1. Introduction
Antibiotics have been included in a poultry diet for many years to enhance growth rates, stabilize gut microbiota, and protect against some enteric diseases that cause significant loss in productivity. Due to the increase in the microbial resistance to antibiotics and residues in chicken meat products that are known to be harmful to consumers, the European Union (EU) has forbidden the use of antibiotic feed additives in poultry diets [
1]. Therefore, there is a growing interest in finding alternatives (organic feeding) to antibiotics for poultry production [
2]. Probiotics, prebiotics, and phytogenic feed additives with different ratios and combinations are important alternatives to antibiotics [
3,
4]. Moreover, lysozyme is considered as the most recent potentially effective feed additive that can be used to substitute for antibiotics [
5].
It is well known that intestinal microbiota of chickens has a wide metabolic potential and it affects both the nutrition and health of the host. Increased counts of some harmful pathogens, such as Clostridium perfringens and Escherichia coli, may directly affect gut health, reduce nutrient absorption, bird growth, body weight gain (BWG), livability of birds and increase the feed conversion ratio (FCR). Therefore, it is considered as a strong indicator for poor intestinal integrity and digestion. This overgrowth of harmful bacteria might be related to decrease of beneficial bacteria as Lactobacillus counts.
Probiotics are live and harmless micro-organisms that assist in modulating intestinal microbiota for improving growth and utilization of feed in broiler chickens [
6,
7]. The use of probiotics in poultry should be based on their functions through the ability to competitively exclude pathogens from colonizing the intestine, which improves the gut flora and enhances the immunomodulatory activity, as well as the growth rate of the chicks.
Clostridium butyricum (
C. butyricum) is a gram-positive, anaerobic, spore-forming bacterium, isolated from soil, healthy animals, and human fecal matter, which produces butyric acid [
8]. It can resist low pH and high bile concentrations. Moreover,
C. butyricum can be used as an alternative to antibiotics as it can protect against infections [
9]. Dietary supplementation of
C. butyricum has been demonstrated by several researchers to promote growth performance [
8,
10], improve immune function [
11,
12], modulate nitrogen emissions, improve gut morphology, and maintain a balanced intestinal microflora in chickens [
11,
13].
Saccharomyces cerevisiae (
S. cerevisiae) is one of the most widely distributed species of yeast used in animal nutrition. Upon addition of yeast to their diets at the end of the rearing period, the growth rate and feed utilization were enhanced in chicks [
14,
15], villus height in the ileum was increased [
9], and through colonization of beneficial intestinal microflora, species was maintained through competitive exclusion [
16,
17]. Many studies have evaluated the effects of individual or a mixture of probiotic microbes of the same species. Chapman et al. [
18] reported an increased response to multistrain, in comparison to monospecies probiotics which could be attributed to their synergistic interactions. A proper mixture of multimicrobe probiotics is an effective strategy to elicit the beneficial effect of
C. butyricum and
S. cerevisiae. Therefore, the present study aimed to evaluate the effect of
C. butyricum or
S. cerevisiae, individually and in combination, on growth, gut health, and immunity of broilers.
4. Discussion
The beneficial effects of individual supplementation with
C. butyricum or S. cerevisiae probiotics or their mixture on the growth measurements are similar to results by Bostami et al. [
30], who compared the growth stimulatory effects among birds fed an antibiotic and birds that were administered a probiotic mixture (comprising different combination of
Bacillus,
Lactobacillus,
Saccharomyces,
Streptococcus,
Enterococcus, and
Clostridium) with feed and water. In addition, these authors included multistrain species probiotics composed of
Bacillus,
Lactobacillus,
Saccharomyces, and
Rhodopseudomonas in the diet [
31], and concluded that the
Rhodopseudomonas-based probiotic mixture had the ability to be used as an alternative feed additive to antibiotics in broilers.
The improvement in Bw, BWG, FCR, and PER values in G4 chicks might be attributed to the positive effects of probiotics. The probiotics assisted in stabilizing beneficial gut microbes, increasing the digestion of nutrients, stabilizing beneficial gut microbes, enhancing immuno-modulation, and increasing the intestinal villi length, thereby increasing the surface area of absorption, and improving bird health [
12,
32]. The growth promoting effect observed in the probiotic mixture group (G4) could be attributed to the synergistic actions of
C. butyricum and
S. cerevisiae, which contained mannan oligosaccharides (derived from the cell wall of
S. cerevisiae; affect through fermentation of different sugars and synthesis of enzymes that can help in better utilization of nutrients and act as a substrate for
C. butyricum in the gut of broilers) [
18,
33]. In addition, probiotic bacteria can protect against several harmful pathogens.
The results of the present study showed a reduction in the mortality rate of the probiotic-supplemented chicks; this result is similar to that of Cmiljanic et al. [
34] , who reported that mortality percentage could be decreased by the dietary inclusion of Paciflor-C
® as
Arbor Acres broilers. The percentage of decreased mortality might be related to the defensive function of the probiotics on the gut wall, thereby enhancing the immune response [
35]. In addition, the
C. butyricum-based probiotic had more beneficial effects on the performance parameters in comparison to the addition of
S. cerevisiae, emphasizing the improved results of the probiotic mixture group due to the synergistic action between the two probiotic strains. These effects could be attributed to the fact that
C. butyricum produces butyric acid that promotes nutrient metabolism and modulates gut microbiota [
13]. Moreover, Nakanishi et al. [
36] found that
C. butyricum could produce large amounts of short-chain fatty acids, such as butyrate and acetate, which are important energy resources for animals and stimulate colonic sodium and fluid absorption.
Kim et al. [
4] and Sen et al. [
37] found that dietary probiotic supplementation increased the villus height and villus height/crypt depth ratio, but decreased the crypt depth in broilers. These results support our findings in
Table 3. In addition, the results of the present study are in agreement with those of Zhang et al. [
8], who found that dietary inclusion of
C. butyricum in diets increased the jejunal villus height and relative length of the cecum in broilers. These results could be attributed to the production of butyric acid by
C. butyricum that might provide the energy required for epithelial growth [
38]. Our results suggest that dietary inclusion of
C. butyricum in broiler chicks, separately or in combination with
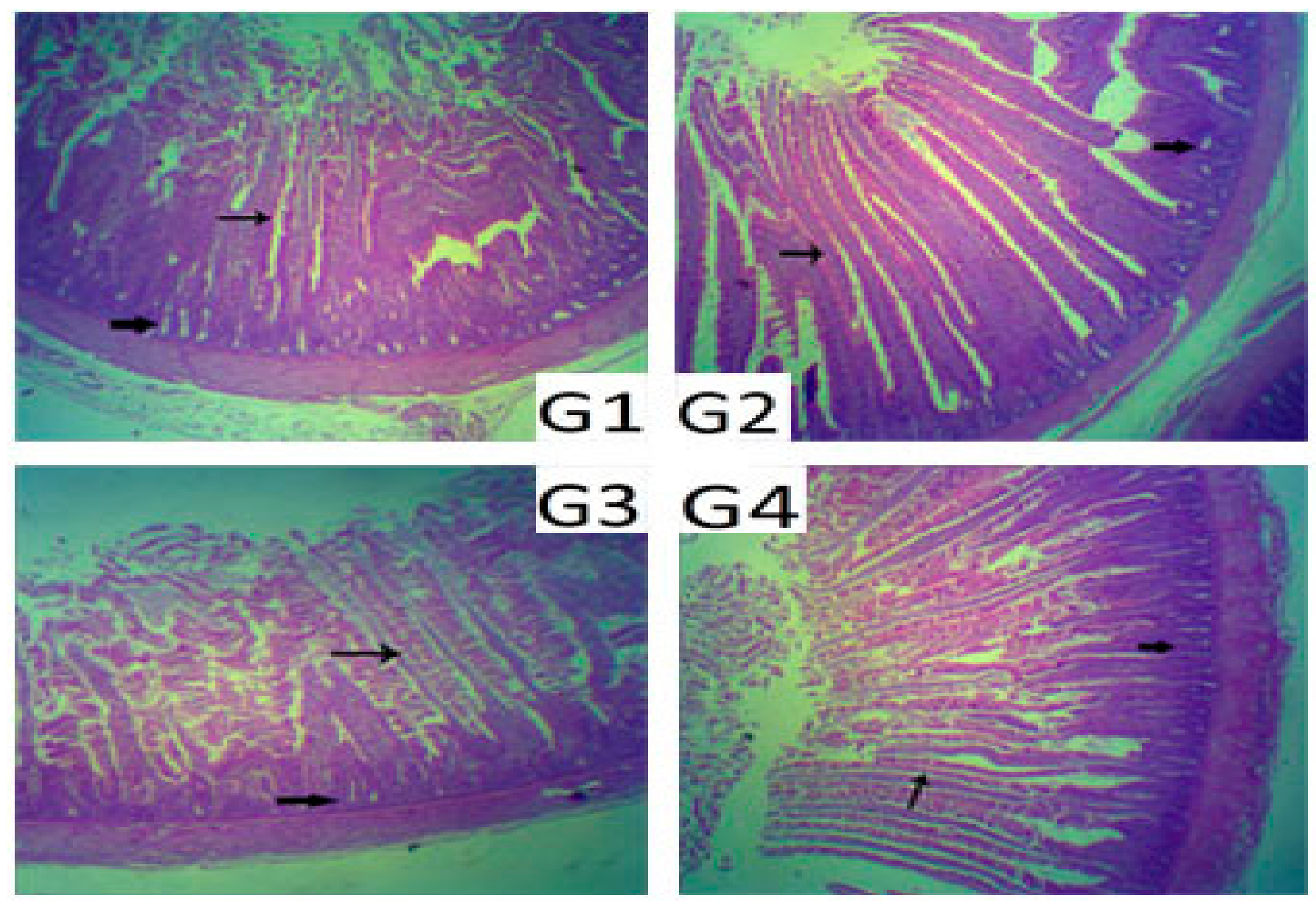
S. cerevisiae, was beneficial to the intestinal morphology in broiler chickens. The enhancements of villus length and crypt depth in broiler chickens are important in facilitating the absorption of nutrients and improving the efficiency of the gut. This might explain the better growth performance of the G3 and G4 chicks in the present study in comparison to the other treatment groups.
Results of intestinal microbiota (
Table 4) indicated a significant decrease (
p ≤ 0.05) in both total coliform and clostridial counts in birds of G2 and G4 compared to the other two groups. Regarding
Lactobacillus counts, the main significant increase (
p ≤ 0.05) was in birds of G4 as 7.4 log
10 CFU/g. These results are supported by Zhang et al. [
9] who concluded that
C. butyricum inhibited the growth of harmful microbes, such as
Escherichia coli (
E. coli), whereas the growth of beneficial bacteria, such as
Lactobacillus and
Bifidobacterium, were enhanced. Moreover,
Clostridium can produce elevated levels of short-chain fatty acids, which exhibit therapeutic, bactericidal, and anti-inflammatory effects. This helps in increasing intestinal acidity, which suppresses the growth of harmful intestinal microbes, such as
E. coli [
12], and inhibits the production of Shiga-like toxins and secretion of substances, such as bacteriocins, organic acids, and hydrogen peroxides [
39].
Consistent with the present observation, Gunal et al. [
40] found decreased total bacterial counts in broilers in response to probiotic mixture. The reduction of total bacterial count into the ileum and cecum is assumed to be due to the suppression of potentially pathogenic micro-organisms into the gastrointestinal tract of broiler [
41]. Competitive exclusion of beneficial micro-organisms prevents attachment of pathogenic bacteria by lowering intestinal pH content, which inhibits the growth of pathogenic micro-organisms in the intestine [
39]. Probiotics or Beneficial Microbes (BM) are involved in protection against a variety of pathogens in chickens including
E. coli,
Campylobacter and
Salmonella and can reduce the mortality of birds [
42].
C. butericum can inhibit the growth of pathogenic bacteria such as
E. coli and inhibit the production of Shiga-like toxins, improving the growth performances, humoral immune response and reduced mortality [
39].
It has been previously discovered that the intestinal immunity plays a major role in bird’s immune response [
43]. Here, we measured NDV HI titers as one of the indicators for effective intestinal immune response (gut-associated lymphoid tissues (GALT)) after using a live vaccination for NDV through eye drops and an increase of HI titers in the G2 and G4 chicks compared to those in G3 and control chicks, which are consistent with those reported by Yang et al. [
12], who found that dietary inclusion of
C. butyricum as an alternative to antibiotic/antibacterial agents, such as colistin sulfate, stimulated immune response and modulated the gut microbiota of broilers. Probiotics exert a defensive function on the intestinal wall and immune system [
35], which might help to reduce the mortality. Zhang et al. [
9] reported that dietary inclusion of
C. butyricum exhibited similar or better results in alleviating the immune stress in
E. coli K88-challenged broilers. In addition,
Clostridium is more effective in stimulating immune response and reducing mortality in chicks, owing to its synergistic and biotherapeutic effects [
39].
Regarding carcass traits, similar findings were reported by Rezaeipour et al. [
44], who stated that carcass traits were not affected by L-threonine or
S. cerevisiae supplementations in broilers. However, our results showed that the percentage of abdominal fat was significantly lower (
p < 0.05) in the G4 group than those of the other groups. In conformity with our findings, Liao et al. [
45] showed that supplementing broiler diets with 1 × 10
9 CFU of
C. butyricum/kg decreased the percentage of abdominal fat (
p ˂ 0.05) compared to that of the controls. These varying results might be attributed to the different doses or types of probiotics used, strain of broilers, BD, or environmental conditions. In partial agreement, Mohamed et al. [
46] stated that addition of yeast to broiler diets did not affect the percentage of dressing and relative weight of heart, gizzard, and abdominal fat; however, all the carcass parameters and weights of the internal organs were significantly (
p ≤ 0.05) affected by the dietary treatments, which were also supported by Paryad and Mahmoudi [
47]. Similar to our findings, Liao et al. [
45] showed that supplementing broiler diets with 1 × 10
9 CFU of
C. butyricum/kg decreased the percentage of abdominal fat (
p ˂ 0.05) compared to that of the controls. These varying results might be attributed to the different doses or types of probiotics used, strain of broilers, BD, or environmental conditions.