Garlic (Allium Sativum) Supplementation Improves Respiratory Health but Has Increased Risk of Lower Hematologic Values in Horses
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Geographical Area and Climate Conditions
2.2. Horses and Housing Conditions
2.3. Experimental Feeding
2.4. Respiratory Tract Examination and Blood Sampling
2.5. Statistical Analysis
3. Results and Discussion
3.1. Housing Conditions
3.2. Respiratory Health
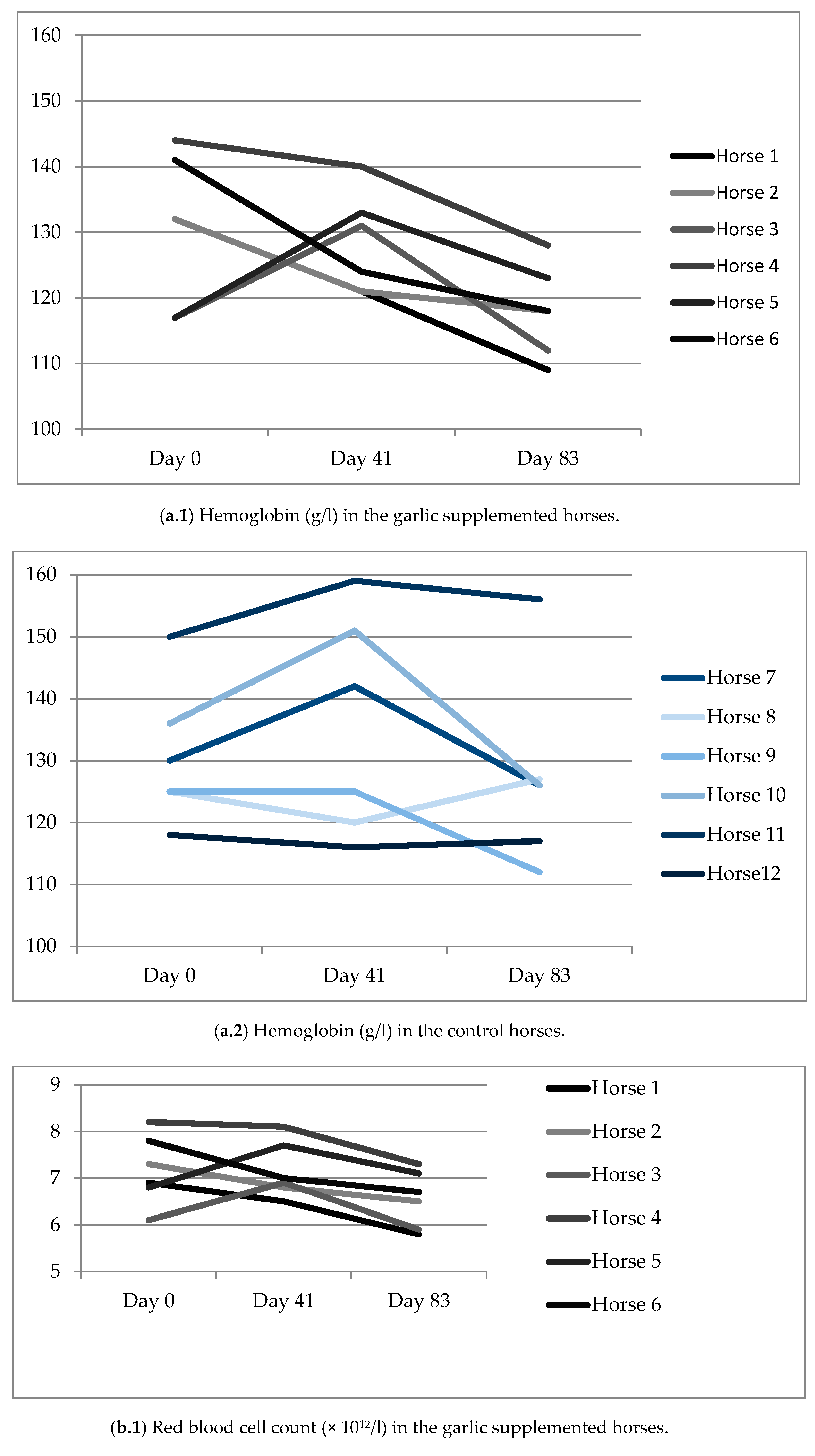
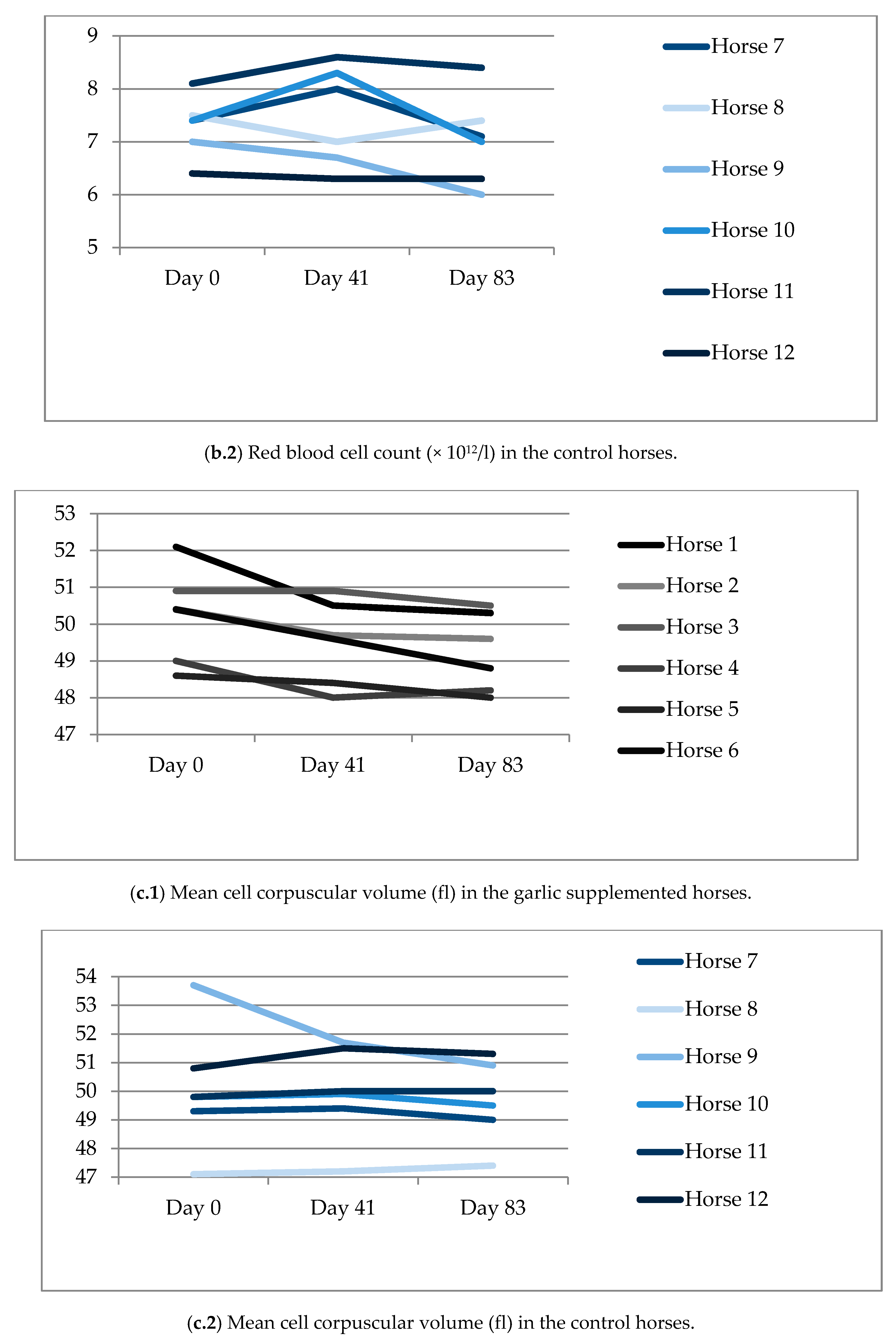
3.3. Blood Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rahman, M.S. Allicin and other functional active components in garlic: Health benefits and bioavailability. Int. J. Food Prop. 2007, 10, 245–268. [Google Scholar] [CrossRef]
- McGorum, B.C.; Ellison, J.; Cullen, R.T. Total and respirable airborne dust endotoxin concentrations in three equine management systems. Equine Vet. J. 1998, 30, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Berndt, A.; Derksen, F.J.; Robinson, N.E. Endotoxin concentrations within the breathing zone of horses are higher in stables than on pasture. Vet. J. 2010, 183, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Raymond, S.L.; Curtis, E.F.; Winfield, L.M.; Clarke, A.F. A comparison of respirable particles associated with various forage products for horses. Equine Pract. 1997, 19, 23–26. [Google Scholar]
- Vandeput, S.; Istasse, L.; Nicks, B.; Lekeux, P. Airborne dust and aeroallergen concentrations in different sources of feed and bedding for horses. Vet. Q. 1997, 19, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Elfman, L.; Wålinder, R.; Riihimäki, M.; Pringle, J. Air quality in horse stables. In Chemistry, Emission Control, Radioactive Pollution and Indoor Air Quality; Mazzeo, D., Ed.; Intech: Rijeka, Croatia, 2011; pp. 655–680. [Google Scholar]
- Munday, R.; Munday, C.M. Relative activities of organosulfur compounds derived from onions and garlic in increasing tissue activities of quinone reductase and glutathione transferase in rat tissues. Nutr. Cancer 2001, 40, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Wade, L.L.; Newman, S.J. Hemoglobinuric nephrosis and Hepastoplenic eryrhophagocytosis in a dusky-headed conure (Aratinga weddeli) after ingestion of garlic (Allium sativum). J. Avian Med. Surg. 2004, 18, 155–161. [Google Scholar] [CrossRef]
- Ruwende, C.; Hill, A. Glucose-6-phosphate dehydrogenase deficiency and malaria. J. Mol. Med. 1998, 76, 581–588. [Google Scholar] [CrossRef]
- Harvey, J.W.; Racker, D. Experimental onion-induced hemolytic anemia in dogs. Vet. Pathol. 1985, 22, 387–392. [Google Scholar] [CrossRef]
- Hutchison, T.W.S. Onions as a cause of Heinz body anaemia and death in cattle. Can. Vet. J. 1977, 18, 358–360. [Google Scholar]
- Pearson, W.; Boermans, H.J.; Bettler, W.J.; McBride, B.W.; Lindinger, M.I. Association of maximum voluntary dietary intake of freeze-dried garlic with Heinz body anemia in horses. Am. J. Vet. Res. 2005, 66, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Lamprecht, E.D. Some commonly fed herbs and other functional foods in equine nutrition: A review. Vet. J. 2008, 178, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, F.; Caspasso, R.; Izzo, A.A. Garlic (Allium sativum L.): Adverse effects and drug interactions in humans. Mol. Nutr. Food Res. 2007, 51, 1386–1397. [Google Scholar] [CrossRef] [PubMed]
- Elghandour, M.M.Y.; Reddy, P.R.; Salem, A.Z.M.; Reddy, P.P.R.; Hyder, I.; Barbabosa-Pliego, A.; Yasawini, D. Plant bioactives and extracts as feed additives in Horse nutrition. J. Equine Vet. Sci. 2018, 69, 66–77. [Google Scholar] [CrossRef]
- Pearson, W. Ethnoveterinary medicine: The Science of botanicals in equine health and disease. In Proceedings of the Second Annual European Equine Health and Nutrition Congress, Lelystad, The Netherlands, 19–20 March 2003; pp. 31–40. [Google Scholar]
- Kook, S.; Gun-Hee, K.; Choi, K. The antidiabetic effect of onion and garlic in experimental diabetic rats: Meta-analysis. J. Medic. Food 2009, 12, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Soffar, S.A.; Mokhtar, G.M. Evaluation of the antiparasitic effect of aqueous garlic (Allium sativum) extract in Hymenolepiasis nana and Giardia. J. Egypt. Soc. Parasitol. 1991, 21, 497–502. [Google Scholar] [PubMed]
- Cellini, L.; Di Campli, E.; Masuli, M. Inhibition of Helicobacter pyroli by garlic extract (Allium sativum) FEMS. Immunol. Med. Microbiol. 1996, 13, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Bergero, D.; Valle, E. A critical analysis on the use of herbs and herbal extracts in feeding sport horses. Pferdeheilkunde 2006, 22, 550–557. [Google Scholar] [CrossRef]
- The National Academies Report in Brief. Safety of Dietary Supplements for Horses, Dogs and Cats; The National Academy of Sciences; The National Academies Press: Washington, DC, USA, 2008; 260p. [Google Scholar]
- Saastamoinen, M.; Särkijärvi, S.; Hyyppä, S. Reducing respiratory health risks to horses and workers: A comparison of two stall bedding materials. Animals 2015, 5, 967–977. [Google Scholar] [CrossRef]
- Luke. Feed Tables and Nutrition Recommendations. Natural Resources Institute Finland. 2018. Available online: https://portal.mtt.fi/portal/page/portal/Rehutaulukot/feed_tables_english or http://urn.fi/URN:ISBN:978-952-326-054-2 (accessed on 15 October 2018).
- Saastamoinen, M.; Hellämäki, M. Forage analysis as a basis of feeding of horses. In Forages and Grazing in Horse Nutrition; Saastamoinen, M., Fradinho, M.J., Santos, A.S., Miraglia, N., Eds.; EAAP Publication 132; Wageningen Academic Publishers: Wageningen, The Netherlands, 2012; pp. 304–314. [Google Scholar]
- Clarke, E.G.C.; Clarke, M.L. Garner’s Veterinary Toxicology, 3rd ed.; Ballieri & Tindal: London, UK, 1967. [Google Scholar]
- Riihimäki, M.; Raine, A.; Elfman, L.; Pringle, J. Markers of respiratory inflammation in horses in relation to seasonal changes in air quality in a conventional racing stable. Can. J. Vet. Res. 2008, 72, 432–439. [Google Scholar]
- Pösö, A.R.; Soveri, T.; Oksanen, H.E. The effect of exercise on blood parameters in Standardbred and Finnish-bred horses. Acta Vet. Scand. 1983, 24, 170–184. [Google Scholar] [PubMed]
- Movet. Laboratory Handbook. Available online: www.movet.fi (accessed on 15 October 2018). (In Finnish).
- Saastamoinen, M.T. Propionic acid treated grain (oats) in the diet of horses. Agric. Sci. Finl 1994, 3, 161–168. [Google Scholar] [CrossRef]
- Lindner, A. Laboratory Diagnosis for Sport Horses; Wageningen Academic Publishers: Wageningen, The Netherlands, 1998; 64p. [Google Scholar]
- Hu, Q.; Yang, Q.; Yamoto, O.; Yamasiki, M.; Meade, Y.; Yoshihara, T. Isolation and identification of organosulfur compounds oxidizing canine erythrocytes from garlic (Allium sativum). J. Agric. Food. Chem. 2002, 50, 1059–1062. [Google Scholar] [CrossRef] [PubMed]
- Stevens, H. Suspected wild garlic poisoning in sheep. Vet. Rec. 1984, 115, 363. [Google Scholar] [CrossRef] [PubMed]
- Miyazawa, K.; Ito, M.; Ohsaki, K. An equine case of urticaria associated with dry garlic feeding. J. Vet. Med. Sci. 1991, 53, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Ostrowska, E.; Nicholas, K.G.; Sterling, S.J.; Brendan, G.T.; Rodney, B.J.; Earling, D.R.; Jois, M.; Dunshea, F.R. Consumption of brown onions (Allium cepa var. cavalier and var. density) moderately modulates blood lipids, haematological and haemostatic variables in healthy pigs. Br. J. Nutr. 2004, 91, 211–218. [Google Scholar]
- Ogawa, E.; Shinoki, T.; Akahori, F.; Masaka, T. Effect of onion ingestion on anti-oxidizing agents in dog erythrocytes. Jpn. J. Vet. Sci. 1986, 48, 685–691. [Google Scholar] [CrossRef]
- Heidarpour, M.; Fakrieh, M.; Aslani, M.R.; Mohri, M.; Keywanloo, M. Oxidative effects of long-term onion (Allium cepa) feeding on goat erythrocytes. Comp. Clin. Pathol. 2011, 22, 195–202. [Google Scholar] [CrossRef]
- Pierce, K.R.; Joyce, J.R.; England, R.B.; Jones, P. Acute hemolytic anemia caused by wild onion poisoning in horses. Am. Vet. Med. Ass. J. 1972, 160, 323–327. [Google Scholar]
| Housing Conditions | November | December | January |
|---|---|---|---|
| Temperature °C | |||
| Mean | 9.6 | 7.3 | 10.7 |
| Range | 8–11 | 3–10 | 4–12 |
| Humidity % | |||
| Mean | 71.5 | 65.1 | 66.8 |
| Range | 58–82 | 46–82 | 43–85 |
| Experimental Group; Horse | Initial (Day 0) | Day 41 | Day 83 | Control Group; Horse | Initial (Day 0) | Day 41 | Day 83 |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 0 | 1 | 7 | 1 | 1 | 1 |
| 2 | 1 | 0 | 0 | 8 | 0 | 1 | 0 |
| 3 | 1 | 1 | 0 | 9 | 1 | 0 | 0 |
| 4 | 0 | 1 | 0 | 10 | 1 | 1 | 1 |
| 5 | 1 | 1 | 1 | 11 | 1 | 0 | 1 |
| 6 | 1 | 0 | 0 | 12 | 0 | 0 | 0 |
| Total | 5 | 3 | 2 | Total | 4 | 3 | 3 |
| Experimental Group; Horse | Initial (Day 0) | Day 41 | Day 83 | Control Group; Horse | Initial (Day 0) | Day 41 | Day 83 |
|---|---|---|---|---|---|---|---|
| 1 | ++ | ++ | +++ | 7 | + | +++ | ++ |
| 2 | – | (+) | – | 8 | – | – | ++ |
| 3 | +++ | ++ | + | 9 | ++ | – | (+) |
| 4 | (+) | – | – | 10 | +++ | +++ | +++ |
| 5 | + | +++ | + | 11 | + | + | +++ |
| 6 | – | – | (+) | 12 | – | (+) | – |
| Hematology Parameter | Experimental Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Initial (Day 0) | Day 41 | Day 83 | Initial (Day 0) | Day 41 | Day 83 | |
| Hb, g/L | 130.5 (11.5) | 128.3 (7.6) | 118.0 (7.0) | 130.7 (11.2) | 135.5 (17.7) | 127.3 (15.3) |
| HcT, % | 36.9 (0.0) | 35.4 (0.0) | 32.3 (0.0) | 36.4 (0.0) | 37.2 (0.0) | 34.9 (0.0) |
| RBC, × 1012/L | 7.17 (0.7) | 7.15 (0.6) | 6.57 (0.6) | 7.29 (0.6) | 7.46 (1.0) | 7.04 (0.8) |
| MCV, fl | 50.0 (1.3) | 49.5 (1.1) | 49.2 (1.1) | 50.1 (2.2) | 50.0 (1.6) | 49.7 (1.4) |
| WBC, × 109/L | 8.15 (1.3) | 7.52 (0.7) | 6.97 (0.7) | 7.76 (1.3) | 6.95 (0.9) | 7.52 (1.3) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saastamoinen, M.; Särkijärvi, S.; Hyyppä, S. Garlic (Allium Sativum) Supplementation Improves Respiratory Health but Has Increased Risk of Lower Hematologic Values in Horses. Animals 2019, 9, 13. https://doi.org/10.3390/ani9010013
Saastamoinen M, Särkijärvi S, Hyyppä S. Garlic (Allium Sativum) Supplementation Improves Respiratory Health but Has Increased Risk of Lower Hematologic Values in Horses. Animals. 2019; 9(1):13. https://doi.org/10.3390/ani9010013
Chicago/Turabian StyleSaastamoinen, Markku, Susanna Särkijärvi, and Seppo Hyyppä. 2019. "Garlic (Allium Sativum) Supplementation Improves Respiratory Health but Has Increased Risk of Lower Hematologic Values in Horses" Animals 9, no. 1: 13. https://doi.org/10.3390/ani9010013
APA StyleSaastamoinen, M., Särkijärvi, S., & Hyyppä, S. (2019). Garlic (Allium Sativum) Supplementation Improves Respiratory Health but Has Increased Risk of Lower Hematologic Values in Horses. Animals, 9(1), 13. https://doi.org/10.3390/ani9010013





