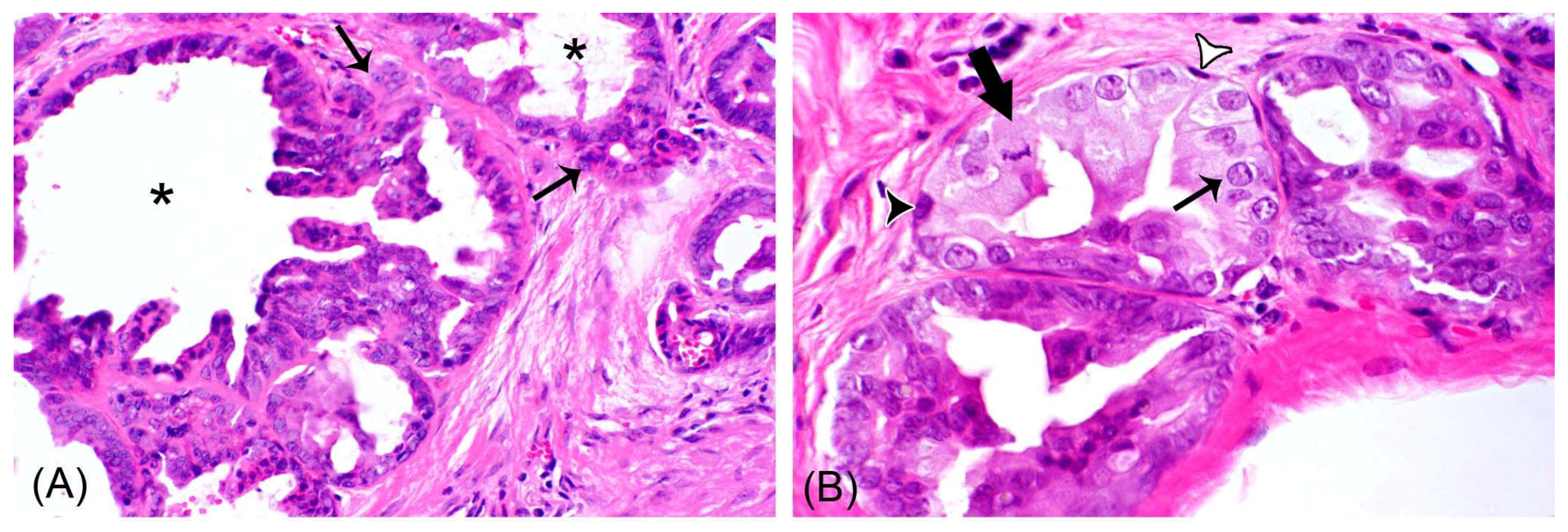
3.1. Histopathological Features
3.2. Measurement of the Immunopositive Tumor Area
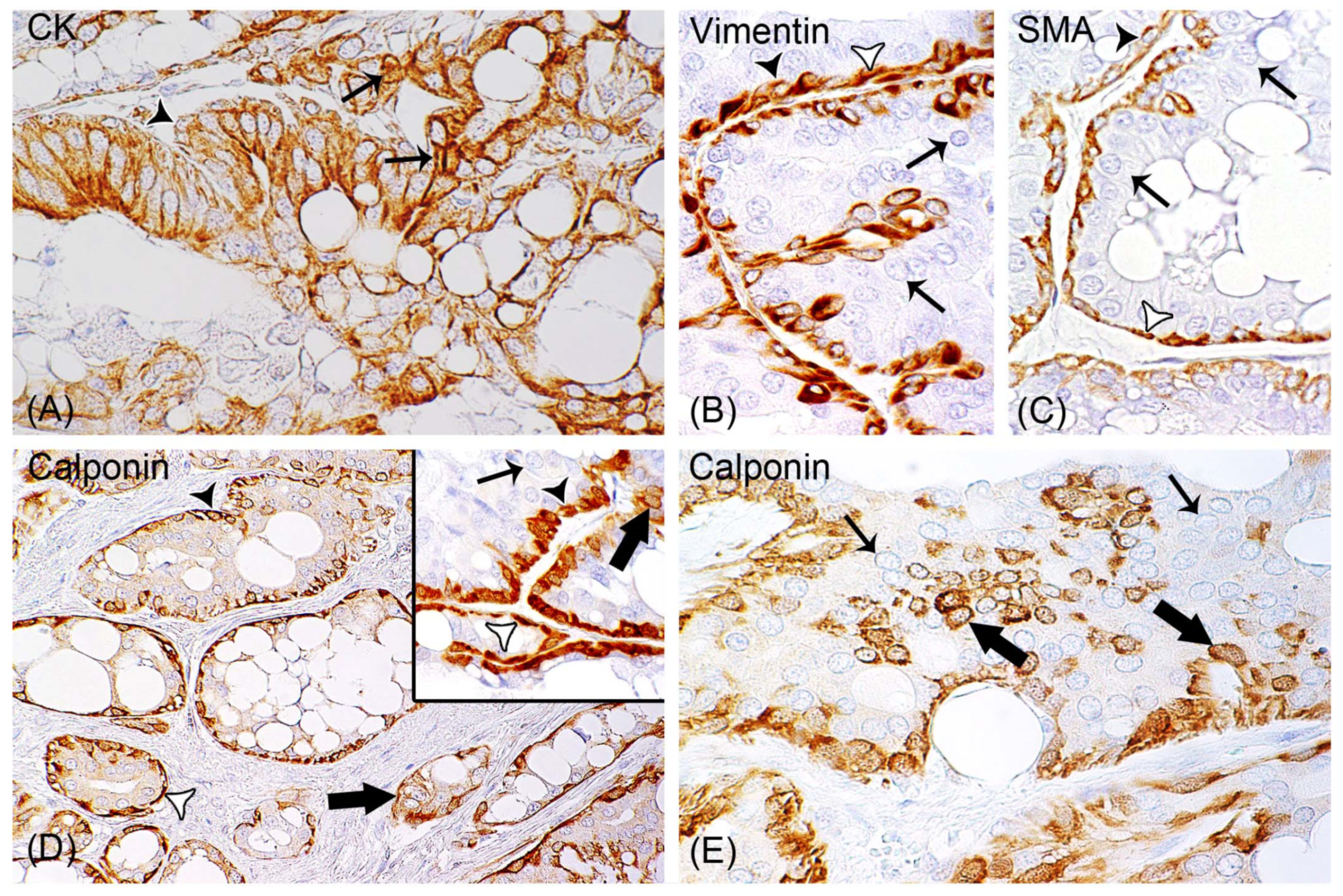
All Rabbit Mammary Carcinomas Contained Epithelial Areas Positive for Vimentin, SMA, and Calponin
3.3. Determination of Immunopositive Cells
3.3.1. Non-Neoplastic Myoepithelial Cells Were Observed in all Mammary Carcinomas
3.3.2. Calponin-Positive Tumor Cells Were Detected in 93% (111/119) of Carcinomas
4. Discussion
4.1. Calponin and its Cellular Functions
4.2. Differences in the Size of the Tumor Areas Stained for CK, Calponin, SMA, and Vimentin
4.3. All Rabbit Mammary Carcinomas Contained Non-Neoplastic Myoepithelial Cells
4.4. A Higher Number of Hypertrophic Myoepithelial Cells Was Associated with Increasing Age of the Rabbits
4.5. Most Carcinomas Contained Calponin-Positive Tumor Cells
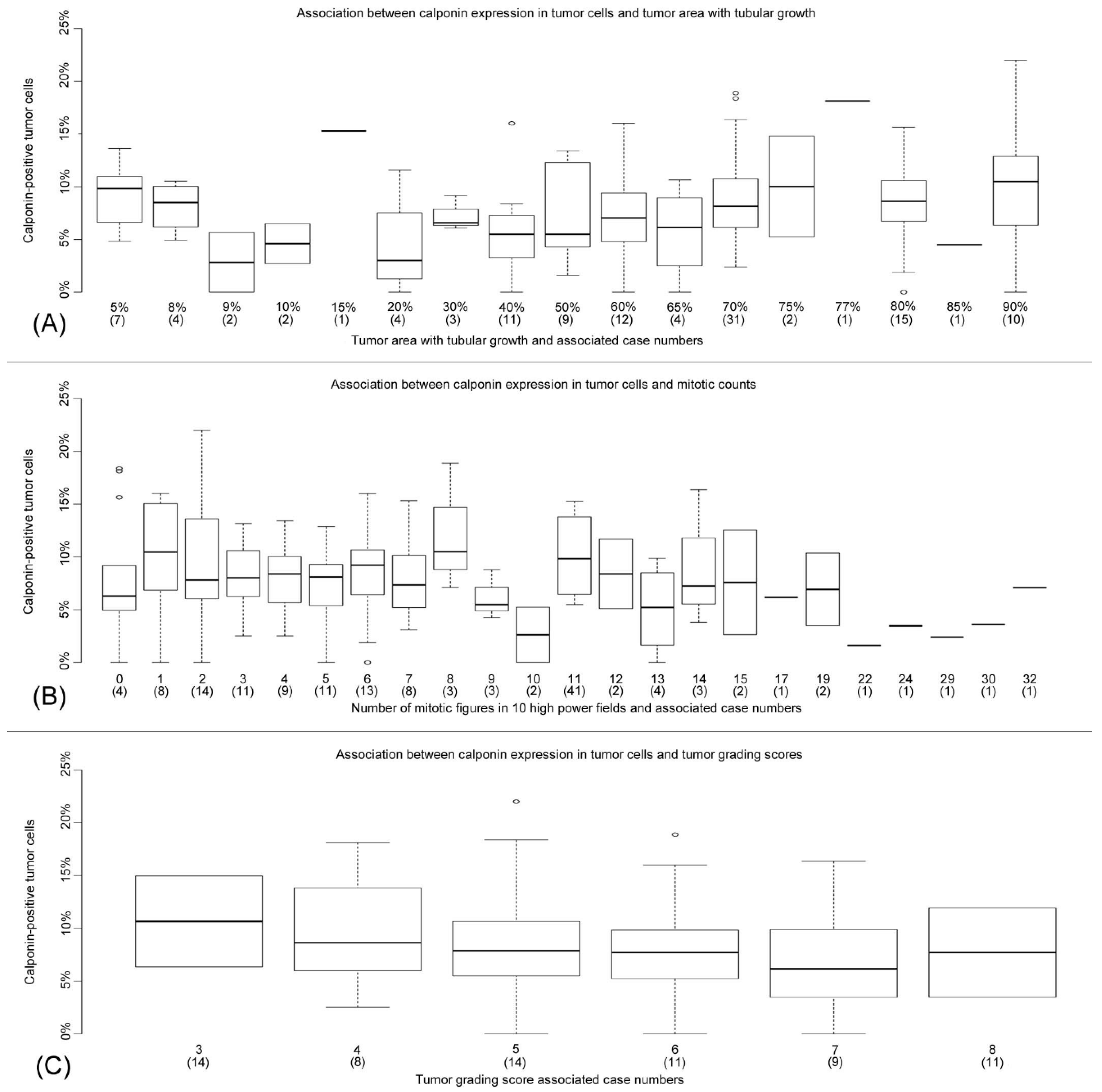
4.6. A Correlation Exists between an Increase in Calponin-Positive Tumor Cells and Histological Features Associated with Reduced Proliferation and Higher Differentiation/Lower Tumor Grade
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeMello, M. Rabbits multiplying like rabbits: The rise in the worldwide popularity of rabbits as pets. In Companion Animals in Everyday Life; Pręgowski, M.P., Ed.; Palgrave Macmillan: New York, NY, USA, 2016; pp. 91–107. [Google Scholar]
- Barthold, S.W.; Griffey, S.M.; Percy, D.H. Pathology of Laboratory Rodents and Rabbits, 4th ed.; John Wiley & Sons: Ames, IA, USA, 2016; pp. 253–323. [Google Scholar]
- Schöniger, S.; Horn, L.C.; Schoon, H.A. Tumors and tumor-like lesions in the mammary gland of 24 pet rabbits: A histomorphological and immunohistochemical characterization. Vet. Pathol. 2014, 51, 569–580. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.; Hewicker-Trautwein, M. Classification and epidemiology of mammary tumours in pet rabbits (Oryctolagus cuniculus). J. Comp. Pathol. 2015, 152, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Degner, S.; Schoon, H.-A.; Laik-Schandelmaier, C.; Aupperle-Lellbach, H.; Schöniger, S. Estrogen receptor-α and progesterone receptor expression in mammary proliferative lesions of female pet rabbits. Vet. Pathol. 2018, 55, 838–848. [Google Scholar] [CrossRef] [PubMed]
- Shahbazfar, A.A.; Mohammadpour, H.; Isfahani, H.R.E. Mammary gland carcinoma in a New Zealand white rabbit (Oryctolagus cuniculus). Acta Sci. Vet. 2012, 40, 1–4. [Google Scholar]
- Arrington, L.R.; Kelly, K.C. Domestic Rabbit Biology and Production; University of Florida Press: Gainsville, FL, USA, 1976; p. 47. [Google Scholar]
- Dewar, R.; Fadare, O.; Gilmore, H.; Gown, A.M. Best practices in diagnostic immunohistochemistry: Myoepithelial markers in breast pathology. Arch. Pathol. Lab. Med. 2011, 135, 422–429. [Google Scholar] [CrossRef]
- Pandey, P.R.; Saidou, J.; Watabe, K. Role of myoepithelial cells in breast tumor progression. Front. Biosci. 2010, 15, 226–236. [Google Scholar] [CrossRef]
- Sirka, O.K.; Shamir, E.R.; Ewald, A.J. Myoepithelial cells are a dynamic barrier to epithelial dissemination. J. Cell Biol. 2018, 217, 3368–3381. [Google Scholar] [CrossRef]
- Adriance, M.C.; Inman, J.L.; Petersen, O.W.; Bissell, M.J. Myoepithelial cells: Good fences make good neighbors. Breast Cancer Res. 2005, 7, 190–197. [Google Scholar] [CrossRef]
- Gama, A.; Alves, A.; Gartner, F.; Schmitt, F. p63: A novel myoepithelial cell marker in canine mammary tissues. Vet. Pathol. 2003, 40, 412–420. [Google Scholar] [CrossRef]
- De las Mulas, J.M.; Reymundo, C.; de los Monteros, A.E.; Millán, Y.; Ordás, J. Calponin expression and myoepithelial cell differentiation in canine, feline and human mammary simple carcinomas. Vet. Comp. Oncol. 2004, 2, 24–35. [Google Scholar] [CrossRef]
- Beha, G.; Sarli, G.; Brunetti, B.; Sassi, F.; Ferrara, D.; Benazzi, C. Morphology of the myoepithelial cell: Immunohistochemical characterization from resting to motile phase. Sci. World J. 2012, 2012, 252034. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Céspedes, R.; Millán, Y.; Guil-Luna, S.; García-Monterde, J.; Reymundo, C.; de los Monteros, A.E.; de las Mulas, J.M. Myoepithelial cell layer integrity in canine mammary carcinoma. J. Comp. Pathol. 2011, 145, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Zappulli, V.; Caliari, D.; Rasotto, R.; Ferro, S.; Castagnaro, M.; Goldschmidt, M. Proposed classification of the feline ‘‘complex’’ mammary tumors as ductal and intraductal papillary mammary tumors. Vet. Pathol. 2013, 50, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Ordóñez, N.G. Broad-spectrum immunohistochemical epithelial markers: A review. Hum. Pathol. 2013, 44, 1195–1215. [Google Scholar] [CrossRef] [PubMed]
- Grandi, D.; Campanini, N.; Becchi, G.; Lazzaretti, M. On the myoepithelium of human salivary glands. An immunocytochemical study. Eur. J. Morphol. 2000, 38, 249–255. [Google Scholar] [CrossRef]
- Gugliotta, P.; Sapino, A.; Macrí, L.; Skalli, O.; Gabbiani, G.; Bussolati, G. Specific demonstration of myoepithelial cells by anti-alpha smooth muscle actin antibody. J. Histochem. Cytochem. 1988, 36, 659–663. [Google Scholar] [CrossRef]
- Yamin, R.; Morgan, K.G. Deciphering actin cytoskeletal function in the contractile vascular smooth muscle cell. J. Physiol. 2012, 590, 4145–4154. [Google Scholar] [CrossRef]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. Histopathology 1991, 19, 403–410. [Google Scholar] [CrossRef]
- Aupperle, H.; Ozgen, S.; Schoon, H.A.; Schoon, D.; Hoppen, H.O.; Sieme, H.; Tannapfel, A. Cyclical endometrial steroid hormone receptor expression and proliferation intensity in the mare. Equine Vet. J. 2000, 32, 228–232. [Google Scholar] [CrossRef]
- Ellis, I.O.; Lee, A.H.S.; Pinder, S.E.; Rakha, E.A. Tumors of the breast. In Diagnostic Histopathology of Tumors, 4th ed.; Fletcher, C.D.M., Ed.; Churchill Livingstone Elsevier: Edinburgh, UK, 2013; Volume 2, pp. 1057–1145. [Google Scholar]
- Liu, R.; Jin, J.P. Calponin isoforms CNN1, CNN2 and CNN3: Regulators for actin cytoskeleton functions in smooth muscle and non-muscle cells. Gene. 2016, 585, 143–153. [Google Scholar] [CrossRef]
- Taniguchi, S. Suppression of cancer phenotypes through a multifunctional actin-binding protein, calponin, that attacks cancer cells and simultaneously protects the host from invasion. Cancer Sci. 2005, 96, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Wang, X.; Bergan, R.C.; Jin, J.P. Diminished expression of h2-calponin in prostate cancer cells promotes cell proliferation, migration and the dependence of cell adhesion on substrate stiffness. FEBS Open Bio 2014, 24, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Los de Monteros, A.E.; Millán, M.Y.; Ordás, J.; Carrasco, L.; Reymundo, C.; las de Mulas, J.M. Immunolocalization of the smooth muscle-specific protein calponin in complex and mixed tumors of the mammary gland of the dog: Assessment of the morphogenetic role of the myoepithelium. Vet. Pathol. 2002, 39, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Polyak, K.; Hu, M. Do myoepithelial cells hold the key for breast tumor progression? J. Mammary Gland Biol. Neopl. 2005, 10, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Miyano, M.; Sayaman, R.W.; Stoiber, M.H.; Lin, C.H.; Stampfer, M.R.; Brown, J.B.; LaBarge, M.A. Age-related gene expression in luminal epithelial cells is driven by a microenvironment made from myoepithelial cells. Aging (Albany NY) 2017, 9, 2026–2051. [Google Scholar] [CrossRef] [PubMed]
- Petersen, O.W.; Nielsen, H.L.; Gudjonsson, T.; Villadsen, R.; Rønnov-Jessen, L.; Bissell, M.J. The plasticity of human breast carcinoma cells is more than epithelial to mesenchymal conversion. Breast Cancer Res. 2001, 3, 213–217. [Google Scholar] [CrossRef]
- Kesse-Adu, R.; Shousha, S. Myoepithelial markers are expressed in at least 29% of oestrogen receptor negative invasive breast carcinoma. Mod. Pathol. 2004, 17, 646–652. [Google Scholar] [CrossRef]
- Kaufmann, O.; Fietze, E.; Mengs, J.; Dietel, M. Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am. J. Clin. Pathol. 2001, 116, 823–830. [Google Scholar] [CrossRef]
- Yoshimura, H.; Nakahira, R.; Kishimoto, T.E.; Michishita, M.; Ohkusu-Tsukada, K.; Takahashi, K. Differences in indicators of malignancy between luminal epithelial cell type and myoepithelial cell type of simple solid carcinoma in the canine mammary gland. Vet. Pathol. 2014, 51, 1090–1095. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Ross, K.N.; Lander, E.S.; Golub, T.R. A molecular signature of metastasis in primary solid tumors. Nat. Genet. 2003, 33, 49–54. [Google Scholar] [CrossRef]
- Qiu, Z.; Chu, Y.; Xu, B.; Wang, Q.; Jiang, M.; Li, X.; Wang, G.; Yu, P.; Liu, G.; Wang, H.; et al. Increased expression of calponin 2 is a positive prognostic factor in pancreatic ductal adenocarcinoma. Oncotarget 2017, 8, 56428–56442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Takeoka, M.; Ehara, T.; Sagara, J.; Hashimoto, S.; Taniguchi, S. Calponin h1 induced a flattened morphology and supressed the growth of human fibrosarcoma HT1080 cells. Eur. J. Cancer. 2002, 38, 436–442. [Google Scholar] [CrossRef]
- Lakhani, S.R.; O’Hare, M.J. The mammary myoepithelial cell—Cinderella or ugly sister? Breast Cancer Res. 2001, 3. [Google Scholar] [CrossRef]
- Rakha, E.A.; Reis-Filho, J.S.; Ellis, I.O. Basal-like breast cancer: A critical review. J. Clin. Oncol. 2008, 26, 2568–2581. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, S.R.; Ellis, I.O.; Schnitt, S.J.; Tan, P.H.; van de Vijver, M.J. WHO Classification of Tumors of the Breast, 4th ed.; International Agency for Research on Cancer: Lyon, France, 2012; pp. 1–123. [Google Scholar]
| Antibody | Monoclonal (Mouse) | Clone | Company | Dilution | Antigen Retrieval |
|---|---|---|---|---|---|
| Anti-cytokeratin | antihuman | AE1/AE3 | Dako * | 1:50 | 95 °C, citrate buffer |
| Anti-vimentin | antiporcine | V9 | Dako * | 1:100 | None |
| Anti-calponin | antihuman | SPM 169 | Zytomed * | 1:200 | 95 °C, citrate buffer |
| Anti-SMA | antihuman | 1A4 | Dako * | 1:100 | None |
| Parameter 1 | Parameter 2 | Cases | Tests | p-Value/ρ-Value |
|---|---|---|---|---|
| Age | Calponin-positive SME | n = 97 | lm, Src | p = 0.049/ρ = −0.172 |
| Calponin-positive HME | n = 97 | lm, Src | p = 0.039/ρ = 0.139 | |
| Calponin-positive NME | n = 97 | lm | p = 0.710 | |
| Age categories ≤5 years (n = 56) >5 years (n = 41) | Calponin-positive SME | n = 97 | lm | p = 0.063 |
| Calponin-positive HME | n = 97 | lm | p = 0.060 | |
| Calponin-positive NME | n = 97 | lm | p = 0.842 | |
| sex categories f (n = 85) fs (n = 22) | Calponin-positive SME | n = 97 | lm | p = 0.730 |
| Calponin-positive HME | n = 107 | lm | p = 0.943 | |
| Calponin-positive NME | n = 107 | lm | p = 0.164 | |
| Percentage of tubular growth | Calponin-positive SME | n = 119 | lm | p = 0.366 |
| Calponin-positive HME | n = 115 | lm | p = 0.723 | |
| Calponin-positive NME | n = 111 | lm, Src | p = 0.044/ρ = 0.214 | |
| Mitotic figures/ 10 HPFs | Calponin-positive SME | n = 119 | lm | p = 0.605 |
| Calponin-positive HME | n = 115 | lm | p = 0.185 | |
| Calponin-positive NME | n = 111 | lm, Src | p = 0.026/ρ = −0.147 | |
| Grading scores 3–9 | Calponin-positive SME | n = 119 | lm | p = 0.993 |
| Calponin-positive HME | n = 115 | lm | p = 0.547 | |
| Calponin-positive NME | n = 111 | lm, Src | p = 0.048/p = −0.157 |
| Immunostaining/ Marker | Epithelial Tumor Area Range (Median Value) | IRSarea Range (Median Value) | H-scorearea Range (Median Value) |
|---|---|---|---|
| Cytokeratin | 13.75% to 100.00% (78.09%) | 0.28 to 5.35 (2.06) | 17.64 to 199.73 (110.44) |
| Calponin | 5.55% to 87.62% (52.53%) | 0.06 to 3.09 (0.96) | 5.55 to 126.42 (76.20) |
| Vimentin | 2.80% to 84.18% (47.95%) | 0.03 to 2.53 (1.07) | 2.80 to 114.05 (67.41) |
| Smooth muscle actin | 1.31% to 88.82% (45.08%) | 0.01 to 2.64 (0.78) | 1.31 to 124.45 (56.93) |
| Immunostaining/ Marker | Spindle-Shaped ME (n = 119) Range (Median Value) | Hypertrophic ME (n = 115) Range (Median Value) | Tumor Cells (n = 111) Range (Median Value) |
|---|---|---|---|
| Smooth muscle actin | 20.64–100.00% (67.17%) | 0.00–79.36% (32.83%) | 0.00% |
| Vimentin | 19.85–100.00% (67.41%) | 0.00–80.15% (32.59%) | 0.00% |
| Calponin | 13.07–100.00% (53.22%) | 0.00–70.90% (39.01%) | 0.00–21.99% (7.42%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Degner, S.; Schoon, H.-A.; Degner, S.; Baudis, M.; Schandelmaier, C.; Aupperle-Lellbach, H.; Schöniger, S. Expression of Myoepithelial Markers in Mammary Carcinomas of 119 Pet Rabbits. Animals 2019, 9, 740. https://doi.org/10.3390/ani9100740
Degner S, Schoon H-A, Degner S, Baudis M, Schandelmaier C, Aupperle-Lellbach H, Schöniger S. Expression of Myoepithelial Markers in Mammary Carcinomas of 119 Pet Rabbits. Animals. 2019; 9(10):740. https://doi.org/10.3390/ani9100740
Chicago/Turabian StyleDegner, Sophie, Heinz-Adolf Schoon, Sebastian Degner, Mathias Baudis, Claudia Schandelmaier, Heike Aupperle-Lellbach, and Sandra Schöniger. 2019. "Expression of Myoepithelial Markers in Mammary Carcinomas of 119 Pet Rabbits" Animals 9, no. 10: 740. https://doi.org/10.3390/ani9100740
APA StyleDegner, S., Schoon, H.-A., Degner, S., Baudis, M., Schandelmaier, C., Aupperle-Lellbach, H., & Schöniger, S. (2019). Expression of Myoepithelial Markers in Mammary Carcinomas of 119 Pet Rabbits. Animals, 9(10), 740. https://doi.org/10.3390/ani9100740






