Consequences and Management of Canine Brachycephaly in Veterinary Practice: Perspectives from Australian Veterinarians and Veterinary Specialists
Simple Summary
Abstract
1. Introduction
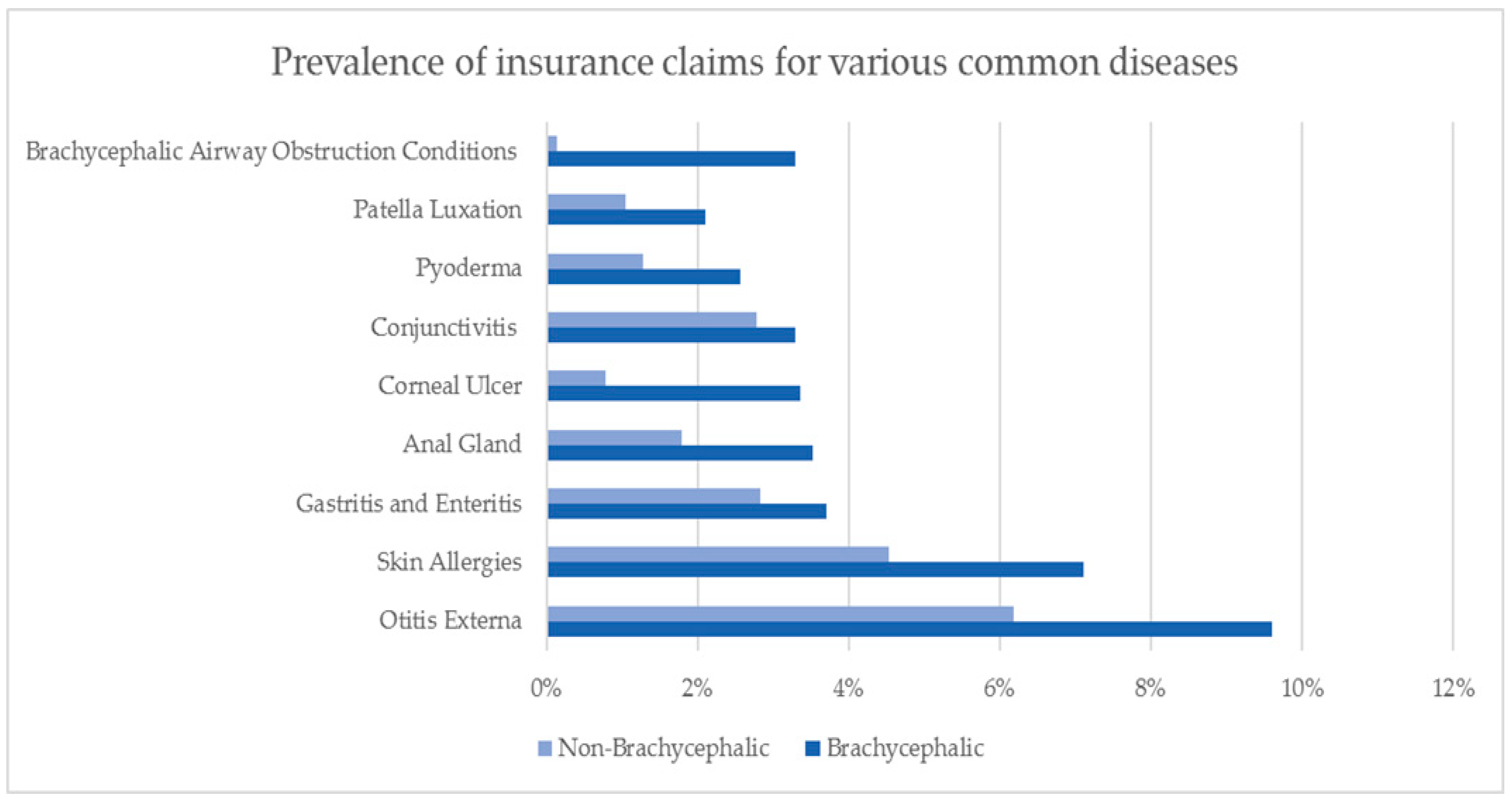
2. Australian Insurance Data
3. Stabilization of Respiratory Distress
3.1. Intubation
3.2. Thermoregulation
3.3. Complications
3.4. Extubation
3.5. Monitoring
4. Sedation and Anaesthesia
4.1. Peri-Anaesthetic Morbidity and Mortality
4.2. Anaesthetic Considerations
4.2.1. Upper Respiratory Obstruction and Poor Oxygenation
4.2.2. Poor Ventilation and Anaesthetic Uptake
4.2.3. Regurgitation
4.2.4. Agitated Recovery and Postoperative Inflammation
5. Surgical Treatment of Airway Abnormalities
5.1. Surgical Therapy
5.1.1. Stenotic Nares
5.1.2. Turbinectomy
5.1.3. Hyperplastic Soft-Palate
5.1.4. Everted Laryngeal Saccules
5.1.5. Laryngeal Collapse
5.2. Prognosis After Surgical Therapy
6. Effects of Brachycephaly on the Brain and Associated Neurologic Abnormalities
6.1. Clinical Signs and Diagnosis
6.2. Treatment
6.3. Other Neurological Conditions
7. Dermatological Conditions
8. Other Conditions
9. Behavioural Consequences of Canine Brachycephaly
10. Ethical Challenges Associated with Brachycephalic Breeds
“…the vast majority of us work in general practice and our income is based on mending people’s animals and getting paid for it, and, like it or not, a large number of those clients have brachycephalic dogs. In my practice alone we have a number of pug, shih-tzu and bulldog breeders and dozens of owners with squashed-nosed pets…If I stood up and told the truth about these breeds, I would immediately alienate them and they would up sticks and move to the neighbouring practice where the vet was not as outspoken. Vets in general practice simply cannot afford to be honest and to speak out. You would be hard-pushed to find a general practitioner who likes the concept of a brachycephalic dog but you would be equally hard-pushed to find one being openly critical of them because this would put their livelihood on the line.”[101]
11. The Veterinarian’s Role
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Packer, R.M.; Hendricks, A.; Tivers, M.S.; Burn, C.C. Impact of facial conformation on canine health: Brachycephalic obstructive airway syndrome. PLoS ONE 2015, 10, e0137496. [Google Scholar] [CrossRef] [PubMed]
- British Veterinary Association. BVA Position Statement on Brachycephalic Dogs. 2018. Available online: https://www.bva.co.uk/News-campaigns-and-policy/Newsroom/News-releases/BVA-and-BSAVA-statement-on-brachycephalic-breeds/ (accessed on 19 January 2018).
- Teng, K.T.; McGreevy, P.D.; Toribio, J.A.; Dhand, N.K. Trends in popularity of some morphological traits of purebred dogs in Australia. Canine Genet. Epidemiol. 2016, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Australian National Kennel Club. National Registration Statistics; ANKC: London, UK, 2018; Available online: www.ankc.org.au (accessed on 3 December 2018).
- Davis, M.S.; Cummings, S.L.; Payton, M.E. Effect of brachycephaly and body condition score on respiratory thermoregulation of healthy dogs. J. Am. Vet. Med. Assoc. 2017, 251, 1160–1165. [Google Scholar] [CrossRef] [PubMed]
- Pink, J.J.; Doyle, R.S.; Hughes, J.M.; Tobin, E.; Bellenger, C.R. Laryngeal collapse in seven brachycephalic puppies. J. Small Anim. Pract. 2006, 47, 131–135. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, D.G.; Jackson, C.; Guy, J.H.; Church, D.B.; McGreevy, P.D.; Thomson, P.C.; Brodbelt, D.C. Epidemiological associations between brachycephaly and upper respiratory tract disorders in dogs attending veterinary practices in England. Canine Genet. Epidemiol. 2015, 2, 10. [Google Scholar] [CrossRef] [PubMed]
- Ladlow, J.; Liu, N.C.; Kalmar, L.; Sargan, D. Brachycephalic obstructive airway syndrome. Vet. Rec. 2018, 182, 375–378. [Google Scholar] [CrossRef] [PubMed]
- Hoareau, G.L.; Jourdan, G.; Mellema, M.; Verwaerde, P. Evaluation of arterial blood gases and arterial blood pressures in brachycephalic dogs. J. Vet. Internal Med. 2012, 26, 897–904. [Google Scholar] [CrossRef]
- Wang, S.; Laloe, D.; Missant, F.M.; Malm, S.; Lewis, T.; Verrier, E.; Strandberg, E.; Bonnett, B.N.; Leroy, G. Breeding policies and management of pedigree dogs in 15 national kennel clubs. Vet. J. 2018, 234, 130–135. [Google Scholar] [CrossRef]
- Crispin, S. The advisory council on the welfare issues of dog breeding. Vet. J. 2011, 189, 129–131. [Google Scholar] [CrossRef]
- International Partnership for Dogs. The Brachycephalic Issue. Available online: https://dogwellnet.com/content/hot-topics/brachycephalics/the-brachycephalic-issue-r308/ (accessed on 29 November 2018).
- Australian National Kennel Club. French Bulldogs Taskforce. Available online: http://ankc.org.au/MemberNewsItemDetail/?id=2693 (accessed on 3 December 2018).
- Roy Morgan. Over 600,000 Pet Owners Have Pet Insurance. Roy Morgan, 2018. Available online: http://www.roymorgan.com/findings/7615-over-600000-pet-owners-have-pet-insurance-201806080622 (accessed on 10 December 2018).
- Feng, T.; McConnell, C.; O’hara, K.; Chair, J.; Spadadofori, G. Brachycephalic Breed Disease Prevalence Study. Available online: http://nationwidedvm.com/wp-content/uploads/2017/03/NWBrachycelphalicStudy0317.pdf (accessed on 19 December 2018).
- Drobatz, K. Heat stroke. In Small Animal Critical Care Medicine, 2nd ed.; Silverstein, D.C., Hopper, K., Eds.; Saunders Elsevier: St Louis, MO, USA, 2014; pp. 795–799. [Google Scholar]
- Bruchim, Y.; Klement, E.; Saragusty, J.; Finkeilstein, E.; Kass, P.; Aroch, I. Heat stroke in dogs: A retrospective study of 54 cases (1999–2004) and analysis of risk factors for death. J. Vet. Internal Med. 2006, 20, 38–46. [Google Scholar]
- Senn, D.; Sigrist, N.; Forterre, F.; Howard, J.; Spreng, D. Retrospective evaluation of postoperative nasotracheal tubes for oxygen supplementation in dogs following surgery for brachycephalic syndrome: 36 cases (2003–2007). J. Vet. Emerg. Crit. Care 2011, 21, 261–267. [Google Scholar] [CrossRef]
- Nicholson, I.; Baines, S. Complications associated with temporary tracheostomy tubes in 42 dogs (1998 to 2007). J. Small Anim. Pract. 2012, 53, 108–114. [Google Scholar] [CrossRef]
- Ree, J.J.; Milovancev, M.; MacIntyre, L.A.; Townsend, K.L. Factors associated with major complications in the short-term postoperative period in dogs undergoing surgery for brachycephalic airway syndrome. Can. Vet. J. 2016, 57, 976–980. [Google Scholar] [PubMed]
- Poncet, C.M.; Dupre, G.P.; Freiche, V.G.; Estradak, M.M.; Poubanne, Y.A.; Bouvy, B.M. Prevalence of gastrointestinal tract lesions in 73 brachycephalic dogs with upper respiratory syndrome. J. Small Anim. Pract. 2005, 46, 273–279. [Google Scholar] [CrossRef]
- Savvas, I.; Raptopoulos, D.; Rallis, T. A “light meal” three hours preoperatively decreases the incidence of Gastro-Esophageal Reflux in dogs. J. Am. Anim. Hosp. Assoc. 2016, 52, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Panti, A.; Bennett, R.C.; Corletto, F.; Brearley, J.; Jeffery, N.; Mellanby, R.J. The effect of omeprazole on oesophageal ph in dogs during anaesthesia. J. Small Anim. Pract. 2009, 50, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.V.; Evans, A.T.; Mauer, W.A. Influence of metoclopramide on gastroesophageal reflux in anesthetized dogs. Am. J. Vet. Res. 2006, 67, 26–31. [Google Scholar] [CrossRef]
- Johnson, R.A. Maropitant prevented vomiting but not gastroesophageal reflux in anesthetized dogs premedicated with acepromazine-hydromorphone. Vet. Anaesth. Analg. 2014, 41, 406–410. [Google Scholar] [CrossRef]
- Brodbelt, D.C.; Pfeiffer, D.U.; Young, L.E.; Wood, J.L. Results of the confidential enquiry into perioperative small animal fatalities regarding risk factors for anesthetic-related death in dogs. J. Am. Vet. Med. Assoc. 2008, 233, 1096–1104. [Google Scholar] [CrossRef]
- Emmerson, T. Brachycephalic obstructive airway syndrome: A growing problem. J. Small Anim. Pract. 2014, 55, 543–544. [Google Scholar] [CrossRef]
- Lodato, D.L.; Hedlund, C.S. Brachycephalic airway syndrome: Management. Compendium 2012, 34, E4. [Google Scholar] [PubMed]
- Oechtering, T.; Oechtering, G.; Noller, C. Structural characteristics of the nose in brachycephalic dog breeds analysed by computed tomography. Tierarztl. Prax. Ausg. Klein. Heim. 2007, 35, 177–187. [Google Scholar] [CrossRef]
- Dupre, G.; Heidenreich, D. Brachycephalic syndrome. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 691–707. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, D.; Gradner, G.; Kneissl, S.; Dupre, G. Nasopharyngeal dimensions from computed tomography of pugs and French bulldogs with brachycephalic airway syndrome. Vet. Surg. 2016, 45, 83–90. [Google Scholar] [CrossRef]
- Haimel, G.; Dupre, G. Brachycephalic airway syndrome: A comparative study between pugs and French bulldogs. J. Small Anim. Pract. 2015, 56, 714–719. [Google Scholar] [CrossRef] [PubMed]
- Lodato, D.L.; Hedlund, C.S. Brachycephalic airway syndrome: Pathophysiology and diagnosis. Compendium 2012, 34, E3. [Google Scholar] [PubMed]
- Broux, O.; Clercx, C.; Etienne, A.L.; Busoni, V.; Claeys, S.; Hamaide, A.; Billen, F. Effects of manipulations to detect sliding hiatal hernia in dogs with brachycephalic airway obstructive syndrome. Vet. Surg. 2018, 47, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Reeve, E.J.; Sutton, D.; Friend, E.J.; Warren-Smith, C.M.R. Documenting the prevalence of hiatal hernia and oesophageal abnormalities in brachycephalic dogs using fluoroscopy. J. Small Anim. Pract. 2017, 58, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Oechtering, G.U.; Pohl, S.; Schlueter, C.; Lippert, J.P.; Alef, M.; Kiefer, I.; Ludewig, E.; Schuenemann, R. A novel approach to brachycephalic syndrome. 1. Evaluation of anatomical intranasal airway obstruction. Vet. Surg. 2016, 45, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Oechtering, G.U.; Pohl, S.; Schlueter, C.; Schuenemann, R. A novel approach to brachycephalic syndrome. 2. Laser-assisted turbinectomy (late). Vet. Surg. 2016, 45, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.C.; Oechtering, G.U.; Adams, V.J.; Kalmar, L.; Sargan, D.R.; Ladlow, J.F. Outcomes and prognostic factors of surgical treatments for brachycephalic obstructive airway syndrome in 3 breeds. Vet. Surg. 2017, 46, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Dunie-Merigot, A.; Bouvy, B.; Poncet, C. Comparative use of CO2 laser, diode laser and monopolar electrocautery for resection of the soft palate in dogs with brachycephalic airway obstructive syndrome. Vet. Rec. 2010, 167, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Findji, L.; Dupre, G. Folded flap palatoplasty for treatment of elongated soft palates in 55 dogs. Wiener Tierarztliche Monatsschrift 2008, 95, 56–63. [Google Scholar]
- White, R.N. Surgical management of laryngeal collapse associated with brachycephalic airway obstruction syndrome in dogs. J. Small Anim. Pract. 2012, 53, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Cantatore, M.; Gobbetti, M.; Romussi, S.; Brambilla, G.; Giudice, C.; Grieco, V.; Stefanello, D. Medium term endoscopic assessment of the surgical outcome following laryngeal saccule resection in brachycephalic dogs. Vet. Rec. 2012, 170, 518. [Google Scholar] [CrossRef] [PubMed]
- Mehl, M.L.; Kyles, A.E.; Pypendop, B.H.; Filipowicz, D.E.; Gregory, C.R. Outcome of laryngeal web resection with mucosal apposition for treatment of airway obstruction in dogs: 15 cases (1992–2006). J. Am. Vet. Med. Assoc. 2008, 233, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.R.; Kaye, B.M.; Beswick, A.R.; Ter Haar, G. Complications following laryngeal sacculectomy in brachycephalic dogs. J. Small Anim. Pract. 2018, 59, 16–21. [Google Scholar] [CrossRef]
- Kocsube, S.; Perrone, G.; Magista, D.; Houbraken, J.; Varga, J.; Szigeti, G.; Hubka, V.; Hong, S.B.; Frisvad, J.C.; Samson, R.A. Aspergillus is monophyletic: Evidence from multiple gene phylogenies and extrolites profiles. Stud. Mycol. 2016, 85, 199–213. [Google Scholar] [CrossRef]
- Worth, D.B.; Grimes, J.A.; Jimenez, D.A.; Koenig, A.; Schmiedt, C.W. Risk factors for temporary tracheostomy tube placement following surgery to alleviate signs of brachycephalic obstructive airway syndrome in dogs. J. Am. Vet. Med. Assoc. 2018, 253, 1158–1163. [Google Scholar] [CrossRef]
- Poncet, C.M.; Dupre, G.P.; Freiche, V.G.; Bouvy, B.M. Long-term results of upper respiratory syndrome surgery and gastrointestinal tract medical treatment in 51 brachycephalic dogs. J. Small Anim. Pract. 2006, 47, 137–142. [Google Scholar] [CrossRef]
- Rusbridge, C. Chiari-like malformation and syringomyelia. Eur. J. Companion Anim. Prac. 2013, 23, 70–89. [Google Scholar]
- Schmidt, M.J.; Volk, H.; Klingler, M.; Failing, K.; Kramer, M.; Ondreka, N. Comparison of closure times for cranial base synchondroses in mesaticephalic, brachycephalic, and Cavalier King Charles Spaniel dogs. Vet. Radiol. Ultrasound 2013, 54, 497–503. [Google Scholar] [CrossRef]
- Shaw, T.A.; McGonnell, I.M.; Driver, C.J.; Rusbridge, C.; Volk, H.A. Increase in cerebellar volume in Cavalier King Charles Spaniels with Chiari-like malformation and its role in the development of syringomyelia. PLoS ONE 2012, 7, e33660. [Google Scholar] [CrossRef] [PubMed]
- Cerda-Gonzalez, S.; Olby, N.J.; Griffith, E.H. Medullary position at the craniocervical junction in mature Cavalier King Charles Spaniels: Relationship with neurologic signs and syringomyelia. J. Vet. Internal Med. 2015, 29, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Wijnrocx, K.; Van Bruggen, L.W.L.; Eggelmeijer, W.; Noorman, E.; Jacques, A.; Buys, N.; Janssens, S.; Mandigers, P.J.J. Twelve years of Chiari-like malformation and syringomyelia scanning in Cavalier King Charles Spaniels in the Netherlands: Towards a more precise phenotype. PLoS ONE 2017, 12, e0184893. [Google Scholar] [CrossRef] [PubMed]
- Rusbridge, C.; Knowler, S.P.; Pieterse, L.; McFadyen, A.K. Chiari-like malformation in the Griffon Bruxellois. J. Small Anim. Pract. 2009, 50, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Freeman, A.C.; Platt, S.R.; Kent, M.; Huguet, E.; Rusbridge, C.; Holmes, S. Chiari-like malformation and syringomyelia in American Brussels griffon dogs. J. Vet. Internal Med. 2014, 28, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.J.; Loughin, C.A.; Dewey, C.W.; Marino, L.J.; Sackman, J.J.; Lesser, M.L.; Akerman, M.B. Morphometric features of the craniocervical junction region in dogs with suspected Chiari-like malformation determined by combined use of magnetic resonance imaging and computed tomography. Am. J. Vet. Res. 2012, 73, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Knowler, S.P.; Kiviranta, A.M.; McFadyen, A.K.; Jokinen, T.S.; La Ragione, R.M.; Rusbridge, C. Craniometric analysis of the hindbrain and craniocervical junction of chihuahua, Affenpinscher and Cavalier King Charles Spaniel dogs with and without syringomyelia secondary to Chiari-like malformation. PLoS ONE 2017, 12, e0169898. [Google Scholar] [CrossRef]
- Kiviranta, A.M.; Rusbridge, C.; Laitinen-Vapaavuori, O.; Hielm-Bjorkman, A.; Lappalainen, A.K.; Knowler, S.P.; Jokinen, T.S. Syringomyelia and craniocervical junction abnormalities in chihuahuas. J. Vet. Internal Med. 2017, 31, 1771–1781. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.E.; Knowler, S.P.; Rusbridge, C.; Noorman, E.; Jeffry, N.D. Prevalence of asymptomatic syringomyelia in Cavalier King Charles Spaniels. Vet. Rec. 2011, 168, 667–669. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.; Rusbridge, C.; Knowler, P.; Blott, S.; Woolliams, J.A. Heritability of syringomyelia in Cavalier King Charles Spaniels. Vet. J. 2010, 183, 345–347. [Google Scholar] [CrossRef] [PubMed]
- Lemay, P.; Knowler, S.P.; Bouasker, S.; Nedelec, Y.; Platt, S.; Freeman, C.; Child, G.; Barreiro, L.B.; Rouleau, G.A.; Rusbridge, C.; et al. Quantitative trait loci (QTL) study identifies novel genomic regions associated to Chiari-like malformation in Griffon Bruxellois dogs. PLoS ONE 2014, 9, e89816. [Google Scholar] [CrossRef] [PubMed]
- Knowler, S.P.; v/d Berg, H.; McFadyen, A.; La Ragione, R.M.; Rusbridge, C. Inheritance of Chiari-like malformation: Can a mixed breeding reduce the risk of syringomyelia? PLoS ONE 2016, 11, e0151280. [Google Scholar] [CrossRef]
- Knowler, S.P.; McFadyen, A.K.; Rusbridge, C. Effectiveness of breeding guidelines for reducing the prevalence of syringomyelia. Vet. Rec. 2011, 169, 681. [Google Scholar] [CrossRef] [PubMed]
- Driver, C.J.; De Risio, L.; Hamilton, S.; Rusbridge, C.; Dennis, R.; McGonnell, I.M.; Volk, H.A. Changes over time in craniocerebral morphology and syringomyelia in Cavalier King Charles Spaniels with Chiari-like malformation. BMC Vet. Res. 2012, 8, 215. [Google Scholar] [CrossRef] [PubMed]
- Rusbridge, C.; Carruthers, H.; Dube, M.P.; Holmes, M.; Jeffery, N.D. Syringomyelia in Cavalier King Charles Spaniels: The relationship between syrinx dimensions and pain. J. Small Anim. Pract. 2007, 48, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.W.; Marino, D.J.; Bailey, K.S.; Loughin, C.A.; Barone, G.; Bolognese, P.; Milhorat, T.H.; Poppe, D.J. Foramen magnum decompression with cranioplasty for treatment of caudal occipital malformation syndrome in dogs. Vet. Surg. 2007, 36, 406–415. [Google Scholar] [CrossRef]
- Rusbridge, C. Chiari-like malformation with syringomyelia in the Cavalier King Charles Spaniel: Long-term outcome after surgical management. Vet. Surg. 2007, 36, 396–405. [Google Scholar] [CrossRef]
- Mitchell, T.J.; Knowler, S.P.; van den Berg, H.; Sykes, J.; Rusbridge, C. Syringomyelia: Determining risk and protective factors in the conformation of the Cavalier King Charles Spaniel dog. Canine Genet. Epidemiol. 2014, 1, 9. [Google Scholar] [CrossRef]
- Guevar, J.; Penderis, J.; Faller, K.; Yeamans, C.; Stalin, C.; Gutierrez-Quintana, R. Computer-assisted radiographic calculation of spinal curvature in brachycephalic “screw-tailed” dog breeds with congenital thoracic vertebral malformations: Reliability and clinical evaluation. PLoS ONE 2014, 9, e106957. [Google Scholar] [CrossRef] [PubMed]
- Inglez de Souza, M.C.C.M.; Ryan, R.; ter Haar, G.; Packer, R.M.A.; Volk, H.A.; De Decker, S. Evaluation of the influence of kyphosis and scoliosis on intervertebral disc extrusion in French bulldogs. BMC Vet. Res. 2018, 14, 5. [Google Scholar] [CrossRef] [PubMed]
- Dewey, C.W.; Davies, E.; Bouma, J.L. Kyphosis and kyphoscoliosis associated with congenital malformations of the thoracic vertebral bodies in dogs. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Song, R.B.; Glass, E.N.; Kent, M. Spina bifida, meningomyelocele, and meningocele. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 327–345. [Google Scholar] [CrossRef]
- Miller, W.H.; Griffin, C.E.; Campbell, K.L. Muller and Kirk’s Small Animal Dermatology, 7th ed.; Elsevier Mosby: St Louis, MO, USA, 2013. [Google Scholar]
- Webb Milum, A.N.; Griffin, C.E.; Blessing, K.S. A cross-sectional study of show English bulldogs in the united states: Evaluating paw lesions, cytological findings, pruritic behaviours and gastrointestinal signs. Vet. Dermatol. 2018, 29, 395-e130. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Pooch, A.S.; Liu, H. A genetic assessment of the English bulldog. Canine Genet. Epidemiol. 2016, 3, 6. [Google Scholar] [CrossRef]
- Becskei, C.; Cuppens, O.; Mahabir, S.P. Efficacy and safety of sarolaner against generalized demodicosis in dogs in European countries: A non-inferiority study. Vet. Dermatol. 2018, 29, 203-e72. [Google Scholar] [CrossRef]
- Olivry, T.; DeBoer, D.J.; Favrot, C.; Jackson, H.A.; Mueller, R.S.; Nuttall, T.; Prélaud, P.; International Committee on Allergic Diseases of Animals. Treatment of canine atopic dermatitis: 2015 updated guidelines from the international committee on allergic diseases of animals (ICADA). BMC Vet. Res. 2015, 11, 210. [Google Scholar] [CrossRef]
- Negre, A.; Bensignor, E.; Guillot, J. Evidence-based veterinary dermatology: A systematic review of interventions for Malassezia dermatitis in dogs. Vet. Dermatol. 2009, 20, 1–12. [Google Scholar] [CrossRef]
- Packer, R.M.A.; Hendricks, A.; Burn, C.C. Impact of facial conformation on canine health: Corneal ulceration. PLoS ONE 2015, 10, e0123827. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Meeson, R.L.; Sheridan, A.; Church, D.B.; Brodbelt, D.C. The epidemiology of patellar luxation in dogs attending primary-care veterinary practices in England. Canine Genet. Epidemiol. 2016, 3, 4. [Google Scholar] [CrossRef]
- Coppinger, R.; Schneider, R. Evolution of working dogs. In The Domestic Dog: Its Evolution, Behaviour and Interactions with People; Serpell, J., Ed.; Cambridge University Press: Cambridge, UK, 1995; pp. 21–47. [Google Scholar]
- Georgevsky, D.; Carrasco, J.J.; Valenzuela, M.; McGreevy, P.D. Domestic dog skull diversity across breeds, breed groupings and genetic clusters. J. Vet. Behav. 2014, 9, 228–234. [Google Scholar] [CrossRef]
- Carrasco, J.J.; Georgevsky, D.; Valenzuela, M.; McGreevy, P.D. A pilot study of sexual dimorphism in the head morphology of domestic dogs. J. Vet. Behav. 2014, 9, 43–46. [Google Scholar] [CrossRef]
- Ghirlanda, S.; Acerbi, A.; Herzog, H.; Serpell, J.A. Fashion vs. Function in cultural evolution: The case of dog breed popularity. PLoS ONE 2013, 8, e74770. [Google Scholar] [CrossRef] [PubMed]
- Asher, L.; Diesel, G.; Summers, J.F.; McGreevy, P.D.; Collins, L.M. Inherited defects in pedigree dogs. Part 1: Disorders related to breed standards. Vet. J. 2009, 182, 402–411. [Google Scholar] [CrossRef] [PubMed]
- Goodwin, D.; Bradshaw, J.W.S.; Wickens, S.M. Paedomorphosis affects visual signals of domestic dogs. Anim. Behav. 1997, 53, 297–304. [Google Scholar] [CrossRef]
- McGreevy, P.D. A Modern Dog’s Life; UNSW Press: Sydney, Australia, 2009. [Google Scholar]
- Regodon, S.; Vivo, J.M.; Franco, A.; Guillen, M.T.; Robina, A. Craniofacial angle in dolicho-, meso- and brachycephalic dogs: Radiological determination and application. Ann. Anat. 1993, 175, 361–363. [Google Scholar] [CrossRef]
- Dickie, A.M.; Sullivan, M. The effect of obliquity on the radiographic appearance of the temporomandibular joint in dogs. Vet. Radiol. Ultrasound 2001, 42, 205–217. [Google Scholar] [CrossRef]
- Cerda-Gonzalez, S.; Olby, N.J.; McCullough, S.; Pease, A.P.; Broadstone, R.; Osborne, J.A. Morphology of the caudal fossa in Cavalier King Charles Spaniels. Vet. Radiol. Ultrasound 2009, 50, 37–46. [Google Scholar] [CrossRef]
- Roberts, T.; McGreevy, P.; Valenzuela, M. Human induced rotation and reorganization of the brain of domestic dogs. PLoS ONE 2010, 5, e11946. [Google Scholar] [CrossRef]
- McGreevy, P.; Grassi, T.D.; Harman, A.M. A strong correlation exists between the distribution of retinal ganglion cells and nose length in the dog. Brain Behav. Evol. 2004, 63, 13–22. [Google Scholar] [CrossRef] [PubMed]
- McGreevy, P.D.; Georgevsky, D.; Carrasco, J.; Valenzuela, M.; Duffy, D.L.; Serpell, J.A. Dog behavior co-varies with height, bodyweight and skull shape. PLoS ONE 2013, 8, e80529. [Google Scholar] [CrossRef] [PubMed]
- Rollin, B.E. A New Basis for Animal Ethics: Telos and Common Sense; University of Missouri Press: Columbia, MI, USA, 2016. [Google Scholar]
- Beausoleil, N.J.; Mellor, D.J. Introducing breathlessness as a significant animal welfare issue. N. Z. Vet. J. 2015, 63, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Roedler, F.S.; Pohl, S.; Oechtering, G.U. How does severe brachycephaly affect dog’s lives? Results of a structured preoperative owner questionnaire. Vet. J. 2013, 198, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Mosley, B. “Short-Faced” Dogs More Prone to Death in Flight, According to Dot Data. 2010. Available online: https://www.transportation.gov/sites/dot.dev/files/docs/Canine_Deaths_Press_Release.pdf (accessed on 19 January 2018).
- QANTAS. Travelling with Pets; QANTAS: Masters, Australia, 2018. [Google Scholar]
- Neff, M.W.; Rine, J. A fetching model organism. Cell 2006, 124, 229–231. [Google Scholar] [CrossRef]
- Sandoe, P.; Kondrup, S.V.; Bennett, P.C.; Forkman, B.; Meyer, I.; Proschowsky, H.F.; Serpell, J.A.; Lund, T.B. Why do people buy dogs with potential welfare problems related to extreme conformation and inherited disease? A representative study of Danish owners of four small dog breeds. PLoS ONE 2017, 12, e0172091. [Google Scholar] [CrossRef]
- Ryan, S.; Bacon, H.; Endenburg, N.; Hazel, S.; Jouppi, R.; Lee, N.; Seksel, K.; Takashima, G. WSAVA Animal Welfare Guidelines for Companion Animal Practitioners and Veterinary Teams; WSAVA: Jerusalem, Israel, 2018. [Google Scholar]
- Anonymous. Pugs are anatomical disasters. Vets must speak out—Even if it’s bad for business. The Guardian, 22 September 2016. [Google Scholar]
- Hernandez, E.; Fawcett, A.; Brouwer, E.; Rau, J.; Turner, P.V. Speaking up: Veterinary ethical responsibilities and animal welfare issues in everyday practice. Animals 2018, 8, 15. [Google Scholar] [CrossRef]
- Coghlan, S. Strong patient advocacy and the fundamental ethical role of veterinarians. Strong Patient Advocacy Fundam. Ethical Role Vet. 2018, 31, 349–368. [Google Scholar] [CrossRef]
- Centre for Veterinary Education. Veterinary oaths [online]. In One Welfare Portal; Centre for Veterinary Education: Sydney, Australia, 2017. [Google Scholar]
- British Veterinary Association. Vets Speaking up for Animal Welfare: BVA Animal Welfare Strategy; BVA: London, UK, 2016. [Google Scholar]
- Beauchamp, T.L.; Childress, J.F. Principles of Biomedical Ethics, 7th ed.; Oxford University Press: New York, NY, USA; Oxford, UK, 2013. [Google Scholar]
- Packer, R.M.; Hendricks, A.; Burn, C.C. Do dog owners perceive the clinical signs related to conformational inherited disorders as ‘normal’ for the breed? A potential constraint to improving canine welfare. Anim. Welf. 2012, 21, 81–93. [Google Scholar] [CrossRef]
- Packer, R.M.A.; Murphy, D.; Farnworth, M.J. Purchasing popular purebreds: Investigating the influence of breed-type on the pre-purchase motivations and behaviour of dog owners. Anim. Welf. 2017, 26, 191–201. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L. The Principles of Humane Experimental Technique; Metheun: London, UK, 1959. [Google Scholar]
- NHMRC. Australian Code for the Care and Use of Animals for Scientific Purposes; NHMRC: Canberra, Australia, 2013. [Google Scholar]
- McCausland, C. The five freedoms of animal welfare are rights. J. Agric. Environ. Ethics 2014, 27, 649–662. [Google Scholar] [CrossRef]
- Farstad, W. Ethics in animal breeding. Reprod. Domest. Anim. 2018, 53, 4–13. [Google Scholar] [CrossRef] [PubMed]
- British Veterinary Association. Information on Advertising Policy re Bulldogs, French Bulldogs and Pugs. Available online: https://veterinaryrecord.bmj.com/pages/wp-content/uploads/sites/50/2017/04/Vet-record-Letter-for-advertisers-amended-for-website.pdf (accessed on 19 January 2018).
- Latter, M. Ava moves away from brachycephalic breeds in advertising. Aust. Vet. J. 2017, 95, N4. [Google Scholar]
- RIS, R.d.b. Animal Protection Act TSCHG § 5. Available online: https://www.ris.bka.gv.at/Dokument.wxe?Abfrage=Bundesnormen&Dokumentnummer=NOR40192428 (accessed on 3 December 2018).
- Bundesamt für Justiz. Animal Protection Act § 11b; Bundesamt für Justiz: Bonn, Germany, 2018; Volume Act § 11b. [Google Scholar]
- Federal Assembly of the Swiss Confederation. Animal protection act. In The Federal Council: The Portal of the Swiss Government; Federal Assembly of the Swiss Confederation: Geneva, Switzerland, 2017; Volume 455. [Google Scholar]
- Ryan, J. Trade me ban on pugs, English and French bulldogs. In Trade Me Trust and Safety Blog; Available online: https://www.trademe.co.nz/trust-safety/2018/01/17/pugs-and-bulldogs-ban/ (accessed on 19 January 2018).
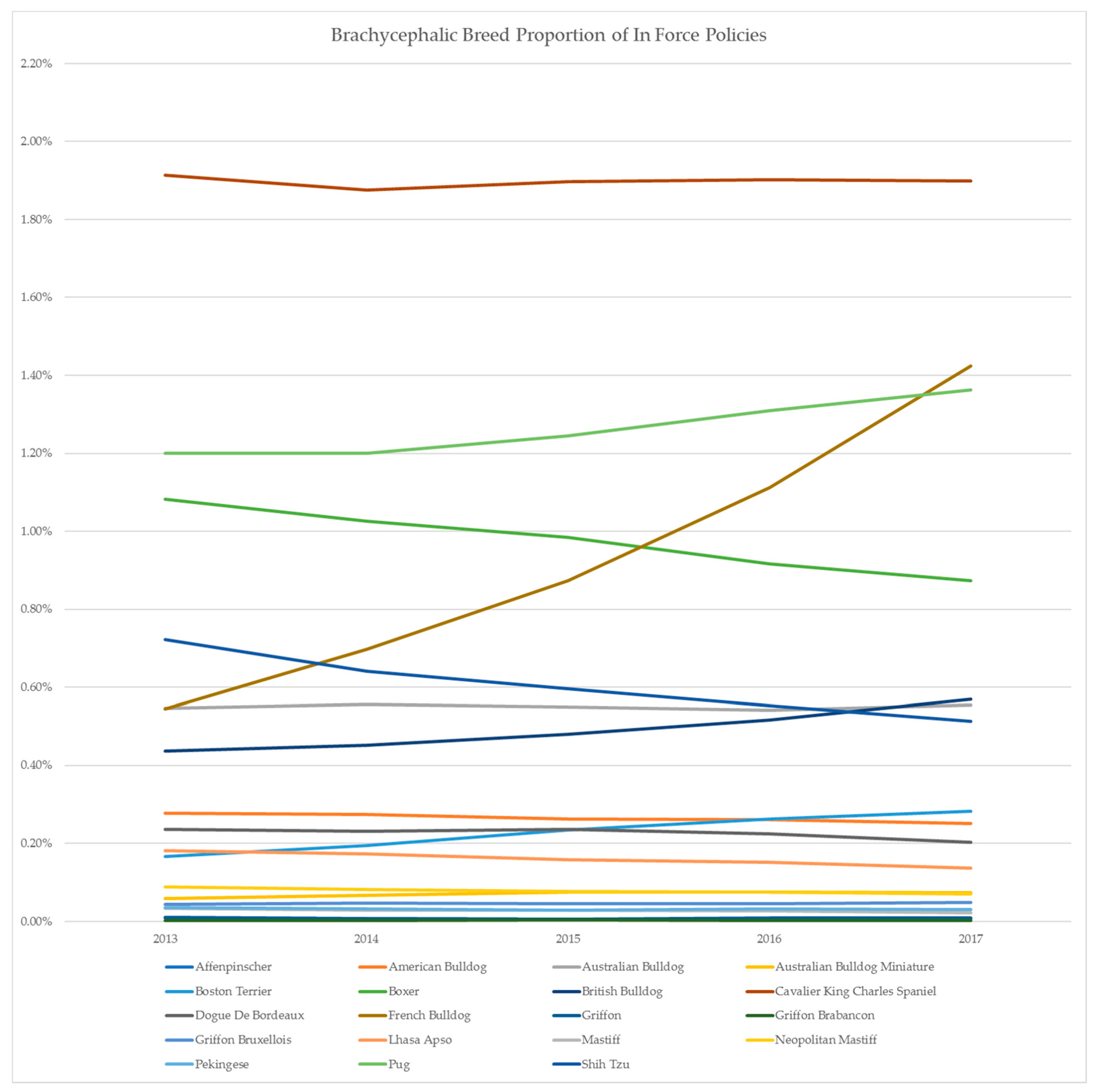
| Breed | Year on Year Growth | |||
|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | |
| Affenpinscher | −19.29% | −17.65% | −3.60% | 35.59% |
| American Bulldog | −0.91% | −4.33% | −0.95% | −3.97% |
| Australian Bulldog | 1.70% | −1.23% | −1.51% | 2.42% |
| Australian Bulldog Miniature | 14.45% | 13.29% | 0.98% | −3.05% |
| Boston Terrier | 17.04% | 20.04% | 12.02% | 7.38% |
| Boxer | −5.24% | −3.91% | −6.89% | −4.73% |
| British Bulldog | 3.35% | 6.05% | 7.81% | 10.21% |
| Cavalier King Charles Spaniel | −2.05% | 1.20% | 0.27% | −0.20% |
| Dogue De Bordeaux | −2.43% | 2.46% | −4.78% | −10.08% |
| French Bulldog | 28.00% | 25.04% | 27.44% | 28.08% |
| Griffon | −30.29% | −17.65% | 41.56% | 7.23% |
| Griffon Brabancon | 13.00% | 29.40% | −13.24% | −4.57% |
| Griffon Bruxellois | 6.75% | −2.92% | 0.52% | 4.66% |
| Lhasa Apso | −5.38% | −8.67% | −3.31% | −9.84% |
| Mastiff | −15.92% | −2.28% | −5.44% | −15.23% |
| Neopolitan Mastiff | −7.57% | −6.83% | −1.36% | −5.82% |
| Pekingese | −10.56% | −7.61% | 7.51% | −1.78% |
| Pug | −0.01% | 3.70% | 5.25% | 4.07% |
| Shih Tzu | −11.28% | −7.01% | −7.40% | −7.18% |
| Total | 0.00% | 2.53% | 3.06% | 3.85% |
| Virtue | Manifestation |
|---|---|
| Care | The veterinarian has an emotional commitment to, and the willingness to act on behalf of persons and patients. |
| Compassion | The veterinarian has an active regard for both the animal and owner’s welfare, with imaginative awareness and sympathy, tenderness and discomfort at another’s suffering. The ability to identify and motivation to address suffering. |
| Discernment | The veterinarian is able to make appropriate judgements and decisions without undue influence of fears, personal attachments or inducements. |
| Trustworthiness | The veterinarian can be trusted to give an honest, informed opinion about the patient’s condition, potential causes and contributing factors, and prognosis, and to declare any conflicts of interest. |
| Integrity | The veterinarian is faithful to his or her moral values, and will defend these when necessary. |
| Conscientiousness | The veterinarian works conscientiously to do what is right: to provide the best possible care to the individual patient, and to future patients by remaining up-to-date with scientific evidence. The conscientious veterinarian strives to prevent disease at the level of the individual, as well as that of the population. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fawcett, A.; Barrs, V.; Awad, M.; Child, G.; Brunel, L.; Mooney, E.; Martinez-Taboada, F.; McDonald, B.; McGreevy, P. Consequences and Management of Canine Brachycephaly in Veterinary Practice: Perspectives from Australian Veterinarians and Veterinary Specialists. Animals 2019, 9, 3. https://doi.org/10.3390/ani9010003
Fawcett A, Barrs V, Awad M, Child G, Brunel L, Mooney E, Martinez-Taboada F, McDonald B, McGreevy P. Consequences and Management of Canine Brachycephaly in Veterinary Practice: Perspectives from Australian Veterinarians and Veterinary Specialists. Animals. 2019; 9(1):3. https://doi.org/10.3390/ani9010003
Chicago/Turabian StyleFawcett, Anne, Vanessa Barrs, Magdoline Awad, Georgina Child, Laurencie Brunel, Erin Mooney, Fernando Martinez-Taboada, Beth McDonald, and Paul McGreevy. 2019. "Consequences and Management of Canine Brachycephaly in Veterinary Practice: Perspectives from Australian Veterinarians and Veterinary Specialists" Animals 9, no. 1: 3. https://doi.org/10.3390/ani9010003
APA StyleFawcett, A., Barrs, V., Awad, M., Child, G., Brunel, L., Mooney, E., Martinez-Taboada, F., McDonald, B., & McGreevy, P. (2019). Consequences and Management of Canine Brachycephaly in Veterinary Practice: Perspectives from Australian Veterinarians and Veterinary Specialists. Animals, 9(1), 3. https://doi.org/10.3390/ani9010003





