Influence of Liver Condition and Copper on Selective Parameters of Post-Mortem Dog Tissue Samples
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Analysis
2.3. Liver Pathology Classification
2.4. Statistical Analysis
3. Results
3.1. Copper Correlation with Plasma Metabolites
3.2. Plasma Metabolites of Different Liver Pathology Conditions
3.3. Blood Chemistry and Complete Cell Count of Different Liver Conditions
4. Discussion
4.1. Effect of Copper Concentration on Plasma Metabolites
4.2. Effect of Liver Condition on Plasma Metabolites
4.3. Effect of Liver Condition on Blood Chemistry and Cell Blood Count
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meyer, H.; Twedt, D.; Roudebush, P.; Dill-Macky, E. Hepatobiliary disorders. In Small Animal Clinical Nutrition, 5th ed.; Hand, M., Thatcher, C., Remillard, R., Roudebush, P., Novotny, B., Eds.; Mark Morris Institute: Topeka, KS, USA, 2010; pp. 1155–1194. [Google Scholar]
- Poldervaart, J.H.; Favier, R.P.; Penning, L.C.; van den Ingh, T.S.; Rothuizen, J. Primary hepatitis in dogs: A retrospective review (2002–2006). J. Vet. Intern. Med. 2009, 23, 72–80. [Google Scholar] [CrossRef]
- Wedeking, K.; Yu, S.; Kats, L.; Paetau-Robinson, I.; Cowell, C. Micronutrients: Minerals and vitamins. In Small Animal Clinical Nutrition; Hand, M., Thatcher, C., Remillard, R., Roudebush, P., Novotny, B., Eds.; Mark Morris Institute: Topeka, KS, USA, 2010; pp. 107–148. [Google Scholar]
- Fieten, H.; Penning, L.C.; Leegwater, P.A.; Rothuizen, J. New canine models of copper toxicosis: Diagnosis, treatment, and genetics. Ann. N. Y. Acad. Sci. 2014, 1314, 42–48. [Google Scholar] [CrossRef]
- Klotz, L.O.; Kroncke, K.D.; Buchczyk, D.P.; Sies, H. Role of copper, zinc, selenium and tellurium in the cellular defense against oxidative and nitrosative stress. J. Nutr. 2003, 133, 1448S–1451S. [Google Scholar] [CrossRef]
- Hoffmann, G.; van den Ingh, T.S.; Bode, P.; Rothuizen, J. Copper-associated chronic hepatitis in labrador retrievers. J. Vet. Intern. Med. 2006, 20, 856–861. [Google Scholar] [CrossRef]
- Spee, B.; Arends, B.; van den Ingh, T.S.; Penning, L.C.; Rothuizen, J. Copper metabolism and oxidative stress in chronic inflammatory and cholestatic liver diseases in dogs. J. Vet. Intern. Med. 2006, 20, 1085–1092. [Google Scholar] [CrossRef]
- Dirksen, K.; Fieten, H. Canine copper-associated hepatitis. Vet. Clin. N. Am. Small Anim. Pract. 2017, 47, 631–644. [Google Scholar] [CrossRef]
- Evans, A.M.; Bridgewater, B.R.; Liu, Q.; Mitchell, M.W.; Robinson, R.J.; Dai, H.; Stewart, S.J.; DeHaven, C.D.; Miller, L.A.D. High resolution mass spectrometry improves data quantity and quality as compared to unit mass resolution mass spectrometry in high-throughput profiling metabolomics. Metabolomics 2014, 4, 1. [Google Scholar]
- Aitchison, J. Logratios and natural laws in compositional data analysis. Math. Geol. 1999, 31, 563–580. [Google Scholar] [CrossRef]
- Tvedten, H. Appendix II. In Small Animal Clinical Diagnosis by Laboratory Methods, 4th ed.; Willard, M.D., Tvedten, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2011; pp. 417–418. [Google Scholar]
- Johnston, A.N.; Center, S.A.; McDonough, S.P.; Wakshlag, J.J.; Warner, K.L. Hepatic copper concentrations in Labrador Retrievers with and without chronic hepatitis: 72 cases (1980–2010). J. Am. Vet. Med. Assoc. 2013, 242, 372–380. [Google Scholar] [CrossRef]
- Baker, D.H. Cupric oxide should not be used as a copper supplement for either animals or humans. J. Nutr. 1999, 129, 2278–2279. [Google Scholar] [CrossRef]
- Hall, A.M.; Kou, K.; Chen, Z.; Pietka, T.A.; Kumar, M.; Korenblat, K.M.; Goodwin, B. Evidence for regulated monoacylglycerol acyltransferase expression and activity in human liver. J. Lipid Res. 2012, 53, 990–999. [Google Scholar] [CrossRef] [Green Version]
- Spiteller, P.; Kern, W.; Reiner, J.; Spiteller, G. Aldehydic lipid peroxidation products derived from linoleic acid. Biochim. Biophys. Acta 2001, 1531, 188–208. [Google Scholar] [CrossRef]
- Huster, D.; Finegold, M.J.; Morgan, C.T.; Burkhead, J.L.; Nixon, R.; Vanderwerf, S.M.; Lutsenko, S. Consequences of copper accumulation in the livers of the Atp7b-/- (Wilson disease gene) knockout mice. Am. J. Pathol. 2006, 168, 423–434. [Google Scholar] [CrossRef]
- De Wolski, K.; Fu, X.; Dumont, L.J.; Roback, J.D.; Waterman, H.; Odem-Davis, K.; Zimring, J.C. Metabolic pathways that correlate with post-transfusion circulation of stored murine red blood cells. Haematologica 2016, 101, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Del Carmen Fernández-Tomé, M.; Speziale, E.H.; Sterin-Speziale, N.B. Phospholipase C inhibitors and prostaglandins differentially regulate phosphatidylcholine synthesis in rat renal papilla: Evidence of compartmental regulation of CTP:phosphocholine cytidylyltransferase and CDP-choline:1,2-diacylglycerol cholinephosphotransferase. Biochim. Biophys. Acta 2002, 1583, 185–194. [Google Scholar]
- Jackson, R.C.; Lui, M.S.; Boritzki, T.J.; Morris, H.P.; Weber, G. Purine and pyrimidine nucleotide patterns of normal, differentiating, and regenerating liver and of hepatomas in rats. Cancer Res. 1980, 40, 1286–1291. [Google Scholar]
- Wu, N.; Yang, M.; Gaur, U.; Xu, H.; Yao, Y.; Li, D. Alpha-ketoglutarate: Physiological functions and applications. Biomol. Ther. 2016, 24, 1–8. [Google Scholar] [CrossRef]
- Vatrinet, R.; Leone, G.; De Luise, M.; Girolimetti, G.; Vidone, M.; Gasparre, G.; Porcelli, A.M. The α-ketoglutarate dehydrogenase complex in cancer metabolic plasticity. Cancer Metab. 2017, 5, 3. [Google Scholar] [CrossRef]
- Stipanuk, M.H. Sulfur amino acid metabolism: Pathways for production and removal of homocysteine and cysteine. Annu. Rev. Nutr. 2004, 24, 539–577. [Google Scholar] [CrossRef]
- Jaeken, J. Disorders of Glutamine, Serine and Asparagine Metabolism. In Inborn Metabolic Diseases, 6th ed.; Saudubray, J., Baumgartner, M., Walter, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 357–362. [Google Scholar]
- Newsholme, P.; Procopio, J.; Lima, M.M.R.; Pithon-Curi, T.C.; Curi, R. Glutamine and glutamate—Their central role in cell metabolism and function. Cell Biochem. Funct. 2003, 21, 1–9. [Google Scholar] [CrossRef]
- Kalhan, S.C.; Guo, L.; Edmison, J.; Dasarathy, S.; McCullough, A.J.; Hanson, R.W.; Milburn, M. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism 2011, 60, 404–413. [Google Scholar] [CrossRef] [Green Version]
- Kato, D.-I.; Miyamoto, K.; Ohta, H. Microbial deracemization of α-amino acids. J. Mol. Catal. B Enzym. 2005, 32, 157–165. [Google Scholar] [CrossRef]
- Trauner, M.; Boyer, J.L. Bile salt transporters: Molecular characterization, function, and regulation. Physiol. Rev. 2003, 83, 633–671. [Google Scholar] [CrossRef]
- Crocenzi, F.A.; Sanchez Pozzi, E.J.; Pellegrino, J.M.; Rodriguez Garay, E.A.; Mottino, A.D.; Roma, M.G. Preventive effect of silymarin against taurolithocholate-induced cholestasis in the rat. Biochem. Pharmacol. 2003, 66, 355–364. [Google Scholar] [CrossRef]
- Murphy, G.M.; Jansen, F.H.; Billing, B.H. Unsaturated monohydroxy bile acids in cholestatic liver disease. Biochem. J. 1972, 129, 491–494. [Google Scholar] [CrossRef] [Green Version]
- Arouri, A.; Mouritsen, O.G. Membrane-perturbing effect of fatty acids and lysolipids. Prog. Lipid Res. 2013, 52, 130–140. [Google Scholar] [CrossRef]
- Stafford, R.E.; Fanni, T.; Dennis, E.A. Interfacial properties and critical micelle concentration of lysophospholipids. Biochemistry 1989, 28, 5113–5120. [Google Scholar] [CrossRef]
- Jonas, A. Lecithin cholesterol acyltransferase. Biochim. Biophys. Acta 2000, 1529, 245–256. [Google Scholar] [CrossRef]
- Ozer, J.; Ratner, M.; Shaw, M.; Bailey, W.; Schomaker, S. The current state of serum biomarkers of hepatotoxicity. Toxicology 2008, 245, 194–205. [Google Scholar] [CrossRef]
- Fernandez, N.J.; Kidney, B.A. Alkaline phosphatase: Beyond the liver. Vet. Clin. Pathol. 2007, 36, 223–233. [Google Scholar] [CrossRef]
- Liptak, J.M.; Dernell, W.S.; Monnet, E.; Powers, B.E.; Bachand, A.M.; Kenney, J.G.; Withrow, S.J. Massive hepatocellular carcinoma in dogs: 48 cases (1992–2002). J. Am. Vet. Med. Assoc. 2004, 225, 1225–1230. [Google Scholar] [CrossRef]
- Hastbacka, J.; Pettila, V. Prevalence and predictive value of ionized hypocalcemia among critically ill patients. Acta Anaesthesiol. Scand. 2003, 47, 1264–1269. [Google Scholar] [CrossRef]
- Witters, P.; Freson, K.; Verslype, C.; Peerlinck, K.; Hoylaerts, M.; Nevens, F.; Van Geet, C.; Cassiman, D. Review article: Blood platelet number and function in chronic liver disease and cirrhosis. Aliment. Pharmacol. Ther. 2008, 27, 1017–1029. [Google Scholar] [CrossRef]
- Ceron, J.J.; Eckersall, P.D.; Martynez-Subiela, S. Acute phase proteins in dogs and cats: Current knowledge and future perspectives. Vet. Clin. Pathol. 2005, 34, 85–99. [Google Scholar] [CrossRef]
- Kaneko, J. Serum proteins and the dysproteinemias. In Clinical Biochemistry of Domestic Animals, 4th ed.; Kaneko, J., Harvey, J., Bruss, M., Eds.; Elsevier Science & Technology Books: New York, NY, USA, 1997; pp. 117–138. [Google Scholar]
| Liver Pathology | Normal | Mild | Moderate | Severe | Neoplasia | Total |
|---|---|---|---|---|---|---|
| N | 16 | 19 | 9 | 6 | 5 | 55 |
| Breed (B/L/LM) | 14/2/0 | 11/5/3 | 5/2/2 | 4/1/1 | 3/2/0 | 37/12/6 |
| Age average ± SD (range) | 12.1 ± 4.47 (0.4–16.4) | 12.6 ± 2.82 (4.5–16.3) | 13.5 ± 3.02 (8–16.3) | 13.8 ± 1.74 (11–15.9) | 12.7 ± 2.22 (10.3–15) | 12.8 ± 3.23 (0.4–16.4) |
| Gender (IF/SF/NM) | 1/9/6 | 1/12/6 | 0/5/4 | 0/2/4 | 0/1/4 | 2/29/24 |
| Liver copper (dw; ±SD, range) | 401 ± 667.9 (86.1–2768) | 294 ± 142.2 (142–773) | 207 ± 128.8 (78.8–456) | 212 ± 110.8 (28.7–378) | 203 ± 66.0 (92.5–254) | 294 ± 375.1 (28.6–2768) |
| Plasma Metabolites (N = 55) | R | p-Value |
|---|---|---|
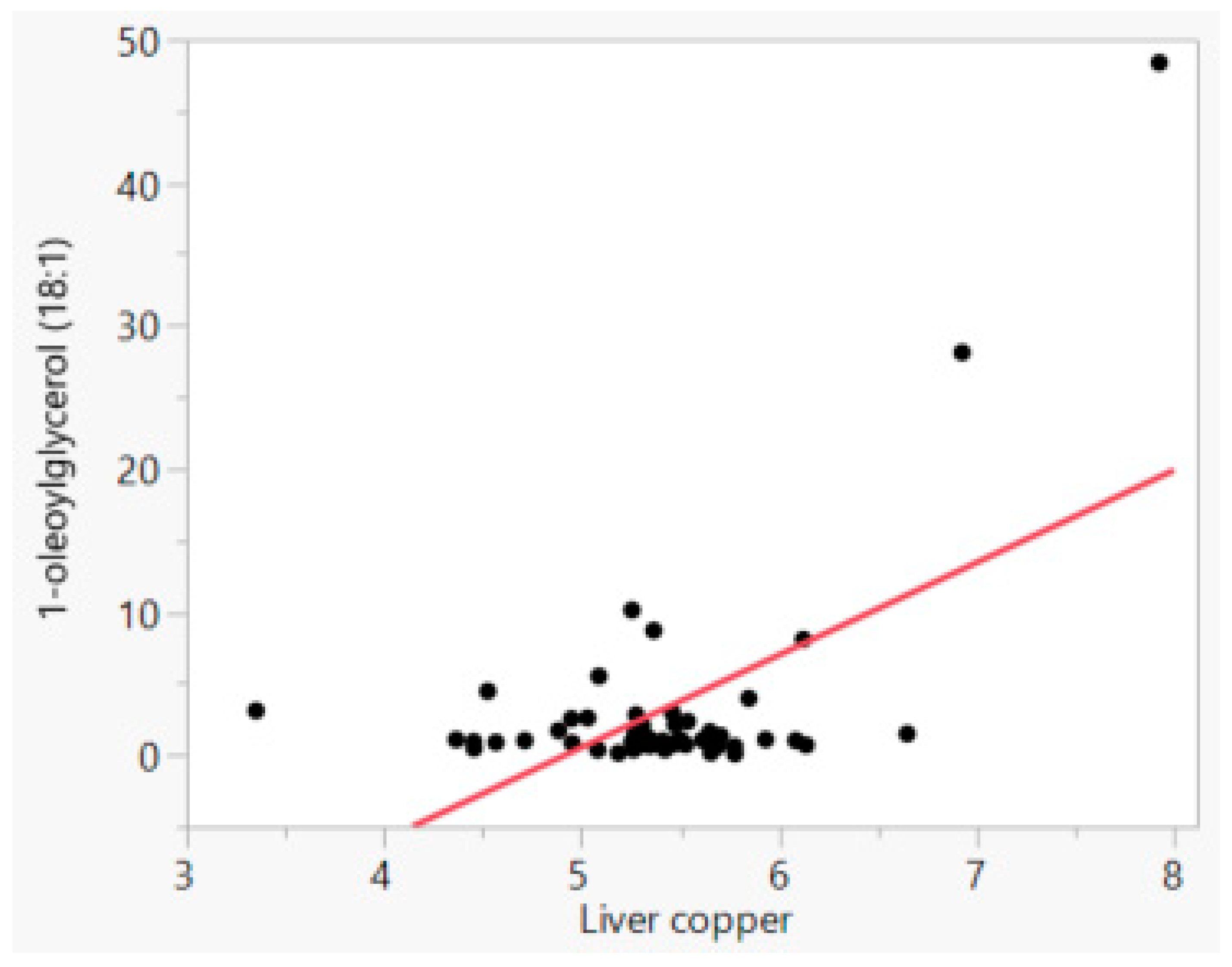
| 1-oleoyl glycerol (18:1) | 0.581 | <0.0001 |
| 1-linoleoylglycerol (18:2) | 0.557 | <0.0001 |
| 2-linoleoylglycerol (2-monolinolein) | 0.504 | <0.0001 |
| 1-arachidonylglycerol | 0.425 | 0.0012 |
| 13-HODE + 9-HODE | 0.436 | 0.0009 |
| Stearoyl-arachidonoyl-glycerophosphocholine (2) | 0.427 | 0.0011 |
| Parameter | Normal | Mild | Moderate | Severe | Neoplasia | p-Value |
|---|---|---|---|---|---|---|
| N | 16 | 19 | 9 | 6 | 5 | |
| Copper 1 | 5.56 ± 0.254 | 5.63 ± 0.238 | 5.19 ± 0.284 | 5.19 ± 0.324 | 5.29 ± 0.344 | 0.3314 |
| 1-methyladenosine | 0.863 b ± 0.0862 | 1.124 b ± 0.0791 | 0.966 b ± 0.1149 | 1.187 b ± 0.1407 | 1.845 a ± 0.1542 | <0.0001 |
| Xanthosine | 1.52 b ± 0.559 | 1.27 b ± 0.513 | 0.62 b ± 0.745 | 0.76 b ± 0.913 | 5.48 a ± 1.000 | 0.0035 |
| N-acetyl-beta-alanine | 1.222 b ± 0.1921 | 1.251 b ± 0.1763 | 1.062 b ± 0.2562 | 0.795 b ± 0.3138 | 2.500 a ± 0.3437 | 0.0073 |
| α-ketoglutarate | 0.946 b ± 0.3185 | 0.982 b ± 0.2923 | 1.211 b ± 0.4247 | 1.325 ab ± 0.5201 | 3.581 a ± 0.5698 | 0.0030 |
| α-ketobutyrate | 1.153 b ± 0.2736 | 1.287 b ± 0.2511 | 1.543 ab ± 0.3648 | 0.837 b ± 0.4468 | 3.165 a ± 0.4895 | 0.0074 |
| Asparagine | 1.07 b ± 0.1476 | 1.01 b ± 0.1355 | 1.01 b ± 0.1968 | 2.11 a ± 0.2411 | 1.28 ab ± 0.2641 | 0.0035 |
| Glutamate | 0.903 b ± 0.3118 | 1.097 ab ± 0.2861 | 1.167 ab ± 0.4157 | 1.921 ab ± 0.5091 | 2.812 a ± 0.5577 | 0.0373 |
| 2-aminoheptanoate | 1.26 b ± 0.719 | 1.62 b ± 0.660 | 1.28 b ± 0.959 | 6.78 a ± 1.175 | 2.29 ab ± 1.287 | 0.0030 |
| Taurolithocholate | 0.306 b ± 0.1711 | 0.341 b ± 0.1570 | 0.277 b ± 0.2281 | 1.669 a ± 0.2794 | 0.399 b ± 0.3061 | 0.0014 |
| Taurocholate | 1.469 b ± 2.4617 | 0.901 b ± 2.2590 | 4.541 ab ± 3.2822 | 16.466 a ± 4.0199 | 1.789 ab ± 4.4036 | 0.0215 |
| 1-Arachidonoyl-GPC (20:4) | 0.765 b ± 0.2542 | 1.134 b ± 0.2333 | 1.360 ab ± 0.3390 | 2.737 a ± 0.4151 | 0.882 b ± 0.4548 | 0.0042 |
| 1-oleoyl-GPC (18:1) | 0.792 b ± 0.2258 | 1.191 b ± 0.2072 | 1.379 ab ± 0.30113 | 2.527 a ± 0.3688 | 1.443 ab ± 0.4040 | 0.0057 |
| 2-palmitoyl-GPC (16:0) | 0.936 b ± 0.2629 | 1.184 ab ± 0.2412 | 1.437 ab ± 0.3505 | 2.921 a ± 0.4293 | 1.180 ab ± 0.4702 | 0.0057 |
| Parameter | Normal | Mild | Moderate | Severe | Neoplasia | p-Value |
|---|---|---|---|---|---|---|
| ALT 1 (N) | 3.38 b ± 0.307 (16) | 4.22 ab ± 0.278 (19) | 5.00 a ± 0.377 (9) | 5.41 a ± 0.452 (6) | 4.55 ab ± 0.491 (5) | 0.0008 |
| ALP 2 (N) | 5.11 b ± 0.307 (16) | 5.39 ab ± 0.282 (19) | 5.93 ab ± 0.410 (9) | 7.04 a ± 0.502 (6) | 5.99 ab ± 0.550 (5) | 0.0240 |
| Calcium 3 (N) | 2.35 a ± 0.025 (16) | 2.32 a ± 0.024 (18) | 2.24 ab ± 0.034 (9) | 2.28 ab ± 0.042 (6) | 2.16 b ± 0.045 (5) | 0.0040 |
| Platelet count 4 (N) | 6.01 a ± 0.172 (16) | 5.88 a ± 0.161 (18) | 6.22 a ± 0.216 (7) | 6.32 a ± 0.228 (6) | 5.14 b ± 0.243 (5) | 0.0016 |
| Globulin, g/dL 5 (N) | 1.010 ab ± 0.1296 (16) | 1.046 ab ± 0.1281 (13) | 1.230 a ± 0.1416 (5) | 1.114 ab ± 0.1466 (6) | 0.836 b ± 0.1466 (5) | 0.0260 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corsato Alvarenga, I.; Aldrich, C.G.; Jewell, D.E. Influence of Liver Condition and Copper on Selective Parameters of Post-Mortem Dog Tissue Samples. Animals 2018, 8, 237. https://doi.org/10.3390/ani8120237
Corsato Alvarenga I, Aldrich CG, Jewell DE. Influence of Liver Condition and Copper on Selective Parameters of Post-Mortem Dog Tissue Samples. Animals. 2018; 8(12):237. https://doi.org/10.3390/ani8120237
Chicago/Turabian StyleCorsato Alvarenga, Isabella, Charles Gregory Aldrich, and Dennis E. Jewell. 2018. "Influence of Liver Condition and Copper on Selective Parameters of Post-Mortem Dog Tissue Samples" Animals 8, no. 12: 237. https://doi.org/10.3390/ani8120237
APA StyleCorsato Alvarenga, I., Aldrich, C. G., & Jewell, D. E. (2018). Influence of Liver Condition and Copper on Selective Parameters of Post-Mortem Dog Tissue Samples. Animals, 8(12), 237. https://doi.org/10.3390/ani8120237




