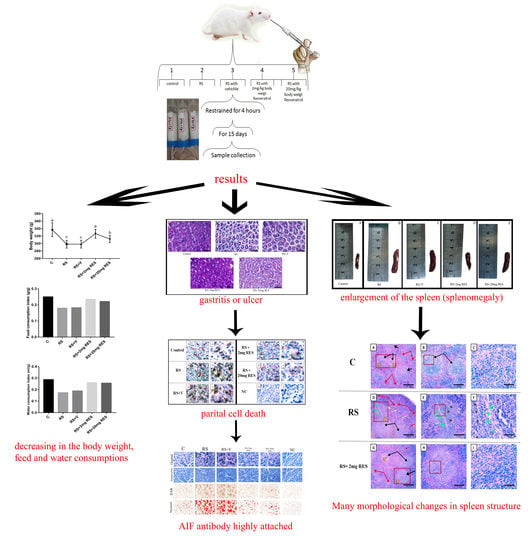
Resveratrol Protects against Restraint Stress Effects on Stomach and Spleen in Adult Male Mice
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Restraint Stress Protocol
2.3. Experimental Design
2.4. Body Weight and Food and Water Consumption Indexes
2.5. Histologic Analysis
2.6. Measurements of Parietal Cell Diameter
2.7. Immunohistochemistry (IHC)
2.8. IHC Quantification through Digital Image Analysis
2.9. Spleen Size, Length, and Weight
2.10. Statistical Analysis
3. Results
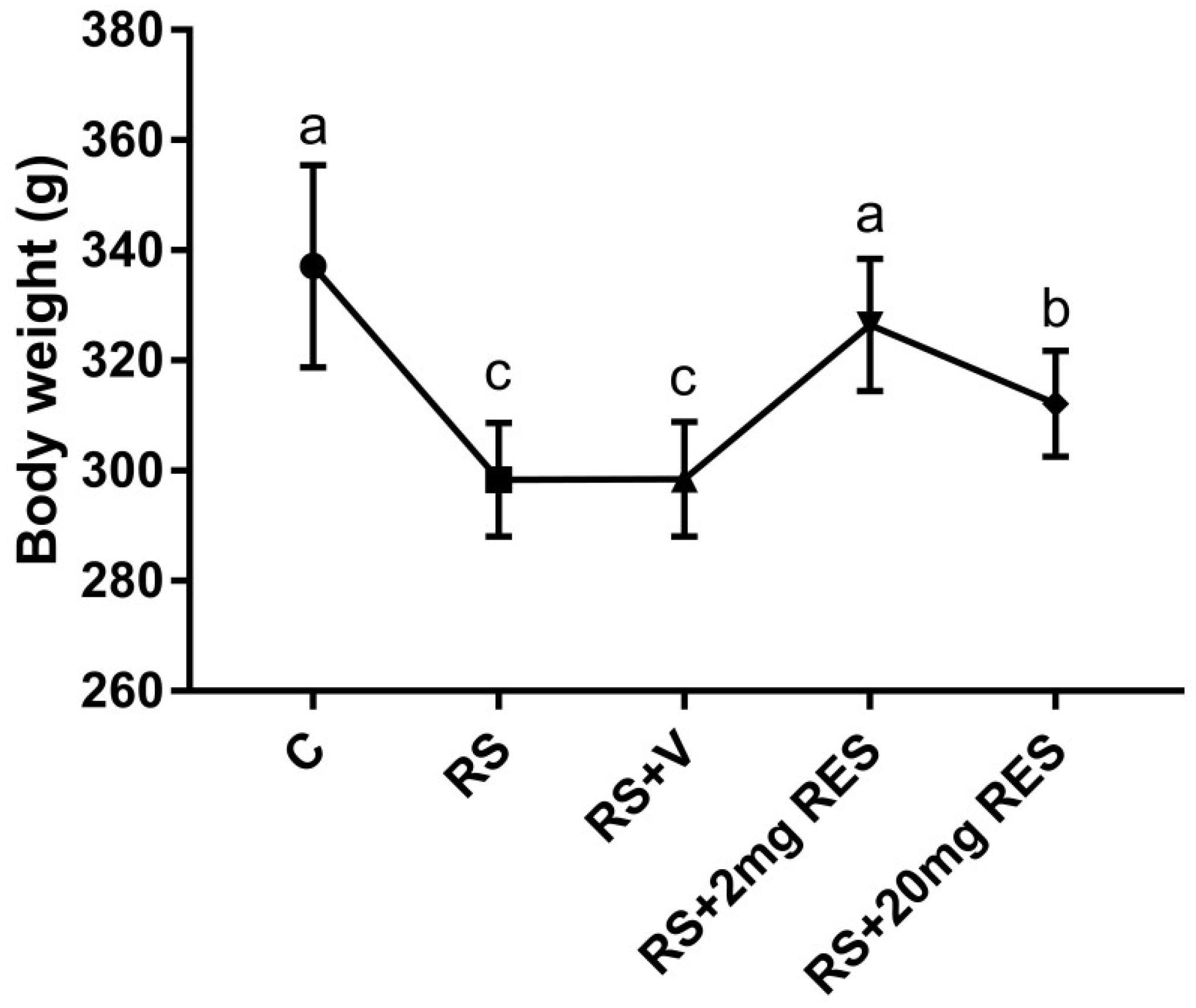
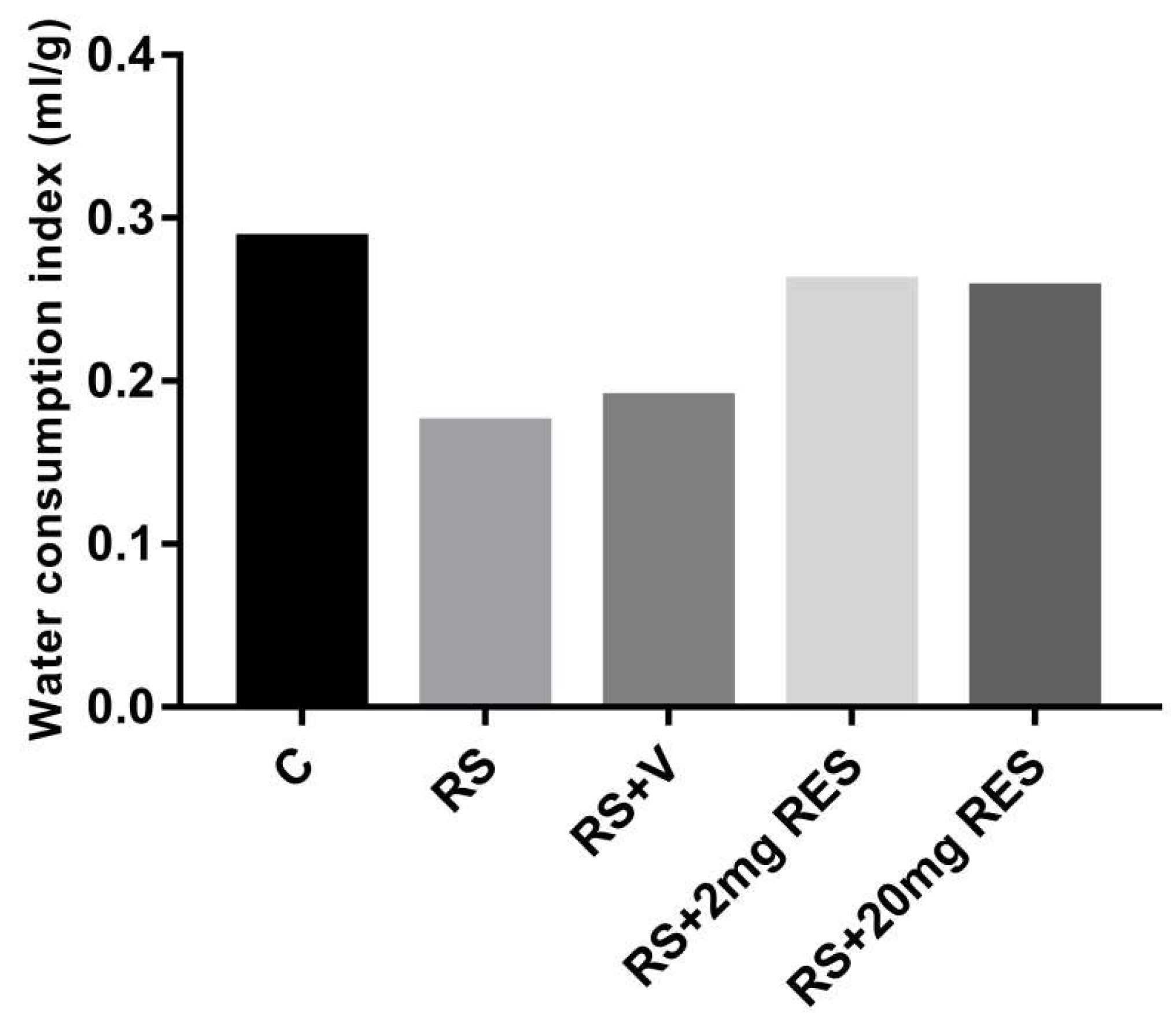
3.1. Effects of Restraint Stress on Body Weight and Food and Water Consumption
3.2. Effects of Resveratrol on Gastric Histology after Restraint Stress
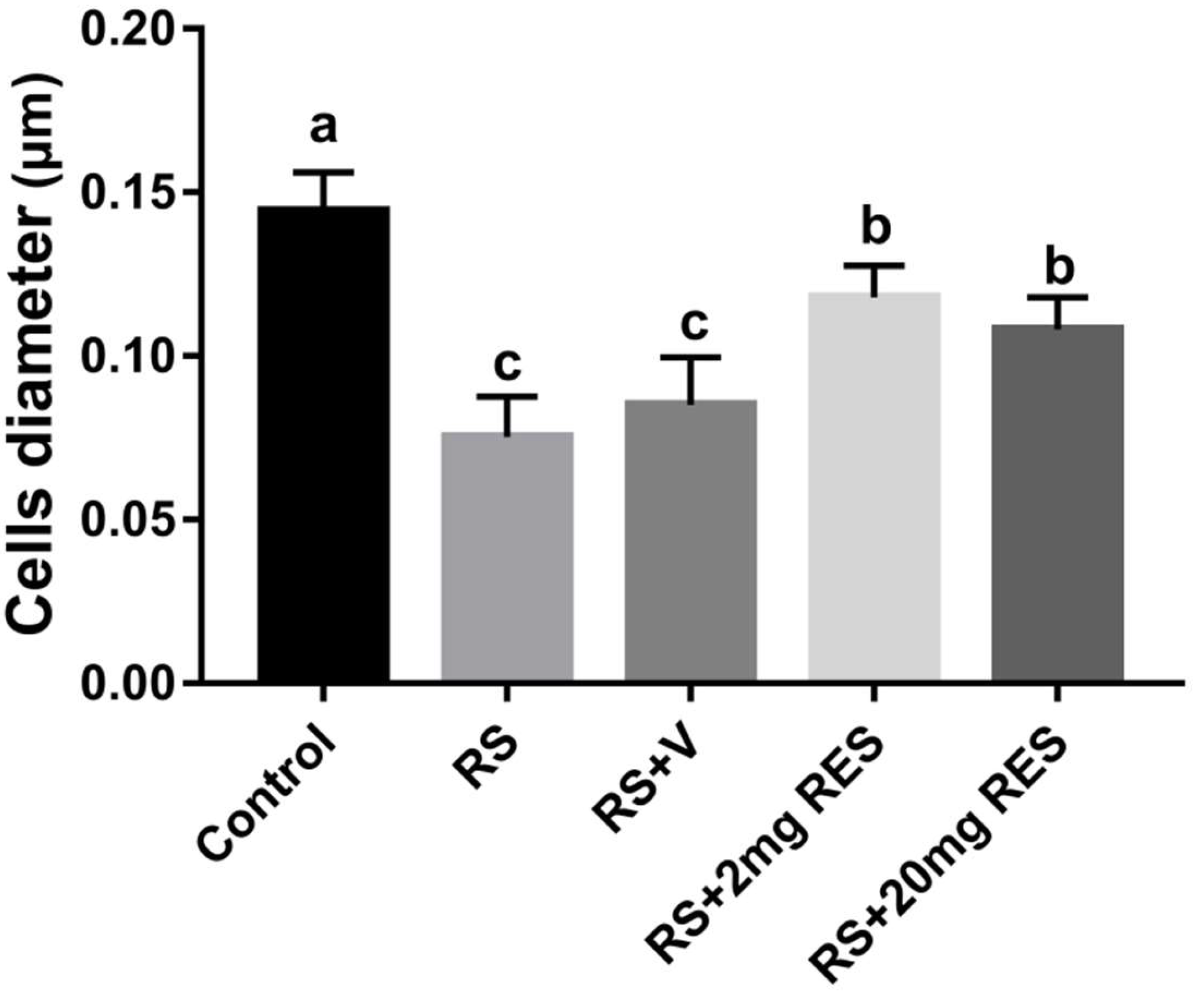
3.3. Effects of Restraint Stress on Parietal Cell Diameter
3.4. Immunohistochemical Staining of Parietal Cells of the Mouse Stomach
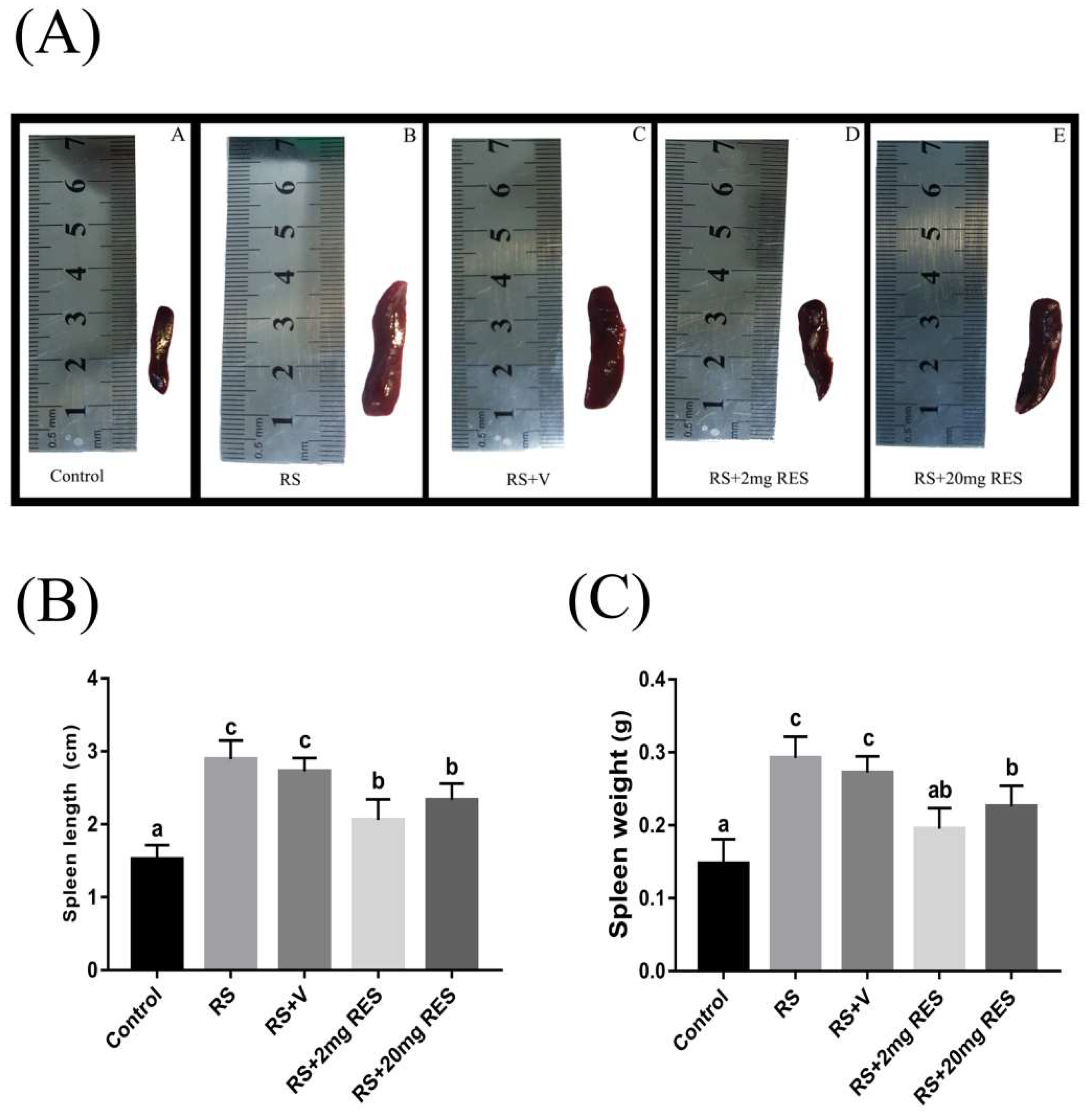
3.5. Effects of Restraint Stress on Spleen Size, Length, and Weight
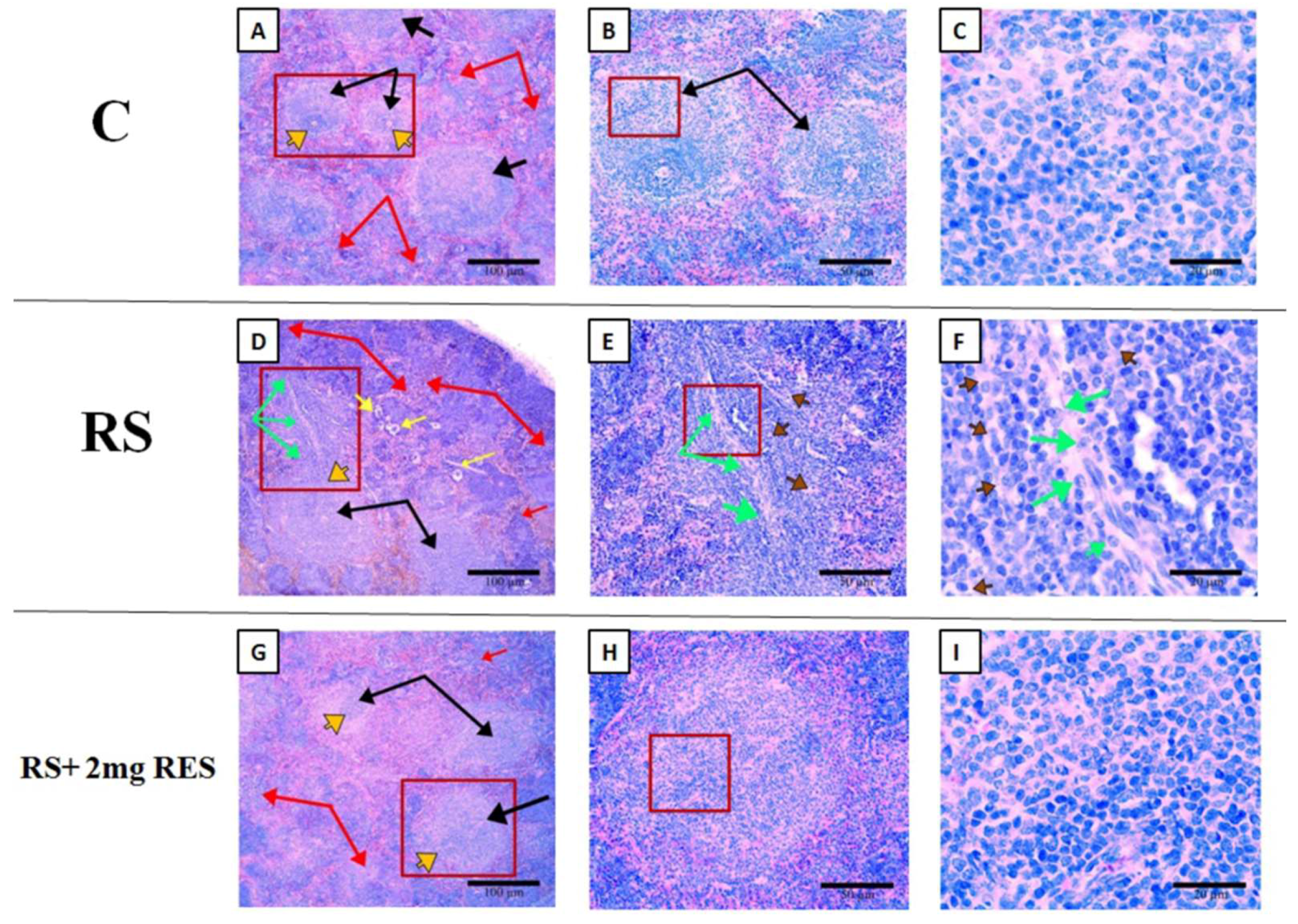
3.6. Effects of Resveratrol on Histologic Alterations in the Spleen Due to Restraint Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muthukumar, K.; Nachiappan, V. Cadmium-Induced Oxidative Stress in Saccharomyces Cerevisiae; NISCAIR-CSIR: New Delhi, India, 2010. [Google Scholar]
- Glavin, G.B.; Paré, W.P.; Sandbak, T.; Bakke, H.-K.; Murison, R. Restraint stress in biomedical research: An update. Neurosci. Biobehav. Rev. 1994, 18, 223–249. [Google Scholar] [CrossRef]
- Pacak, K.; Palkovits, M. Stressor specificity of central neuroendocrine responses: Implications for stress-related disorders. Endocr. Rev. 2001, 22, 502–548. [Google Scholar] [CrossRef] [PubMed]
- Patidar, G.; Shaikh, A. Antistress Potential of Glycyrrhizin in Chronic Immobilization Stress. Biomed. Pharmacol. J. 2015, 5, 273–283. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Gold, P.W. The concepts of stress and stress system disorders: Overview of physical and behavioral homeostasis. JAMA 1992, 267, 1244–1252. [Google Scholar] [CrossRef]
- Chrousos, G.P. Stress and disorders of the stress system. Nat. Rev. Endocrinol. 2009, 5, 374. [Google Scholar] [CrossRef]
- Kessler, R.C. The effects of stressful life events on depression. Annu. Rev. Psychol. 1997, 48, 191–214. [Google Scholar] [CrossRef]
- Moloney, R.D.; Desbonnet, L.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The microbiome: Stress, health and disease. Mamm. Genome 2014, 25, 49–74. [Google Scholar] [CrossRef]
- Hammen, C. Stress and depression. Annu. Rev. Clin. Psychol. 2005, 1, 293–319. [Google Scholar] [CrossRef]
- Wichers, M.; Maes, M. The psychoneuroimmuno-pathophysiology of cytokine-induced depression in humans. Int. J. Neuropsychopharmacol. 2002, 5, 375–388. [Google Scholar] [CrossRef]
- Helander, H.F.; Leth, R.; Olbe, L. Stereological investigations on human gastric mucosa: I. Normal oxyntic mucosa. Anat. Rec. 1986, 216, 373–380. [Google Scholar] [CrossRef]
- Yao, X.; Forte, J.G. Cell biology of acid secretion by the parietal cell. Annu. Rev. Physiol. 2003, 65, 103–131. [Google Scholar] [CrossRef]
- Kulnigg-Dabsch, S. Autoimmune gastritis. Wien. Med. Wochenschr. 2016, 166, 424–430. [Google Scholar] [CrossRef]
- Nomura, S.; Yamaguchi, H.; Ogawa, M.; Wang, T.C.; Lee, J.R.; Goldenring, J.R. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2005, 288, G362–G375. [Google Scholar] [CrossRef]
- Nozaki, K.; Ogawa, M.; Williams, J.A.; Lafleur, B.J.; Ng, V.; Drapkin, R.I.; Mills, J.C.; Konieczny, S.F.; Nomura, S.; Goldenring, J.R. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology 2008, 134, 511–522. [Google Scholar] [CrossRef]
- McKenzie, C.V.; Colonne, C.K.; Yeo, J.H.; Fraser, S.T. Splenomegaly: Pathophysiological bases and therapeutic options. Int. J. Biochem. Cell Biol. 2018, 94, 40–43. [Google Scholar] [CrossRef]
- Wu, X.-N. Current concept of Spleen-Stomach theory and Spleen deficiency syndrome in TCM. World J. Gastroenterol. 1998, 4, 2. [Google Scholar] [CrossRef]
- Jain, S. Ethnobotany and research in medicinal plants in India. Ethnobot. Search New Drugs 1994, 185, 153–168. [Google Scholar]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337. [Google Scholar] [CrossRef]
- Bereswill, S.; Muñoz, M.; Fischer, A.; Plickert, R.; Haag, L.-M.; Otto, B.; Kühl, A.A.; Loddenkemper, C.; Göbel, U.B.; Heimesaat, M.M. Anti-inflammatory effects of resveratrol, curcumin and simvastatin in acute small intestinal inflammation. PLoS ONE 2010, 5, e15099. [Google Scholar] [CrossRef]
- Joza, N.; Pospisilik, J.A.; Hangen, E.; Hanada, T.; Modjtahedi, N.; Penninger, J.M.; Kroemer, G. AIF: Not just an apoptosis-inducing factor. Ann. N. Y. Acad. Sci. 2009, 1171, 2–11. [Google Scholar] [CrossRef]
- Candé, C.; Cohen, I.; Daugas, E.; Ravagnan, L.; Larochette, N.; Zamzami, N.; Kroemer, G. Apoptosis-inducing factor (AIF): A novel caspase-independent death effector released from mitochondria. Biochimie 2002, 84, 215–222. [Google Scholar] [CrossRef]
- Bano, D.; Prehn, J.H. Apoptosis-inducing factor (AIF) in physiology and disease: The tale of a repented natural born killer. EBioMedicine 2018, 30, 29–37. [Google Scholar] [CrossRef]
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441. [Google Scholar] [CrossRef]
- Norbury, C.J.; Hickson, I.D. Cellular responses to DNA damage. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 367–401. [Google Scholar] [CrossRef]
- Park, E.-J.; Pezzuto, J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2015, 1852, 1071–1113. [Google Scholar] [CrossRef]
- Iwakabe, K.; Shimada, M.; Ohta, A.; Yahata, T.; Ohmi, Y.; Habu, S.; Nishimura, T. The restraint stress drives a shift in Th1/Th2 balance toward Th2-dominant immunity in mice. Immunol. Lett. 1998, 62, 39–43. [Google Scholar] [CrossRef]
- Mehfooz, A.; Wei, Q.; Zheng, K.; Fadlalla, M.B.; Maltasic, G.; Shi, F. Protective roles of Rutin against restraint stress on spermatogenesis in testes of adult mice. Tissue Cell 2018, 50, 133–143. [Google Scholar] [CrossRef]
- Shi, F.; LaPolt, P. Relationship between FoxO1 protein levels and follicular development, atresia, and luteinization in the rat ovary. J. Endocrinol. 2003, 179, 195–204. [Google Scholar] [CrossRef]
- Zhang, W.; Wei, Q.-W.; Wang, Z.-C.; Ding, W.; Wang, W.; Shi, F.-X. Cell-specific expression and immunolocalization of nitric oxide synthase isoforms and the related nitric oxide/cyclic GMP signaling pathway in the ovaries of neonatal and immature rats. J. Zhejiang Univ. Sci. B 2011, 12, 55–64. [Google Scholar] [CrossRef]
- Wei, Q.; Fedail, J.S.; Kong, L.; Zheng, K.; Meng, C.; Fadlalla, M.B.; Shi, F. Thyroid hormones alter estrous cyclicity and antioxidative status in the ovaries of rats. Anim. Sci. J. (Nihon Chikusan Gakkaiho) 2018, 89, 513–526. [Google Scholar] [CrossRef]
- Wei, Q.; Li, J.; Li, X.; Zhang, L.; Shi, F. Reproductive toxicity in acrylamide-treated female mice. Reprod. Toxicol. (Elmsford, NY) 2014, 46, 121–128. [Google Scholar] [CrossRef]
- Wei, Q.; Wu, G.; Xing, J.; Mao, D.; Hutz, R.J.; Shi, F. Roles of poly (ADP-ribose) polymerase 1 activation and cleavage in induction of multi-oocyte ovarian follicles in the mouse by 3-nitropropionic acid. Reprod. Fertil. Dev. 2019. [Google Scholar] [CrossRef]
- Wu, G.; Wei, Q.; Yu, D.; Shi, F. Neonatal genistein exposure disrupts ovarian and uterine development in the mouse by inhibiting cellular proliferation. J. Reprod. Dev. 2019, 65, 7–17. [Google Scholar] [CrossRef]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef]
- Korejo, N.A.; Quanwei, W.; Zheng, K.; Dagan, M.; Korejo, R.A.; Shah, A.H.; Fangxiong, S. Contemporaneous effects of diabetes mellitus and hypothyroidism on spermatogenesis and immunolocalization of Claudin-11 inside the seminiferous tubules of mice. BMC Dev. Biol. 2018, 18, 15. [Google Scholar] [CrossRef]
- Korejo, N.A.; Wei, Q.-W.; Shah, A.H.; Shi, F.-X. Effects of concomitant diabetes mellitus and hyperthyroidism on testicular and epididymal histoarchitecture and steroidogenesis in male animals. J. Zhejiang Univ. Sci. B 2016, 17, 850–863. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Panahi, Y.; Sahraei, H.; Johnston, T.P.; Sahebkar, A. The impact of stress on body function: A review. EXCLI J. 2017, 16, 1057. [Google Scholar]
- Frémont, L. Biological effects of resveratrol. Life Sci. 2000, 66, 663–673. [Google Scholar] [CrossRef]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493. [Google Scholar] [CrossRef]
- Vallès, A.; Martí, O.; García, A.; Armario, A. Single exposure to stressors causes long-lasting, stress-dependent reduction of food intake in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2000, 279, R1138–R1144. [Google Scholar] [CrossRef]
- Cordeiro, A.; de Souza, L.L.; Oliveira, L.S.; Faustino, L.C.; Santiago, L.A.; Bloise, F.F.; Ortiga-Carvalho, T.M.; dos Santos Almeida, N.A.; Pazos-Moura, C.C. Thyroid hormone regulation of Sirtuin 1 expression and implications to integrated responses in fasted mice. J. Endocrinol. 2013, 216, 181–193. [Google Scholar] [CrossRef]
- Ferreira, E.; Silva, A.; Serakides, R.; Gomes, A.; Cassali, G.D. Model of induction of thyroid dysfunctions in adult female mice. Arquivo Brasileiro de Medicina Veterinária e Zootecnia 2007, 59, 1245–1249. [Google Scholar] [CrossRef]
- Jia, Y.-T.; Wei, W.; Ma, B.; Xu, Y.; Liu, W.-J.; Wang, Y.; Lv, K.-Y.; Tang, H.-T.; Wei, D.; Xia, Z.-F. Activation of p38 MAPK by reactive oxygen species is essential in a rat model of stress-induced gastric mucosal injury. J. Immunol. 2007, 179, 7808–7819. [Google Scholar] [CrossRef]
- Fatemeh, N.; Mohammad, V.; Hedayat, S.; Soheila, A.; Ehsan, S. Physical and psychological stress have similar effects on gastric acid and pepsin secretions in rat. J. Stress Physiol. Biochem. 2011, 7, 164. [Google Scholar]
- Bitgul, G.; Tekmen, I.; Keles, D.; Oktay, G. Protective effects of resveratrol against chronic immobilization stress on testis. ISRN Urol. 2013, 2013. [Google Scholar] [CrossRef]
- Jiang, Y.-G.; Tao, P.; Yong, L.; Li, M.-C.; Lin, Y.-H. Resveratrol reestablishes spermatogenesis after testicular injury in rats caused by 2, 5-hexanedione. Chin. Med J. 2008, 121, 1204–1209. [Google Scholar] [CrossRef]
- Wenzel, E.; Somoza, V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005, 49, 472–481. [Google Scholar] [CrossRef]
- Leonard, S.S.; Xia, C.; Jiang, B.-H.; Stinefelt, B.; Klandorf, H.; Harris, G.K.; Shi, X. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochem. Biophys. Res. Commun. 2003, 309, 1017–1026. [Google Scholar] [CrossRef]
- Larrosa, M.; Yañéz-Gascón, M.A.J.; Selma, M.A.V.; Gonzélez-Sarrías, A.; Toti, S.; Cerón, J.J.N.; Tomás-Barberán, F.; Dolara, P.; Espín, J.C. Effect of a low dose of dietary resveratrol on colon microbiota, inflammation and tissue damage in a DSS-induced colitis rat model. J. Agric. Food Chem. 2009, 57, 2211–2220. [Google Scholar] [CrossRef]
- Ma, Z.-H.; Ma, Q.-Y.; Wang, L.-C.; Sha, H.-C.; Wu, S.-L.; Zhang, M. Effect of resveratrol on peritoneal macrophages in rats with severe acute pancreatitis. Inflamm. Res. 2005, 54, 522–527. [Google Scholar] [CrossRef]
- Repetto, M.; Llesuy, S. Antioxidant properties of natural compounds used in popular medicine for gastric ulcers. Braz. J. Med Biol. Res. 2002, 35, 523–534. [Google Scholar] [CrossRef]
- Dey, A.; Guha, P.; Chattopadhyay, S.; Bandyopadhyay, S.K. Biphasic activity of resveratrol on indomethacin-induced gastric ulcers. Biochem. Biophys. Res. Commun. 2009, 381, 90–95. [Google Scholar] [CrossRef]
- Bredemeyer, A.J.; Geahlen, J.H.; Weis, V.G.; Huh, W.J.; Zinselmeyer, B.H.; Srivatsan, S.; Miller, M.J.; Shaw, A.S.; Mills, J.C. The gastric epithelial progenitor cell niche and differentiation of the zymogenic (chief) cell lineage. Dev. Biol. 2009, 325, 211–224. [Google Scholar] [CrossRef]
- Syder, A.J.; Guruge, J.L.; Li, Q.; Hu, Y.; Oleksiewicz, C.M.; Lorenz, R.G.; Karam, S.M.; Falk, P.G.; Gordon, J.I. Helicobacter pylori attaches to NeuAcα2, 3Galβ1, 4 glycoconjugates produced in the stomach of transgenic mice lacking parietal cells. Mol. Cell 1999, 3, 263–274. [Google Scholar] [CrossRef]
- Lee, J.W.; Jeong, E.G.; Soung, Y.H.; Kim, S.Y.; Nam, S.W.; Kim, S.H.; Lee, J.Y.; Yoo, N.J.; Lee, S.H. Immunohistochemical analysis of apoptosis-inducing factor (AIF) expression in gastric carcinomas. Pathol. Res. Pract. 2006, 202, 497–501. [Google Scholar] [CrossRef]
- Konturek, P.C.; Brzozowski, T.; Konturek, S.; Pajdo, R.; Konturek, J.; Kwiecień, S.; Taut, A.; Hahn, E. Apoptosis in gastric mucosa with stress-induced gastric ulcers. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 1999, 50, 211–225. [Google Scholar]
- Yuluğ, E.; Türedi, S.; Alver, A.; Türedi, S.; Kahraman, C. Effects of resveratrol on methotrexate-induced testicular damage in rats. Sci. World J. 2013, 2013. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Ramprasath, V.R.; Aluko, R.E.; Jones, P.J. Potential of resveratrol in anticancer and anti-inflammatory therapy. Nutr. Rev. 2008, 66, 445–454. [Google Scholar] [CrossRef]
- Bhat, K.P.; Kosmeder, J.W.; Pezzuto, J.M. Biological effects of resveratrol. Antioxid. Redox Signal. 2001, 3, 1041–1064. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Sethi, G.; Vadhan-Raj, S.; Bueso-Ramos, C.; Takada, Y.; Gaur, U.; Nair, A.S.; Shishodia, S.; Aggarwal, B.B. Resveratrol inhibits proliferation, induces apoptosis, and overcomes chemoresistance through down-regulation of STAT3 and nuclear factor-κB–regulated antiapoptotic and cell survival gene products in human multiple myeloma cells. Blood 2007, 109, 2293–2302. [Google Scholar] [CrossRef]
- Campagna, M.; Rivas, C. Antiviral Activity of Resveratrol; Portland Press Limited: London, UK, 2010. [Google Scholar]
- Donnelly, L.E.; Newton, R.; Kennedy, G.E.; Fenwick, P.S.; Leung, R.H.; Ito, K.; Russell, R.E.; Barnes, P.J. Anti-inflammatory effects of resveratrol in lung epithelial cells: Molecular mechanisms. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L774–L783. [Google Scholar] [CrossRef]
- Kim, C.; Baek, S.H.; Um, J.-Y.; Shim, B.S.; Ahn, K.S. Resveratrol attenuates constitutive STAT3 and STAT5 activation through induction of PTPε and SHP-2 tyrosine phosphatases and potentiates sorafenib-induced apoptosis in renal cell carcinoma. BMC Nephrol. 2016, 17, 19. [Google Scholar] [CrossRef]
- Xu, W.; Lu, Y.; Yao, J.; Li, Z.; Chen, Z.; Wang, G.; Jing, H.; Zhang, X.; Li, M.; Peng, J. Novel role of resveratrol: Suppression of high-mobility group protein box 1 nucleocytoplasmic translocation by the upregulation of sirtuin 1 in sepsis-induced liver injury. Shock 2014, 42, 440–447. [Google Scholar] [CrossRef]
- Adamus, G.; Webb, S.; Shiraga, S.; Duvoisin, R.M. Anti-recoverin antibodies induce an increase in intracellular calcium, leading to apoptosis in retinal cells. J. Autoimmun. 2006, 26, 146–153. [Google Scholar] [CrossRef]
- Anekonda, T.S.; Adamus, G. Resveratrol prevents antibody-induced apoptotic death of retinal cells through upregulation of Sirt1 and Ku70. BMC Res. Notes 2008, 1, 122. [Google Scholar] [CrossRef]
- Pozo, A.L.; Godfrey, E.M.; Bowles, K.M. Splenomegaly: Investigation, diagnosis and management. Blood Rev. 2009, 23, 105–111. [Google Scholar] [CrossRef]
- Steplewski, Z.; Vogel, W.H.; Ehya, H.; Poropatich, C.; Smith, J.M. Effects of restraint stress on inoculated tumor growth and immune response in rats. Cancer Res. 1985, 45, 5128–5133. [Google Scholar]
- Li, Y.-F.; He, R.-R.; Tsoi, B.; Li, X.-D.; Li, W.-X.; Abe, K.; Kurihara, H. Anti-stress effects of carnosine on restraint-evoked immunocompromise in mice through spleen lymphocyte number maintenance. PLoS ONE 2012, 7, e33190. [Google Scholar] [CrossRef]
- Takada, T.; Yoshinari, N.; Sugiishi, S.; Kawase, H.; Yamane, T.; Noguchi, T. Effect of restraint stress on the progression of experimental periodontitis in rats. J. Periodontol. 2004, 75, 306–315. [Google Scholar] [CrossRef]
- Alamo, I.G.; Kannan, K.B.; Loftus, T.J.; Ramos, H.; Efron, P.A.; Mohr, A.M. Severe trauma and chronic stress activates extramedullary erythropoiesis. J. Trauma Acute Care Surg. 2017, 83, 144–150. [Google Scholar] [CrossRef]
- Szczepanek, S.M.; McNamara, J.T.; Secor, E.R., Jr.; Natarajan, P.; Guernsey, L.A.; Miller, L.A.; Ballesteros, E.; Jellison, E.; Thrall, R.S.; Andemariam, B. Splenic morphological changes are accompanied by altered baseline immunity in a mouse model of sickle-cell disease. Am. J. Pathol. 2012, 181, 1725–1734. [Google Scholar] [CrossRef]
- Hövelmeyer, N.; Wunderlich, F.T.; Massoumi, R.; Jakobsen, C.G.; Song, J.; Wörns, M.A.; Merkwirth, C.; Kovalenko, A.; Aumailley, M.; Strand, D. Regulation of B cell homeostasis and activation by the tumor suppressor gene CYLD. J. Exp. Med. 2007, 204, 2615–2627. [Google Scholar] [CrossRef]
- Kaul, R.; Murakami, M.; Choudhuri, T.; Robertson, E.S. Epstein-Barr virus latent nuclear antigens can induce metastasis in a nude mouse model. J. Virol. 2007, 81, 10352–10361. [Google Scholar] [CrossRef]
- Pathak, N.; Khandelwal, S. Role of oxidative stress and apoptosis in cadmium induced thymic atrophy and splenomegaly in mice. Toxicol. Lett. 2007, 169, 95–108. [Google Scholar] [CrossRef]
- Jabbarzare, M.; Voon Kin, C.; Talib, H.; Mun Fei, Y.; Siti Khadijah, A.; Hassan, H.; Majid, R.A.; Taib, C.N.M.; Moklas, M.A.M.; Hidayat, M.T. Interleukin-18 antagonism improved histopathological conditions of malaria infection in mice. Iran. J. Parasitol. 2015, 10, 389. [Google Scholar]
- Nai, G.A.; Cabello-Inchausti, B.; Suster, S. Anaplastic large cell lymphoma of the spleen. Pathol. Res. Pract. 1998, 194, 517–522. [Google Scholar] [CrossRef]


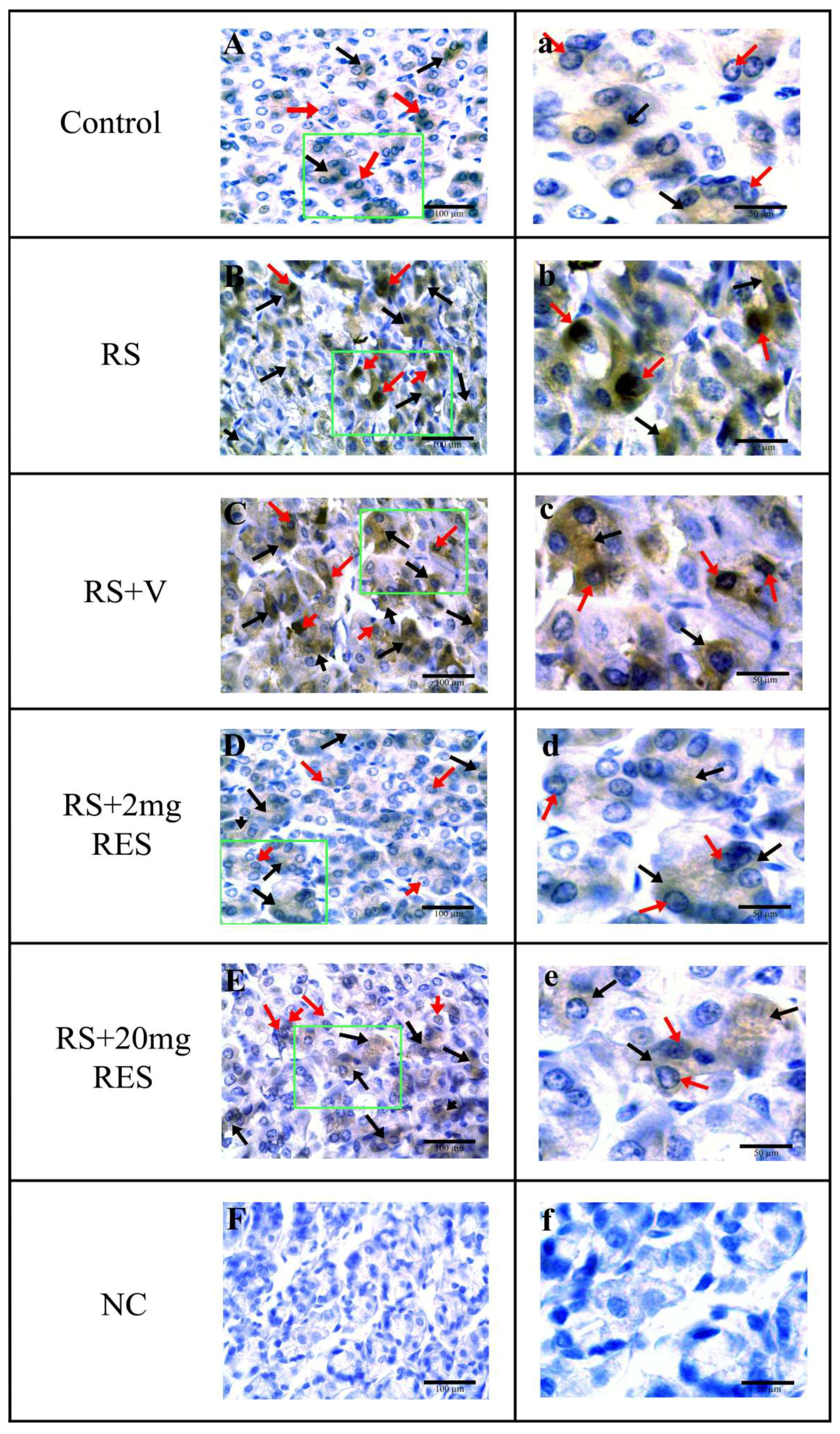
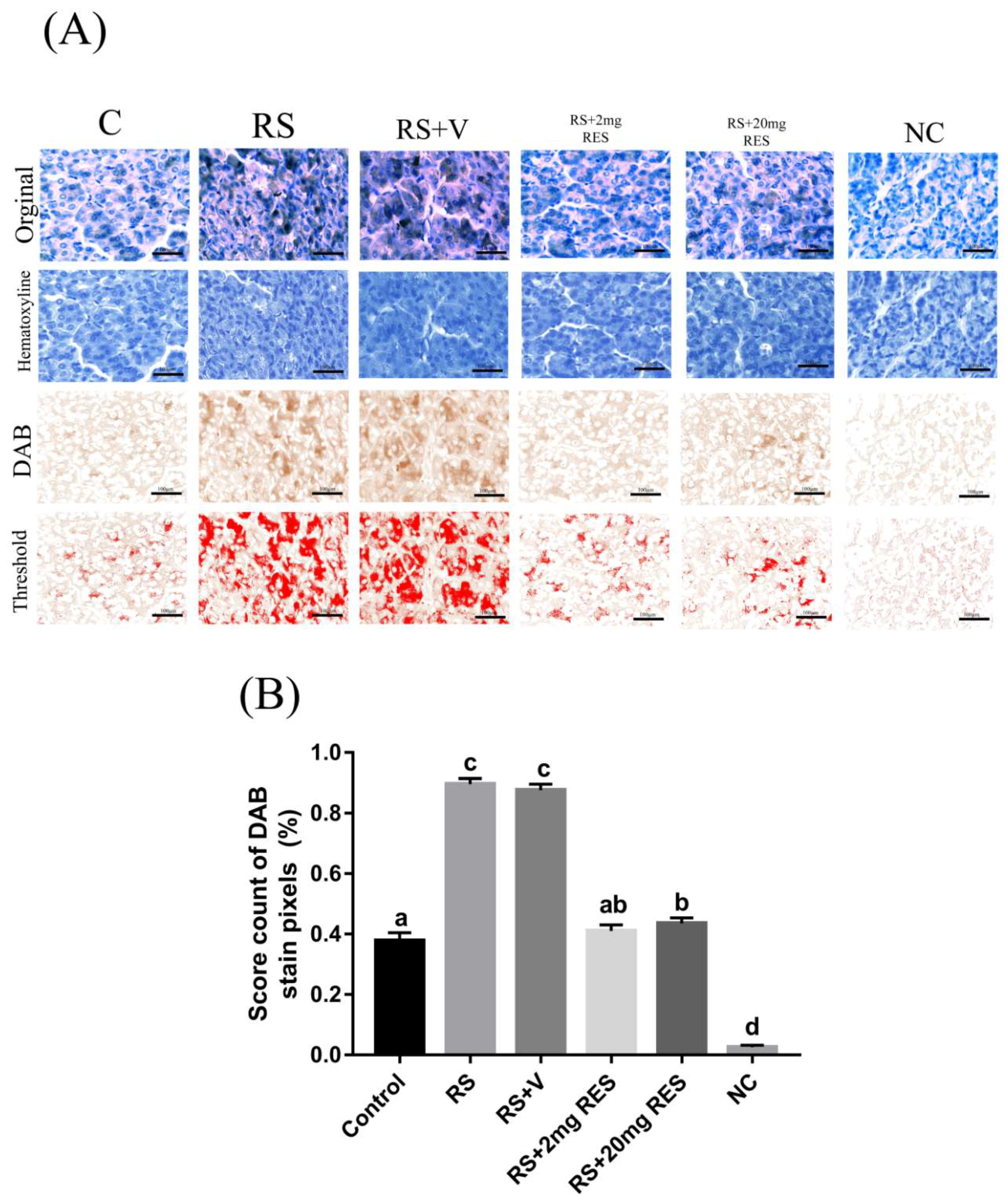
| Experimental Groups | Nucleus a | Cytoplasm a |
|---|---|---|
| Control | − | − |
| RS | +++ | +++ |
| RS + V | +++ | +++ |
| RS + 2mg RES | + | + |
| RS + 20mg RES | ++ | ++ |
| NC | N/A | N/A |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ennab, W.; Mustafa, S.; Wei, Q.; Lv, Z.; Kavita, N.M.X.; Ullah, S.; Shi, F. Resveratrol Protects against Restraint Stress Effects on Stomach and Spleen in Adult Male Mice. Animals 2019, 9, 736. https://doi.org/10.3390/ani9100736
Ennab W, Mustafa S, Wei Q, Lv Z, Kavita NMX, Ullah S, Shi F. Resveratrol Protects against Restraint Stress Effects on Stomach and Spleen in Adult Male Mice. Animals. 2019; 9(10):736. https://doi.org/10.3390/ani9100736
Chicago/Turabian StyleEnnab, Wael, Sheeraz Mustafa, Quanwei Wei, Zengpeng Lv, Ngekure M.X. Kavita, Saif Ullah, and Fangxiong Shi. 2019. "Resveratrol Protects against Restraint Stress Effects on Stomach and Spleen in Adult Male Mice" Animals 9, no. 10: 736. https://doi.org/10.3390/ani9100736
APA StyleEnnab, W., Mustafa, S., Wei, Q., Lv, Z., Kavita, N. M. X., Ullah, S., & Shi, F. (2019). Resveratrol Protects against Restraint Stress Effects on Stomach and Spleen in Adult Male Mice. Animals, 9(10), 736. https://doi.org/10.3390/ani9100736